Abstract
A new series of cationic Schiff bases was synthesized and their chemical structures were confirmed using elemental analysis, infrared spectra and nuclear magnetic resonance. The surface properties of the surfactant solutions including surface tension, effectiveness, efficiency, critical micelle concentration, maximum surface excess and minimum surface area were calculated using surface tension-log concentration profiles. The surface parameters were strongly dependent on the hydrophobic chain length. The thermodynamic properties of the surfactants in their solutions showed the spontaneous behavior of both adsorption and micellization processes. The thermodynamic data revealed that the adsorption of the surfactant molecules at the air/water interface was more favorable than the micellization in the bulk of their solutions. The synthesized surfactants were evaluated with regard to their preventing the corrosion reaction of carbon steel in acidic media and also their acting as antibacterial biocides to inhibit bacterial growth. The data of corrosion and antibacterial evaluations showed the high efficiency and applicability of these compounds in these uses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aggressive acid solutions are widely used for industrial purposes, and inhibitors are commonly used to control metal dissolution as well as consumption. Most acid inhibitors are organic compounds containing oxygen, nitrogen and/or sulfur [1–3]. Acid inhibitors have many important roles as a component in pre-treatment compositions, cleaning solutions and in acidization of oil wells. Compounds with functional groups containing heteroatoms, which can donate lone pair electrons, are found to be particularly useful as inhibitors of metal corrosion. These compounds, in general, are adsorbed onto the metal surface and block the active corrosion sites [4, 5]. Several cationic surfactants have been investigated as corrosion inhibitors for various metals and alloys in acidic media [6, 7].
On the other hand, to overcome the alarming problem of microbial resistance to antibiotics, the discovery of novel active compounds against new targets is a matter of urgency. Many of the crude drugs, which are sources of medicinal preparations, still originate from wild-growing material. However, plant-based drugs have shortened the life span of the source of material. There is a continuous search for more potent and cheaper raw materials to feed the industry. Schiff bases are organic compounds with great utility in important fields such as medicine, agriculture and cosmetic products [8, 9]. Some Schiff bases present anticancer, antitumor and antibacterial activity [10, 11]. They play a prominent part in the enzymatic or unenzymatic transaminating reactions of the carbonyl compounds with amino acids [12]. In the coordinate chemistry field, a lot of Schiff bases operate as ligands [13].
In this study, four new cationic Schiff base surfactants were synthesized, characterized and evaluated as corrosion inhibitors against the corrosion of carbon steel in acidic media and also as antibacterial agents to prevent bacterial growth. The relation between the surface activity and the efficiency of these compounds in the different applications was discussed.
Experimental
Synthesis of Alkyl Fatty Esters of Ketoglutaric Acid (KG)
Ketoglutaric acid (KG) was esterified with different fatty alcohols namely: octyl (8), dodecyl (12), hexadecyl (16) and octadecyl alcohols (18) in a molar ratio of 1:2 in xylene (100 mL) as a solvent and in the presence of p-toluene sulfonic acid (0.1 g) as a dehydrating agent. The reaction was stopped when the water of reaction was obtained. The product was washed with 100 mL of alkaline solution of Na2CO3 (1 N) to remove the catalyst and allowed to separate in a 250 mL separating funnel. Then the aqueous layer was removed and the organic layer was dried in desiccator under CaCl2. The alkyl ketoglutarate esters produced (KG-8, KG-12, KG-16, and KG-18) ranged from liquid to waxy in appearance with the following melting points: 40–42, 71–73, 87–89 and 94–96 °C, respectively.
Synthesis of Schiff Base–Alkyl Ketoglutarate
Alkyl ketoglutarate were reacted with p-aminopyridine in an equimolar ratio in ethanol (100 mL) as a solvent and in presence of p-toluene sulfonic acid (0.1 g) as a dehydrating agent. The reaction mixture was refluxed for 8 h under stirring conditions and allowed to precipitate overnight. The product was filtered off and washed three times with an appropriate amount of ethanol and dried under vacuum at 40 °C. The alkyl ketoglutarate Schiff bases produced (KG-8SB, KG-12SB, KG-16SB, and KG-18SB) exhibited a waxy to crystalline appearance with the following melting points: 62–64, 83–85, 101–103 and 122–124 °C.
