Abstract
Four water-soluble non-ionic ethoxylated surfactants based on vanillin were synthesized (VE15, VE20, VE40, and VE60). The chemical structures of these surfactants were confirmed using FT-IR and 1H-NMR spectra. The molecular weights of the compounds were determined using viscosity measurements and gel permeation chromatography. Surface tension as a function of the concentration of the surfactant in aqueous solution was measured at 25, 40 and 55 °C. From these measurements, the critical micelle concentration (CMC), effectiveness (πcmc), efficiency (pC20), maximum surface (Γmax) excess and minimum surface area (A min), were calculated. The surface activity measurements showed their high tendency towards adsorption and micellization and their good surface tension reduction, and low interfacial tension. The emulsion stability measurements showed the applicability of these surfactants as emulsifying agents. The thermodynamic parameters of micellization (ΔG mic, ΔH mic, ΔS mic) and adsorption (ΔG ads, ΔG ads, ∆S ads) showed their tendency towards adsorption at the interfaces and also micellization in the bulk of their solutions. The biodegradability of the prepared surfactants was tested in river water using die-away method and showed their readily biodegradation in the open environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years there has been a great interest in surfactants derived from natural products. The use of naturally occurring materials is believed to provide improved biodegradability. Furthermore, renewable sources are necessary for long-term sustainable production. The economic importance of nonionic surfactants [1, 2] for detergent formulations has increased considerably in the past three decades, and more attention is being paid to their environmental compatibility, necessitating proof of their biodegradability under natural conditions. Although test criteria exist as required by legislation, detailed knowledge of the environmental fate of nonionic surfactants and of their biodegradability in sewage treatment plants is vital. Important nonionic surfactant families are the poly ethoxylates based on fatty alcohols or alkyl phenols. T-Nonyl phenol ethoxylates have many industrial, commercial, institutional, and domestic uses since they are very efficient and cost-effective surfactants. The presence of alkyl phenols and their ethoxylates in the environment has been reviewed extensively [3]. Nonylphenol ethoxylates degrade slowly and the degradation products are more toxic and persistent than the parent surfactants [4]. Vanillin (4-hydroxy-3-methoxy benzaldehyde) is the major component of natural vanilla, which is one of the most widely used and important flavoring materials worldwide. The source of vanilla is the bean, or pod, of the tropical Vanilla orchid [5]. In common with many other low-molecular weight phenolic compounds, vanillin displays antioxidant and antimicrobial properties and hence has the potential for use as a food preservative [6, 7]. It is active against both Gram-positive and Gram-negative food-spoilage bacteria and has been shown to be effective against both yeasts and molds in fruit purées and laboratory growth media [8–10]. Also vanillin used as pesticide, green corrosion inhibitors for corrosion of different metals [11]. Vanillin has many advantages such as low cost, non-toxicity and easy production with the annual production capability in the world being able reach up to 12,000 ton [12].
In this study, naturally occurring vanillin was chemically modified into nonionic surface active agents containing hydrocarbon chains and different numbers of polyethylene oxide units (15, 20, 40 and 60 units). The surface properties and thermodynamic parameters of the produced vanillin derived surfactants were determined. Further to this, the biodegradability of the new synthesized surfactants was measured.
Materials and Measurements
Chemicals
Commercial vanillin, castor seed oil, monoethanol amine and ethylene oxide, were purchased from El Goumhoria Trade Pharmaceuticals & Chemicals Company, Cairo, Egypt. Sodium hydroxide and hydrochloric acid were analytical grade chemicals obtained from Merck chemical company.
Synthesis
Hydrolysis of Castor Oil
Castor seed oil was hydrolyzed according the procedures in Ref. [13]. In typical procedures, 100 g of castor oil was reacted with sodium hydroxide solution (250 mL of 10 % by weight) and the reaction mixture was heated by a water bath for 2 h. Then bidistilled water (400 mL) was added while stirring for 1.5 h until the mixture became almost clear. After cooling, 300 mL of HCl solution (30 % by weight) was added portion wise under stirring for 3 h. The reaction mixture was allowed to cool at room temperature, and then transferred into a 1-L separating funnel to separate the aqueous layer. The oil phase was then separated, washed three times with bidistilled water to remove the excess acid and salts and then dried under vacuum (0.1 bar) at 40 °C for 24 h. The fatty acids obtained were analyzed using GPC-chromatography and showed the following compositions: ricinoleic acid (89.5 %), linoleic acid (4.2 %), oleic acid (3 %), palmetic acid (1.0 %), and stearic acid (1.0 %). The molecular weight of the produced fatty acid (296 g mol−1) was calculated from the obtained ratio of these acids.