Synthesis of Monoquaternary Ammonium Schiff Base–Alkyl Ketoglutarate (QKG-8SB, QKG-12SB, QKG-16SB, and QKG-18SB)
A 0.1-mol sample of each of the synthesized Schiff bases of alkyl ketoglutarate (KG-8SB, KG-12SB, KG-16SB, and KG-18SB) was refluxed individually in 40 mL ethanol for 4 h in the presence of 25 mL of ethyl iodide. The reaction was left to precipitate, and then the product was filtered off and recrystallized twice from ethanol and dried at 40 °C under a vacuum. The quaternary Schiff bases produced (QKG-8SB, QKG-12SB, QKG-16SB, and QKG-18SB) varied in color between pale yellow to brown crystals with the following melting points: 91–93, 110–112, 147–149 and 163–165 °C, respectively.
Measurements
Surface Tension Measurements
The apparent surface tension values for freshly prepared aqueous Schiff base cationic surfactants solutions with a concentration range of 0.1–0.000001 mol/L were measured using a Krüss K6 platinum ring tensiometer at 25 °C [14].
Weight Loss Measurements
The experiments were performed with carbon steel specimens having a composition (wt%): 0.21 C, 0.035 Si, 0.25 Mn, 0.082 P, and the remainder was Fe. The carbon steel sheets of 2.5 cm × 2.0 cm × 0.6 cm were abraded with a series of emery papers (grades 320, 500, 800 and 1,200) and then washed with distilled water and acetone. After weighing accurately (using a Mettler AG104 0.1 mg Analytical Balance), the specimens were immersed in a 250 mL beaker containing 250 mL hydrochloric acid with and without the addition of different concentrations (100, 200 and 400 ppm by weight) of the tested inhibitors at 25 °C. After different immersion time intervals of 4, 8, 12, and 16 h, the specimens were taken out, washed, dried, and weighed accurately [15].
Biocidal Activity
Microorganisms
The biocidal activity of the synthesized surfactants was tested against different bacterial strains (ATCC American Type Culture Collection) as follows: Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis ATCC 55422, Desulfomonas pigra ATCC 29098 and Salmonella typhimurium ATCC 27948.
Growing of Microorganisms
The bacterial strains were cultured according to the standards of the National Committee for Clinical Laboratory (NCCLS) [16–17]. The bacterial species were grown on nutrient agar medium consisting of beef extract (3.0 g/L), peptone (5.0 g/L), sodium chloride (5.0 g/L), and agar (20.0 g/L). The mixture had been heated until boiling, and the media sterilized by autoclave. The bacterial strains were kept on nutrient agar medium and showed no inhibition zones.
Measurements of Resistance and Susceptibility
For preparation of discs and inoculation, 1.0 mL of inocula was added to 50 mL of agar media (40 °C) and mixed. The agar was poured into 120-mm petri dishes and allowed to cool to room temperature. Wells (6 mm in diameter) were cut in the agar plates using proper sterile tubes and filled up to the surface of the agar with 0.1 mL of the synthesized cationic surfactants dissolved in DMF (1, 2, 5 mg/mL DMF). The plates were left on a level surface, incubated for 24 h at 30 °C and then the diameters of the inhibition zones were measured. The inhibition zone formed by these compounds against the particular test bacterial strain determined qualitatively the antibacterial activities of the synthetic compounds. The mean value obtained for three individual replicates was used to calculate the zone of growth inhibition of each sample. The antimicrobial activity was calculated as a mean of three replicates. The tested compounds were completely compatible with the medium of agar and no turbidity was observed during the mixing process [11].
Minimum Inhibitory Concentration (MIC)
The biocidal activity of the synthesized surfactants against the tested strains was expressed as the minimum inhibitory concentration (MIC) values, defined as the lowest concentration of compounds inhibiting the development of visible growth after 24 h of incubation. The MIC values were determined by the dilution method [17]. The compounds tested were dissolved in a mixture of distilled water/alcohol (3/1; v/v) at various concentrations and a 1 mL aliquot of the cationic surfactants solutions was added to the 14 mL of agar medium. The final concentrations of the tested surfactants in the medium were 300, 200, 100, 40, 20, 10 and 4 μg/mL.