Reaction of Vanillin with Hydrolyzed Castor Seed Oil
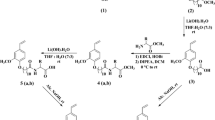
Vanillin (4-hydroxy-3-methoxybenzaldehyde) (0.2 mol) and the hydrolyzed acid of the castor seed oil (0.2 mol) were esterified in 200 mL xylene as a solvent and in the presence of 0.01 % p-toluene sulphonic acid as a catalyst. The mixture was refluxed until the water of the reaction was obtained (3.6 mL) which indicated the end of the reaction. The solvent was stripped off under vacuum using a rotary evaporator. Then, 200 mL of distilled water was added to dissolve the unreacted vanillin and the catalyst, while the oily layer was separated using a separating funnel. Vacuum distillation was then performed to the oily layer to complete drying to afford the vanillin fatty ester (I) [14], Scheme 1.
Reaction of Vanillin Ester with Mono Ethanol Amine
To 0.1 mol of vanillin fatty ester (I), 0.1 mol of monoethanol amine was added and refluxed in 100 mL of ethanol as a solvent for 8 h. The reaction mixture was left to cool and then filtered. The products were recrystallized three times from ethanol and dried in vacuum oven at 40 °C to afford the Schiff base of vanillin fatty ester (II) [15], Scheme 1.
Synthesis of Ethoxylated Nonionic Surfactants
The reaction between vanillin-monoethanol amine Schiff base ester (II) and ethylene oxide was carried out following the procedures of Ref. [16]. In a typical experiment, 1 mol of the synthesized ester was charged into the reaction system (described in Ref. [16]) in the presence of 1 % triethyl amine as a catalyst and then was heated to 150–180 °C with continuous stirring. A stream of nitrogen gas was passed through the system for 2 min to flush out the air. The nitrogen stream was then replaced by an ethylene oxide stream at a fixed rate, which was regulated by monitoring the Hg level of the manometer. The reaction was carried out for different time periods after which the apparatus was filled with nitrogen, cooled and weighed. After cooling, the product obtained was discharged, weighed and the catalyst was evaporated under vacuum (0.1 mm Hg, at 100 °C). The ethoxylated products obtained were brown viscous liquids. The differences in weights indicated the amount of the ethylene oxide units consumed in the reaction, hence, the number of moles of ethylene oxide (n) attached to each mole of the reactants was calculated. The total number of ethylene oxide units attached to the ester was 15, 20, 40, and 60 units. The ethoxylated products obtained were designated as VE15, VE20, VE40, and VE60, where: n = 15, 20, 40 and 60, Scheme 1.
Measurements
Viscometric Measurements
The intrinsic viscosities (η) of the prepared compounds were measured in distilled water at 25 °C using a capillary viscometer (Ubbelohde suspended level type) under thermostated condition (25 ± 0.5 °C) at surfactant concentrations in the range 0.005–5.0 g L−1. The molecular weights (M. Wt.) were calculated using Eq. (1) [17]:
The obtained average molecular weights using viscosity measurements (M. Wt.V) of the different synthesized compounds were compared by the expected molecular weights and listed in Table 1.
Gel permeation Chromatography (GPC)
GPC experiments were carried out using a Supremax 3,000 column (Polymer Standard Service, Mainz, Germany) with ethanol as eluent (1 mL min–1). The system comprised a pump (Hitachi, Darmstadt, Germany), an auto sampler device (Merck Hitachi model AS–2000A) and a vacuum in–line degreaser. The amount of injected sample volume per run was 40 μl. The samples were analyzed with a differential refractive index (RI) detector RI–71 made by Merck. Molecular weights were calculated using Astra software (Wyatt Technology Corp). The standards compounds (M. Wt. of 1,000, 2,000, 3,000, and 6,000) were used for calibration. The average molecular weights (M. Wt.GPC) of the synthesized compounds were obtained relative to the standard compounds (Fatty acid solutions in ethanol) and listed in Table 1. The distribution by GPC was given in term of polydispersity and listed in Table 1.