Results and Discussion
Structure
The chemical structures of the synthesized cationic surfactants were confirmed using elemental analysis data (Table 1) which showed the purity of the produced compounds.
FTIR Spectroscopy
The KG-8, KG-12, KG-16, and KG-18 compounds showed complete disappearance of the characteristic absorption band at 3,400–3,600 cm−1 of the O–H (carboxylic group) and also the appearance of a new band at 1,724–1,728 cm−1 corresponding to the carbonyl group of the ester formed, 1,124 cm−1 corresponds to the C–O ester group. In addition, the absorption band of the C = O group of the ketoglutaric acid (CH2-CO-) appeared at 1,590 cm−1 and shifted to lower values due to the presence of the ester carbonyl in near position. The methyl group (CH3) of the fatty alkyl chains appeared at 2,850 cm−1, while the repeated methylene groups (CH2) appeared at 2,919 cm−1. The absorption band of methylene groups (CO-CH2-CO) appeared at 3,020 cm−1 due to the effect of two carbonyl groups.
KG-12SB, KG-16SB, and KG-18SB were monitored by the disappearance of the carbonyl group at 1,724 cm−1. The products were confirmed by the appearance of two characteristic bands, the first band at 1,621 cm−1 corresponds to the azomethine group (–CH = N–). The bands in 860–930 cm−1 region correspond to the pyridine ring.
Compound QKG-8SB (as a representative sample for the quaternary derivatives) showed a complete disappearance of the sharp and characteristic absorption band at (νC = O at 1,715 cm−1 and also the appearance of a new sharp band (ν–C = N– at 1,626 cm−1. The new absorption band at 3,046 cm−1 represents the formation of the quaternary ammonium group (N+).
1H-NMR Spectroscopy
Compounds KG-8, KG-12, KG-16, and KG-18 in DMSO-d6 showed signals at:δ = 0.96 ppm (t, 6H, CH3), 1.33 ppm (m, 24H, CH2), 3.41 ppm (s, 4H, CO-CH2-CO), 4.08 ppm (t, 4H, CH2-O-). The integration of the signal at 1.33 ppm was as follows: 40H for KG-12, 56H for KG-16 and 64H for KG-18.
Compounds KG-8SB, KG-12SB, KG-16SB, and KG-18SB in DMSO-d6 showed signals at δ = 0.96 ppm (t, 6H, CH3), 1.33 ppm (m, 24H, CH2), 3.41 ppm (s, 4H, CO-CH2-CO), 4.08 ppm (t, 4H, CH2-O-). The integration of the methylene groups at 1.33 ppm was (40H for KG-12SB, 56H for KG-16SB, and 64H for KG-18SB). This represents the distribution of the protons in the chemical structures of the synthesized Schiff bases.
Compounds QKG-8SB, QKG-12SB, QKG-16SB, and QKG-18SB in DMSO-d6 showed the following signals: δ = 0.96 ppm (t, 9H, CH3), 1.33 ppm (m, 24H, CH2), 2.3 ppm (s, 4H, CO-CH2-CO), 2.59 ppm (m, 2H, CH2N+), 4.08 ppm (t, 4H, CH2-O-), 9–9.3 ppm (m, 4H, pyridine ring). While, the integration of the signal at 1.33 ppm corresponding to the repeated methylene groups was: 40H for QKG-12SB, 56H for QKG-16SB and 64H for QKG-18SB.
From the above data, it can be concluded that the chemical structures of the synthesized Schiff bases are as represented in Scheme 1.
Surface Activity
Figure 1 represents the variation of surface tension against –log concentration of the synthesized cationic Schiff base surfactants at 25 °C. The hydrophobic chain length of the various surfactants under consideration plays an effective role on the surface tension reduction of the surfactants at the identical concentration. A cationic surfactant contains a short hydrophobic chain (QKG-8SB) and exhibits a lower depression in the surface tension, while increasing the hydrophobic chain to 12, 16 or 18 methylene groups. This can be attributed to the higher tendency of the longer hydrophobic chains towards adsorption at the air/water interface. The repulsion between the aqueous medium and the hydrophobic chains is increased by increasing the number of repeated methylene groups due to the differences between the later and the former in their polar nature.
At higher surfactant concentrations, the values of the surface tension curve stay almost constant which indicates the critical micelle concentration values of the different surfactants, Table 2. It is clear that the CMC values of the synthesized surfactant decreased by increasing the hydrophobic chain length; the values are lowest for QKG-18SB, viz., 0.79 mmol/L and highest for QKG-8SB, 2.29 mM/L. Obviously, the lower CMC values of the synthesized cationic Schiff bases compared with the conventional quaternary ammonium surfactants (hexadecyltrimethylammonium bromide: 1.9 mM/L) [18, 19] indicate their high surface activity. This can be ascribed to the presence of the azomethine and the two ester groups in the chemical structures of the targeted surfactants which increase the hydrophobicity of the molecules and consequently decreases their CMCs.
The surface tension of the surfactant solution at the critical micelle concentration determines the effectiveness (πCMC). Increasing the hydrophobic chain lengths of the different surfactants increases the depression of the surface tension at the interface, while the efficiency (Pc20) i.e. the concentration of surfactant solution which is required to lower the surface tension of the solution interface to 52 m N/m, is reduced.
The maximum surface excess is another factor determining the surface activity of the surfactant molecules at the interface. The maximum surface excess values of the surfactant solutions were calculated according to Gibb’s adsorption equation (Eq. 1) [19] as follows:
where, dγ/dlogC is the slope of the surface tension profile at the pre-CMC region, R is the universal gas constant and T is the absolute temperature.
The maximum surface excess values of the synthesized surfactants at the interface are increased by increasing the hydrophobic chain length of these molecules. Increasing the chain length increases the hydrophobicity of the molecules which increases the adsorption tendency of surfactant molecules at the interface; as a result, Γmax increased.
On the other hand, the minimum surface area at the interface occupied by each surfactant molecule at the saturation condition of the interface is calculated using Eq. (2) [19]:
where Γmax is the maximum surface excess and N is Avogadro’s number.
The minimum surface area (Amin) provides information about the orientation of the surfactant molecules at the interface and also the arrangement and the compactness of the molecules in the adsorbed layer. Since the cross-sectional area of an aliphatic chain oriented perpendicular to the interface is about 20 Å2, it is apparent that the hydrophobic chains of surfactants adsorbed at the air/water interface are generally not in the close-packed arrangement normal to the interface at saturation adsorption. On the other hand, since the cross-sectional area of the methylene group lying flat in the interface is about 7 Å2, the chains in the surfactant molecules are not lying flat at the interface, but are somewhat tilted with respect to the interface. The calculated Amin values of the synthesized cationic Schiff base surfactants showed that QKG-18SB molecules occupy a lower area at the interface (73.89 Å2) than do QKG-8SB molecules (97.47 Å2). The lower Amin values can be attributed to the higher population of surfactant molecules at the interface. The low Amin values of the synthesized Schiff base derivatives (especially C12, 16, 18) indicates highly packed molecules at the interface, which is an important factor in the effectiveness of these derivatives as foaming, wetting and emulsifying agents. The high packing of the surfactant molecules at the interface is due to the overlapping between their hydrophobic chains. The attraction between the positively charged adsorbed head groups also increases the packing effect of these molecules. It is noticeable from the data listed in Table 2 and the above-mentioned discussion that the gradual increase in the hydrophobic chain length of the different cationic surfactants under investigation gradually increases their surface activity. Furthermore, the most surface active derivative is the QKG-18SB homologue.
Thermodynamics of Interfacial Adsorption and Micelle Formation
The thermodynamic behaviors of the synthesized cationic surfactants in their solutions were studied using the standard free energies of adsorption and micellization at ambient temperature. The standard free energies of adsorption (\( \Updelta {\text{G}}^{\text{o}}_{\text{ads}} \)) and micellization (\( \Updelta {\text{G}}^{\text{o}}_{\text{mic}} \)) of the surfactants at the air/water interface or in the bulk of the solution were evaluated using Eqs. (3, 4) [20].