Surface Tension
Surface tension measurements were obtained using a Du–Noüy tensiometer with a platinum ring. Freshly prepared aqueous solutions of the synthesized nonionic surfactants were measured over a concentration range of 0.01–0.000005 ML–1 at 25, 40, and 55 °C. Apparent surface tension values were an average of three readings with 2-min intervals between the readings [18].
Interfacial Tension
The interfacial tension measurements were obtained between an aqueous solution of the synthesized nonionic surfactants at a concentration of 0.1 % and light paraffin oil at 25 °C using the same procedures as the surface tension measurements [18].
Emulsification Power
The procedure was that 10 mL (0.1 wt%) of each of the different surfactant solutions was individually placed in a 100-mL cylinder and then 10 mL of the paraffin oil was added. The cylinder was shaken vigorously for 10 min and then allowed to settle. The time required to separate 9 mL of pure surfactant solution was recorded (average of three readings) and was taken as an indication of the emulsification power of each surfactant [19].
Cloud Point Measurements
For determining the cloud point (CP), 3 ml of the surfactant solution (0.5 wt%) was placed in a Pyrex glass tube (15 ml capacity), which was then placed in a controlled heating apparatus. The temperature was raised slowly at a rate of 0.1 °C min−1 near the CP, and the temperature at the onset of sudden clouding in the solution was taken as CP [20].
Biodegradability
The biodegradability test (Die away method) in river water of the nonionic surfactant was determined by the surface tension method using a du-Noüy tensiometer (Krüss type K6) [21–23]. In this method, each surfactant was dissolved in river water to a concentration of 100 ppm and incubated at 38 °C. A sample was withdrawn daily (for 28 days), filtered and the surface tension value was measured. The biodegradation percent (D%) was calculated as follows:
where γt is the surface tension at time t, γo is the surface tension at time = 0 (initial surface tension) and γbt is the surface tension of river water without addition of surfactants at time t.
Results and Discussion
Structure
The chemical structures of the synthesized nonionic ethoxylated surfactants (VE n ) were confirmed using FTIR and 1H-NMR spectroscopy as follows (VE15 was taken as a representative sample for the synthesized surfactants):
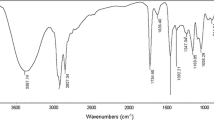
IR spectra: FTIR spectra of the synthesized compounds showed the following absorption bands: 3,392 cm−1 (OH), 2,924 cm−1 (CH3), 2,869 cm−1 (CH2), 1,733 cm−1 (C=O), 1,642 cm−1 (CH=N), 1,458 cm−1 (CH2) n , 1,105 cm−1 (C–O), phenyl groups, 845 cm−1.
1 H-NMR spectra: 1H-NMR spectra of the synthesized compounds in CDCl3, showed signals at: 0.84 ppm (t, 3H, CH 3), 1.26 ppm (m, 24H, CH 2), 1.57 ppm (s, 3H, OCH 3), 2.00 ppm (m, 2H, OCOCH2(CH 2), 3.87 ppm (s, 1H, OH) disappeared by the deuteration, 4.18 ppm (s, 1H, CH=N), 7.25 ppm (m, 3H, C6 H 3). The signal at 3.64 ppm (t, nH, OCH 2CH2) defined the methylene groups in the repeated ethylene oxide units, where n = 60, 80, 160, 240H for VE15, VE20, VE40, VE60, respectively.
Surface Activity
The surface tension versus −log C relationship of the synthesized nonionic surfactant at 25, 40, and 55 °C is shown in Fig. 1a, b (compound VE15 and VE60 were taken as representative for the tested compounds). It is clear that the relationship is characterized by two distinguishable regions. The first at low concentration range and characterized by a fast decrease in the surface tension values, i.e., high slope. While the second (at higher concentrations) where the surface tension variation remains almost constant by increasing the concentration, i.e., the slope is almost constant. The concentration at the break point of these two regions was taken as the critical micelle concentration (CMC).