It is clear from Table 2 that the standard free energies of adsorption and micellization of the synthesized cationic surfactants are always negative. This indicates that both adsorption and micellization processes occurred spontaneously. Moreover, the standard free energies of adsorption of the different surfactant molecules at the air/water interface are more negative than those of the micellization, which reveals that the adsorption process is more favorable than the micellization.
The large difference between \( \Updelta {\text{G}}^{\text{o}}_{\text{ads}} \) and \( \Updelta {\text{G}}^{\text{o}}_{\text{mic}} \) values is attributed to the fact that the adsorbed molecules at the interface are highly compacted; as a result, the water molecules do not interact significantly with these molecules. While in the bulk of the solution, the interaction between the hydrophobic chain and the aqueous medium is increased due to the curved surface of the micelles formed.
The high negativity of \( \Updelta {\text{G}}^{\text{o}}_{\text{ads}} \) of the synthesized cationic surfactants and their high compactness at the interface (as concluded from the Amin values), one can conclude that these surfactants have a surface activity which may result in good applicability in some applications including emulsification, corrosion inhibition and antimicrobial utility.
Corrosion Inhibition Efficiency
The synthesized cationic Schiff base surfactants were evaluated as corrosion inhibitors for protecting carbon steel against the corrosion reaction in an acidic medium (2 N HCl) at 25 °C using the weight loss technique at different doses (100, 200, 400 ppm).
The corrosion rate of the carbon steel is defined as the amount of carbon steel (in milligrams) which dissociates from a unit area of the metal in 1 h, and calculated according to Eq. (5) [15]:
Where, R is the corrosion rate, W is the weight loss of the carbon steel, S the total area in square cm of the specimen, t is immersion time in hours.
Figure 2 shows the variation of the corrosion rates of the carbon steel specimens in the presence of different doses of the surfactants under consideration. It is clear that the corrosion rate is decreased considerably by increasing the doses used of the different surfactants, and the lowest corrosion rates were obtained for the different derivatives at 400 ppm. Furthermore, at a constant dose of the surfactant, the corrosion rate is decreased by increasing the hydrophobic chain length of the surfactants, which favors their adsorption at the metal/liquid interface.
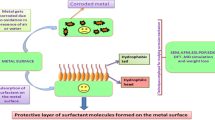
Cationic surfactants have several characteristics which allow them to act as efficient corrosion inhibitors. Cationics are quite stable in acidic media and retain their chemical structure in strong acids [21]. Also, they form a tight and well-arranged adsorbed monolayer, especially at higher doses. That surfactant monolayer acts as a protective shield which protects metal surfaces against the corrosive environments [22].
The origin of the stability of the adsorbed monolayer on the metal surface consists of two forces acting on the inhibitor molecules. First, a repulsion occurs between the hydrophobic chains and the aqueous environment which drives the molecules toward the metal interface. Second, the electrostatic interaction occurs between the negative centers on the metal surface and the positively charged head groups of the cationic surfactant molecules (N+) [23, 24]. As a result, the active centers on the metal surface are blocked and this prevents the acid attack; in addition, the hydrophobic chains form a well-arranged isolating layer that acts as a protective layer for the metal surface from the attack of the acid molecules.
Increasing the hydrophobic chain length of the surfactant molecules increases the overlapping between the chains and also increases the thickness of the adsorbed protective layer which considerably decreases the corrosion process of the carbon steel. In terms of corrosion inhibition efficiency, the high surfactant doses and longer hydrophobic chains increase the corrosion inhibition efficiencies of the targeted surfactants and increase their applicability as corrosion inhibitors (Fig. 3).
In the absence of the corrosion inhibitors, the corrosion rate was 0.136 mg cm−2 h−1. While in their presence, the corrosion rate varied between 0.051 and 0.026 mg cm−2 h−1 at inhibitor dose of 400 ppm and that accompanied by inhibition efficiencies values ranged between 77 and 95%. QKG-18SB inhibitor provides the lowest corrosion rate at 0.026 mg cm−2 h−1 with the maximum inhibition efficiency obtained for the tested inhibitors at 95%.
Antimicrobial Activity
Inhibition Zone Diameter
The potent action of the synthesized cationic Schiff base surfactants (QKG-8SB, QKG-12SB, QKG-16SB, and QKG-18SB) was screened against Gram-positive and Gram-negative bacteria at different concentrations using the values of the inhibition zone diameter tests and the results are summarized in Table 3. The obtained diameters of the inhibition zones are gradually increased by increasing the concentration of the tested surfactants, and the maximum diameters are obtained at 5 mg/mL. Moreover, the antimicrobial activities are gradually increased by increasing the hydrophobic chain length. The octadecyl derivatives (QKG-18SB) showed the maximum antimicrobial activities against the tested bacterial strains. General observation for data in Table 3 indicates that the Gram-negative bacteria are more resistant to the tested compounds compared with the Gram-positive bacteria. The data obtained from the inhibition zone diameter could not be considered as quantitative data. In order to evaluate the synthesized compounds quantitatively as antibacterial agents, minimum inhibitory concentrations were measured.
Minimum Inhibitory Concentration (MIC)
Minimum inhibitory concentration (MIC) values for the synthesized cationic Schiff base biocides (QKG-8SB, QKG-12SB, QKG-16SB, and QKG-18SB) are summarized in Table 4 which also includes the MIC values corresponding to the hexadecyl trimethyl ammonium bromide [25] as a classical antimicrobial surfactant agent. Compared with the classical quaternary ammonium surfactants [25, 26] the tested biocides show moderate activity level against bacteria with MIC values of 4–100 μg/mL (except P. aeruginosa and S. typhimurium: 100–300 and >300 μg/mL, respectively), Table 4. However, the use of quaternary ammonium compounds (QAS) in some fields is limited due to the developed microbial resistance against QAS after longer periods of applications [27], their high acute toxicity and low biodegradability [28]. For medical applications, the use of cationic surfactants with low antimicrobial activity would be necessary because of the need for high biodegradability and less toxicity.
The action mode of the cationic biocides that is generally accepted is that the cationic biocides can adsorb onto negatively charged cell membranes, which will then lead to a decrease in the osmotic stability of the cell and leakage of the intracellular constituents [29, 30]. However, the exact mechanism of the antimicrobial action is still unknown and several other mechanisms that may contribute to the antimicrobial action have also been suggested, such as the formation of an impermeable coat on the bacterial surface [31], uptake of low molecular weight biocides that will interact with electronegative substances in the cell [32], and inhibition of bacterial growth through chelation of trace metals [28]. The mechanism for the interaction may be different for Gram-positive and Gram-negative bacteria.
It is clear from Table 4 that the MIC values of the tested cationic surfactants (except QKG-8SB) against Gram-negative bacteria are lower than the standard used, and the Gram-negative bacteria are resistant to these surfactants in the tested concentration range,. In contrast, the QKG-18SB surfactant presents a similar activity against both Gram-positive and Gram-negative bacteria. That can be attributed to the longer hydrophobic chain length which increases the surface activity of these compounds. Gram-negative bacteria are generally more resistant to antimicrobial agents than are Gram-positive bacteria. This can be explained by the different cell membrane structure of the two bacterial types. The external layer of the outer membrane of the Gram-negative bacteria is almost entirely composed of lipopolysaccharides and proteins that restrict the entrance of biocides and amphiphilic compounds [32]. An important factor that influences the biocidal activity of the different biocides is the net charge on their molecules. Several studies showed that the electrostatic interactions play a key role in the action of cationic biocides, and that a decrease in the charge density of the cationic compounds results in a reduction in adsorption and biocidal efficiency [33–35]. Comparing the MIC values of the synthesized cationic surfactants with the classically antimicrobial surfactant (HTAB), showed their relatively higher biocidal activity is apparent from their relatively lower MIC values. The higher biocidal activity of the synthesized surfactants is accounted to the high average charge on their molecules. In case of HTAB, the head group has one positive charge located on the nitrogen atom (N+). Contrarily, conjugation of electrons over the two pyridinium nuclei sharply increases the charged centers and consequently increases the average charge of the molecules. That increases their tendency towards adsorption in addition to the biocidal activity.