The CMC values determined from Fig. 1 were listed in Table 2. It is obvious that the increase in the number of oxyethylene (EO) units increases the value of the CMC [24]. That can be attributed to the increase in the hydrophilic character of surfactants in water. Such improvements in the solubility lower the tendency for surfactants to form micelles in water, which consequently increases the CMC values.
From the data presented in Table 2, it can be concluded that there is continuous increase in the CMC values on increasing the number of EO units (15, 20, and 40). Also, the reduction of surface tension is increased by increasing the number of EO units within the surfactant molecules. Surfactant containing 60 EO units (VE60) has the lowest CMC values at 25, 40, and 55 °C (Table 2).
The increase in the temperature of the measurements from 25 to 55 °C leads to a moderate decrease in CMC values. This can be attributed to the hydrogen bonds breakdown. As a result of the temperature rising, the surfactant molecules separate from the aqueous phase due to the breakdown of the hydrogen bonds to form the micelles [25].
The maximum surface excess concentration (Γmax) in mol cm−2 was calculated from the following relationship [18]:
where R = gas constant (8.314) and T = t + 273 (°K).
The Γmax values in Table 2 were used to calculate the average minimum area per adsorbed molecule at the aqueous–air interface at saturated condition (A min) using Eq. 4 [18]:
where N A is Avogadro’s number.
By inspecting the data Table 2, it can be concluded that the Γmax values decrease by increasing the number of EO units in the nonionic chain, and similarly does the temperature rising from 25 to 55 °C. It is apparent from Table 2 that A min values increase by increasing the temperature of the measurement, which could be due to the coiling of the nonionic hydrophobic chains at the interface [26].
Cloud Point for the Ethoxylated Synthesized Compounds
The solubility of nonionic surfactants in the aqueous phase is related to the hydrogen bonds formation between their molecules and the water molecules. Increasing the number of the hydrogen bonds increases the solubility of nonionic surfactants in the water phase. Several factors affect the hydrogen bonds formed, including: temperature, added salts and pH. As the temperature of a nonionic surfactant solution is increased, the hydrogen bonds breakdown due to an increase in the activation energy of the system [25–27]. At a certain temperature, known as the cloud point (CP), the surfactant molecules separate out of the solution, causing it to become cloudy. Ultimately, the surfactant solution separates into two immiscible phases: a surfactant rich phase and a surfactant poor phase. Phase separation occurs because of the difference in density of the micelle rich and micelle poor phases.
Data presented in Table 2 revealed that the cloud points of the nonionic surfactant under consideration increased with increasing the ethylene oxide content. This can be attributed to the increase in the number of oxyethylene units. Increases in the number of the oxyethylene units increases the ethereal bonds (–O–) in the molecule which plays a central role in increasing the hydrogen bonds between water molecules and the lone pairs of electrons of the ethereal oxygen atoms [28]. The surfactant which contains 60 oxyethylene units (VE60) requires higher energy to break the hydrogen bonds, which explains its relatively high cloud point at 74–76 °C. Among the other surfactants which contain 15, 20 and/or 40 oxyethylene units, the cloud points were gradually increased from 57–59 °C for VE15 to 60–62 °C for VE40. Since the cloud point is strongly related to the solubility of the surfactant in water, a plot of cloud points against the number of oxyethylene groups (n) was plotted in Fig. 2. It is noteworthy that the plot shows a linear increase in the cloud point with the number of oxyethylene units; when the number of oxyethylene groups is greatly increased from n = 15 (VE15) to n = 60 (VE60).
Emulsification Power
The tendency of the surfactants solution to form oil-in-water or water-in-oil emulsions is a reflection of the ability of surfactant molecules to locate at the boundary surfaces between the different phases. Hence, the most adsorbed surfactant molecules at the interface are the most powerful emulsifying agents. The emulsification efficiency in this study was measured as the time required for separation of 9 mL of pure water from the emulsion formed between surfactant solution (0.1 % wt) and paraffin oil (10 mL: 10 mL). Increasing the time required for separation of the desired amount of water from the emulsified system indicates the stability of the formed emulsion, and vice versa. Table 2 shows the emulsification tendency of the synthesized surfactant, and reveals that nonionic surfactants which contain short oxyethylene chains (VE15, VE20) exhibit low emulsification efficiencies at 180 and 300 s, respectively. While, surfactants contain long oxyethylene chains VE40 and VE60 form more stable emulsions at 600 and 3,000 s, respectively.