The surface activity of the cationic biocides is a vital factor that influences the biocidal activity of the synthesized biocides [36]. The results obtained show that the biocidal activity depends on the surface activity of the tested cationic biocides and mainly on their critical micelle concentration values (CMC). The MIC values of the tested cationic biocides are smaller than their respective CMC values. However, the MIC values of the tested biocides against P. aeruginosa are in the range of their CMC values. This could be explained by the fact that below the CMC values, the biocide molecules participate in the biocidal action towards the microbial membrane. On the other and, above the CMC values, the biocides are in the micellar form and are no longer available to participate in the microorganism destruction [37].
The synthesized cationic biocides were screened for their potency against sulfate reducing bacteria (D. pigra), (Table 4). The data show unequivocal results due to their relatively high efficiency against SRB bacteria. SRB bacteria (anaerobic bacteria) produce H2S gas due to the reduction of sulfate compounds as the sole source of energy. H2S gas increases the acidity of the medium and causes biocorrosion of the pipelines. In addition, H2S is responsible for the formation of sulfide salts which are highly corrosive to stainless steel, even more than H2S gas. To decrease the production of corrosive materials in the petroleum pipeline environments (H2S, sulfide ions), biocides are used. The MIC values of the tested cationic surfactants showed their approved biocidal activity against SRB. It is clear from the data in Table 4 that the gradual increase in the hydrophobic chain length of the synthesized surfactants increases their biocidal activities against SRB.
References
Negm NA, Al Sabagh AM, Migahed MA, Abdel Bary HM, El Din HM (2010) Effectiveness of some diquaternary ammonium surfactants as corrosion inhibitors for carbon steel in 0.5 M HCl solution. Corros Sci 52:2122–2132
Negm NA, Elkholy YM, Zahran MK, Tawfik SM (2010) Corrosion inhibition efficiency and surface activity of surface active benzothiazol-3-ium cationic Schiff base derivatives in hydrochloric acid. Corros Sci 52:3523–3536
Negm NA, Aiad IA (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surf Deterg 10:87–92
Negm NA, Morsy SMI (2005) Corrosion inhibition of triethanolammonium bromide mono- and dibenzoate as cationic inhibitors in an acidic medium. J Surf Deterg 8:1–9
Emregul KC, Akay AA, Atakol O (2005) The corrosion inhibition of steel with Schiff base compounds in 2 M HCl. Mater Chem Phys 93:325–332
Dadgamezhad A (2004) Corrosion inhibitory affects of a new synthetic symmetrical Schiff base on carbon steel in acid media. Corros Sci 51:266–273
Negm NA, Hafiz AA, El-Awady MY (2005) Influence of structure on the cationic polytriethanolammonium bromide derivatives. II. Corrosion inhibition. Egypt J Chem 48:201–210
Badawi AH, Mohamed MAS, Mohamed MZ, Khowdairy MM (2007) Surface and antitumor activity of some novel metal-based cationic surfactants. Assoc Radiat Oncol India 3:198–206
Zhu WR, Hu PZ, Li MY, Huang XL, Wu CT (2003) Synthesis of new Schiff bases containing thiophene moiety. J Nat Sci 8:433–436
Li MY, Hu PZ, Zhu WR, Wang Y (2003) Synthesis and characterization of open ring polycomponent complexes formed in the reaction of lanthanide with m-phenylenediamine and dibenzoyl methane. Asian J Chem 15:38–42
Negm NA, Zaki MF (2008) Structural and biological behaviors of some nonionic Schiff base amphiphiles and their Cu(II) and Fe(III) metal complexes. Colloid Surf B 64:179–183
Scheehan J, Grenda VI (1962) The N-(2-Hydroxyarylidene) protecting group in peptide synthesis. J Am Chem Soc 84:2417–2420
Abd El-Salam FH (2009) Synthesis, antimicrobial activity and micellization of gemini anionic surfactants in a pure state as well as mixed with a conventional nonionic surfactant. J Surf Deterg 12:363–370