Thermodynamics of Micellization and Adsorption of VE15–VE60 Surfactants
The behaviors of the surfactant at the interface and in the bulk of their solutions depend on the thermodynamic parameters of micellization and adsorption. The thermodynamic parameters including standard free energy, entropy and enthalpy (ΔG, ΔS, ΔH) were calculated using Gibbs equations as follows [18], and data are summarized in Table 3:
where R is the universal gas constant (= 8.314 J mol K−1), T the absolute temperature, πcmc is the effectiveness and A min is the minimum surface area.
All the synthesized surfactants showed negative values of the free energies of micellization and adsorption (∆G mic, ∆G ads) indicating that these two processes occurred spontaneously. Comparing the values of ∆G mic, ∆G ads showed a slight increase in ∆G ads values than ∆G mic. The higher negativity of ∆G ads values indicates the adsorptive tendency of these surfactants rather than micellization tendency. That is attributed to the well arrangement of their molecules at the interface, which decreases the repulsion due to the aqueous phase. Increasing the number of EO units in the nonionic surfactant molecules decreases the ∆G mic and ∆G ads values, which could be attributed to the increase in the solubility of the different analogous as the result of hydrogen bonds formation.
The contribution of each oxyethylene group in the two processes was expressed in term of ∆G/nEO, Table 3. Obviously, the contribution of EO units in the adsorption process is more than their contribution in the micellization process. That is due to the predominance of the hydrogen bonds formation at the interface with water molecules. While, in the case of the micellization process, the system requires more energy to introduce the two methylene group into the bulk of the formed micelles. The gradual increase of the oxyethylene units in the surfactant molecules gradually decreases their contributions in the two processes, as a result of increasing the repulsion between the increased number of ethylene groups and the water molecules. On raising the temperature, the negativity of ∆G mic, ∆G ads are increased due to the stability of the adsorbed and micellized surfactant molecules than the freely dispersed ones in the aqueous phase.
Entropy changes of micellization (∆S mic) are low values which point to the ordering of surfactant molecules participating in the micellar phase. Ordering of the molecules shows the compactness at which the hydrophobic chains are coiled in the micellar core in high compatibility, and the hydrophilic groups faced the water phase. That arrangement decreases the repulsion in the surfactant-aqueous phase system and leads to stabilization of the formed micelles. The sequence of the enthalpy changes (Table 3) shows that both the micellization and adsorption processes are occurred and the majority was for the adsorption process than the micellization process.
Biodegradability
Agricultural, industrial and domestic use of surfactants leads to the entry of these compounds into terrestrial and aquatic ecosystems. Synthetic surfactants vary significantly in structure, but most consist of alkyl or alkyl phenol groups attached to nonionic or anionic hydrophilic moieties [29–34]. Permanent use of alkylphenol ethoxylate compounds usually causes pollution problems because they do not undergo biodegradation by micro-organisms present in soil and water. In the environmentally friendly surfactants, bacteria exploit these potentially useful resources of reduced carbon to derive energy and support growth [35–38].
The biodegradability of the synthesized ethoxylated nonionic surfactants was evaluated using a surface tension method. Since all the prepared surfactants under investigation have the same hydrophobic part, hence, hydrophilic oxyethylene chain length is the sole factor affecting this process. It is clear from the results of the biodegradation die-away test in the river water in Table 4 that the biodegradation ratio of all of the prepared compounds ranged from 70 to 89 % after the 28th day of exposure to the microorganisms. Furthermore, the highest biodegradation extent was obtained in the case of VE40 and VE60 at 82 and 89 % compared to VE15 and VE20 surfactants at 70 and 78 %, respectively. As concluded from the biodegradation ratios, the values meet the international recommendation of the biodegradable surfactants in drain water which is 75 % after 28 days [39]. It is clear that there is a direct relationship between the number of attached oxyethylene units and the percentage of biodegradation. Consequently, these surfactants can be classified as biodegradable surfactants. In fact, the biodegradation affinity is due to the presence of a naturally occurring compound, i.e., vanillin, which has the ability to degrade by the action of the environmental microorganisms. Additionally, the presence of the oxyethylene units within the surfactants structure increases their ability towards biodegradation [40].