Negm NA, Salem MAI, Zaki MF (2009) Solubilization behaviors of nonpolar substrates using double tailed cationic surfactants. J Dispers Sci Technol 30:1167–1174
ASTM G1-72 (1990) Practice for preparing, cleaning and evaluating corrosion test specimens, pp 1–8
Hafiz AA, Negm NA, Elawady MY (2005) Influence of structure on the cationic polytriethanolammonium bromide derivatives. III. Biological activity. Egypt J Chem 48:245–250
National committee for clinical laboratory standards M7-A4 (1997) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, pp 92–98
Sehgal P, Doe H, Bakshi MS (2002) Aggregated assemblies of hexadecyltrimethylammonium bromide and phospholipids at the interface and in the bulk solution. J Surf Deterg 5:123–130
Rosen MJ (2001) Surface and interfacial phenomena, 2nd edn. Wiley, NY
Israelachvili JN (1991) Intermolecular and surface forces, 2nd edn. Academic Press, London, p 370
Negm NA, Morsy SMI (2005) Corrosion inhibition of triethanolammonium bromide mono- and dibenzoate as cationic inhibitors in an acidic medium. J Surf Deterg 8:283–287
Bentiss MT, Lagrene M (2000) The substituted 1,3,4-oxadiazoles: a new class of corrosion inhibitors of mild steel in acidic media. Corros Sci 42:127–146
Negm NA, Zaki MF (2008) Corrosion inhibition efficiency of nonionic Schiff base amphiphiles of p-aminobenzoic acid for aluminum in 4 N-HCl. J Colloid Surf A 322:97–102
Badawi AM, Hegazy MA, El Sawy AA, Ahmed HM, Kamel WM (2010) Novel quaternary ammonium hydroxide cationic surfactants as corrosion inhibitors for carbon steel and as biocide for sulfate reducing bacteria. Mater Chem Phys 124:458–465
Negm NA, Aiad IA, Tawfik SM (2010) Screening for potential antimicrobial activities of some cationic uracil biocides against wide-spreading bacterial strains. J Surf Deterg 13:503–511
Zhao T, Sun G (2006) Synthesis and characterization of antimicrobial cationic surfactants: aminopyridinium salts. J Surf Deterg 9:325–330
Davies J (1996) Bacteria on the rampage. Nature 383:219–220
Garcıa MT, Ribosa I, Guindulain T, Leal JS, Rego JV (2001) Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment. Environ Pollut 111:169–175
Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465
Devlieghere F, Vermeulen A, Debevere J (2004) Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol 21:703–714
Zheng LY, Zhu JAF (2003) Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym 54:527–530
Oros G, Cserhati T, Forgacs E (2003) Separation of the strength and selectivity of the microbiological effect of synthetic dyes by spectral mapping technique. Chemosphere 52:185–193
Ringstad L, Schmidtchen A, Malmsten M (2006) Effect of peptide length on the interaction between consensus peptides and DOPC/DOPA bilayers. Langmuir 22:5042–5050
Ringstad L, Kacprzyk L, Schmidtchen A, Malmsten M (2007) Effects of topology, length, and charge on the activity of a kininogen-derived peptide on lipid membranes and bacteria. Biochim Biophys Acta Biomembr 1768:715–727
Makovitzki A, Shai Y (2005) Ultrashort antibacterial and antifungal lipopeptides. Biochemistry 44:9775–9784
Kurup TR, Wan LS, Chan LW (1992) Preservative requirements in emulsions. Pharm Acta Helv 67:204–208
Diz M, Manresa A, Pinazo A, Erra P, Infante MR (1994) Synthesis, surface active properties and antimicrobial activity of new bisquaternary ammonium compounds. J Chem Soc Perkin Trans 2:1871–1876
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negm, N.A., El Farargy, A.F., Al Sabagh, A.M. et al. New Schiff Base Cationic Surfactants: Surface and Thermodynamic Properties and Applicability in Bacterial Growth and Metal Corrosion Prevention. J Surfact Deterg 14, 505–514 (2011). https://doi.org/10.1007/s11743-011-1258-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-011-1258-3