The simplest pathway of the degradation considered in case of the studied compounds is the bacterial attack at the far end of either the hydrophobe or the oxyethylene chain, or a central fission separating the hydrophobe and the EO chain. The attack at the terminal group results in shortening the oxyethylene chain by one unit, which is called (w-EO pathway). That pathway is the most common and has been documented [41, 42].
References
Schick MJ (ed) (1966) Non-ionic surfactants. Marcel Dekker, New York
Lynn JL, Bony BH (1992) Encyclopedia of chemical technology, vol 23, 4th edn. Wiley, New York 510
Bennie DT (1999) Review of the environmental occurrence of alkylphenols and alkylphenol ethoxylates. Water Qual Res J Can 34:79
Jobling S, Sumpter JP (1993) Detergent components in sewage effluent are weakly oestrogenic to fish: an in vitro study using rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol 27:361–372
Walton NJ, Mayer MJ, Narbad A (2003) Molecules of interest: vanillin. Phytochemistry 63:505–515
Burri J, Graf M, Lambelet P, Loliger J (1989) Vanillin: more than a flavouring agent—a potent antioxidant. J Sci Food Agric 48:49–56
Davidson PM, Naidu AS (2000) Phytophenols. In: Naidu AS (ed) Natural food antimicrobial systems. CRC Press, Boca Raton, pp 265–294
Cerrutti P, Alzamora SM, Vidales SL (1997) Vanillin as an antimicrobial for producing shelf-stable strawberry puree. J Food Sci 62:608–610
Lopez MA, Alzamora SM, Argaiz A (1998) Vanillin and pH synergistic effects on mould growth. J Food Sci 63:143–146
Fitzgerald DJ, Stratford M, Narbad A (2003) Analysis of the inhibition of food spoilage yeasts by vanillin. Int J Food Microbiol 68:113–122
Li X, Dengb S, Fu H (2010) Adsorption and inhibition effect of vanillin on cold rolled steel in 3.0 M H3PO4. Prog Org Coat 67:420–426
Negm NA, Kandile NG, Mohamad MA (2011) Synthesis, characterization and surface activity of new eco-friendly schiff bases vanillin derived cationic surfactants. J Surf Deterg 14:325–331
Castor Oil and its Chemistry. http://www.groshea.com
Negm NA (2002) Surface activities and electrical properties of long chain diquaternary bola-form amphiphiles. Egypt J Chem 45:483–499
Erk B, Dilmac A, Baran Y, Balaban A (2000) Preparation, characterization and kinetics of formation of some schiff base chelates of Sn(II) and UO2 (V1) synthesis reactivity. Inorg Metalorganic Chem 30:1929–1938
Wrigley AN, Smith FD, Stirton AJ (1957) Synthetic detergents from animal fats VIII. The ethoxylation of fatty acids and alcohols. J Am Oil Chem Soc 34:39–43
Yan RX (1998) Water-soluble polymers. Chemical Industry Press, Beijing, pp 192–193
Negm NA, Mohamed AA, El-Awady MY (2004) Influence of structure on the cationic polytriethanol ammonium bromide derivatives. I. Synthesis, surface and thermodynamic properties. Egypt J Chem 47:369–375
Negm NA, Elkholy YM, Ghuiba FM, Zahran MK, Mahmoud SA, Tawfik SM (2011) Benzothiazol-3-ium cationic Schiff base surfactants: synthesis, surface activity and antimicrobial applications against pathogenic and sulfur reducing bacteria in oil fields. J Adsorp Sci Technol 32:512–518
Kumar S, Sharam D, Khan ZA, Kabir D (2001) Occurrence of cloud point in SDS–tetra-n-butylammonium bromide system. Langmuir 17:5813–5819
Negm NA, El Farargy AFM, Al Sabagh AM, Abdelrahman NR (2011) New Schiff base cationic surfactants: surface and thermodynamic properties and applicability in bacterial growth and metal corrosion prevention. J Surf Deterg 14:505–514
Wakasman SA, Lechevalver HA (1962) The actinomycetes antibiotic of actinomycetes. Williams & Wilkins, Baltimore, USA, p 430
EL-Sukkary MMA, Sayed NA, Aiad I, Helmy SM, EL-Azab WIM (2009) Molecular surface and thermodynamic properties of nonionic surfactants based on castor oil. Tenside Surf Deterg 46:312–319
Al-Sabagh AM (2000) Surface activity and thermodynamic properties of water-soluble polyester surfactants based on 1,3 dicarboxymethoxy-benzene used for enhanced oil recovery. Polym Adv Technol 11:48–55
Hinze WL, Pramouro EA (1993) Critical review of surfactant-mediated phase separations (cloud-point extractions): theory and applications. Crit Rev Anal Chem 24:133–139
Rosen MJ (1976) The relationship of structure to properties in surfactants. IV. Effectiveness in surface or interfacial tension reduction. J Colloid Interface Sci 56:320–327
Rosen MJ (1978) Surfactant and interfacial phenomena. Wiley, New York
Negm NA (2007) Solubilization, surface active and thermodynamic parameters of Gemini amphiphiles bearing nonionic hydrophilic spacers. J Surf Deterg 10:71–80
Jing KZ (2004) Liquid chromatography–mass spectrometry of nonionic surfactants using electrospray ionization. J Surf Deterg 7:421–423
Negm NA, Aiad IA (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surf Deterg 10:87–92
El-Sukkary MMA, Syed NA, Aiad I, El-Azab WIM (2008) Synthesis and characterization of some alkyl polyglycosides surfactants. J Surf Deterg 11:129–137
Bravo Rodriguez V, Jurado Alameda E, Reyes Requena A, García López AI, Bailón-Moreno R, Cuevas Aranda M (2005) Determination of commercial surfactants: alkylpolyglucosides and fatty alcohol ethoxylates. J Surf Deterg 8:341–346
Abdul-Raouf ME, Abdul-Raheim AM, Maysour NE, Mohamed H (2011) Synthesis, surface-active properties, and emulsification efficiency of trimeric-type nonionic surfactants derived from tris(2-aminoethyl)amine. J Surf Deterg 14:185–193
Jurado E, Serrano MF, Olea JN, Lechuga M (2007) Primary biodegradation of commercial fatty-alcohol ethoxylate surfactants: characteristic parameters. J Surf Deterg 10:145–153
Uppgård LL, Lindgren A, Sjöström M, Wold S (2000) Multivariate quantitative structure–activity relationships for the aquatic toxicity of technical nonionic surfactants. J Surf Deterg 3:33–41
Negm NA, El-Farargy AFM, Mohammed DE, Mohamad HN (2012) Environmentally friendly nonionic surfactants derived from tannic acid: synthesis, characterization and surface activity. J Surf Deterg. doi:10.1007/s11743-011-1326-8
Mohamed MZ, Ismail DA, Mohamed AS (2005) Synthesis and evaluation of new amphiphilic polyethylene glycol-based triblock copolymer surfactants. J Surf Deterg 8:175–180
Bodin A, Linnerborg M, Lars J, Nilsson G, Karlberg AT (2002) Novel hydroperoxides as primary autoxidation products of a model ethoxylated surfactant. J Surf Deterg 5:107–110
Leal JS, Gonzalez JJ, Kaiser KL, Palabrica VS, Comelles F, Garcıa MT (1994) On the toxicity and biodegradation of cationic surfactants (Über die Toxizität und den biologischen Abbau kationischer Tenside). Acta Hydrochim Hydrobiol 22:13–21
Hama I, Sasamoto H, Tamura T, Nakamura T, Miura K (1998) Skin compatibility and ecotoxicity of ethoxylated fatty methyl ester nonionics. J Surf Deterg 1:93–97
Ratledge C (ed) (1994) Biochemistry of microbial degradation, vol 89. Kluwer, Amsterdam
Swisher RD (1987) Surfactant biodegradation, 2nd edn. Dekker, New York, pp 693–719
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sayed, G.H., Ghuiba, F.M., Abdou, M.I. et al. Synthesis, Surface, Thermodynamic Properties of Some Biodegradable Vanillin-Modified Polyoxyethylene Surfactants. J Surfact Deterg 15, 735–743 (2012). https://doi.org/10.1007/s11743-012-1375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1375-7