Abstract
A series of novel nine symmetric diquaternary gemini amphiphiles was synthesized having the formula:
where R1 = C11H23COOCH2CH2, C15H31COOCH2CH2 and/or C17H35COOCH2CH2 alkyl chain, R = CH2CH2OH and n = 10, 15 and 25 ethylene oxide units. Surface active properties at air-aqueous solution were determined using Gibb’s adsorption equations including critical micelle concentration (CMC), effectiveness (π CMC), efficiency (pC20) and minimum surface area (A min). The effects of hydrophobic and hydrophilic chain length on the surface and thermodynamic parameters of the diquaternary surfactants were discussed. Surface tension–concentration profiles of diquaternary amphiphiles display the formation of various aggregative structures, e.g., spherical micelles and lamellar shapes, as well as bearing lower critical micelle concentration than the expected values for the corresponding N+/CH2 ratio of monoquaternary amphiphiles. The calculations of minimum surface area (A min) appear to have higher values for the molecules at the interface, reinforcing the idea of air–water interface attachment of both positively charged nitrogen atoms. Thermodynamic data including, free energy, entropy and enthalpy changes (ΔG, ΔS, ΔH) for adsorption at the air–water interface and also for micellization in the bulk of surfactant solutions were calculated. The data showed a great tendency of the synthesized molecules for adsorption at the interfaces rather than micellization in the bulk of their solutions. Solubilization behaviors of the prepared amphiphiles were described as a vital application of these compounds. The effect of their structures on the solubilization process towards polar and nonpolar solubilizates was also explained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gemini-surfactants or gemini amphiphiles are a unique type of surfactant and they are characterized by two conventional single-tail surfactants whose head groups are joined covalently by a spacer moiety with variable lengths. In aqueous solutions, gemini surfactants spontaneously aggregate into micelles whose shape and size are highly sensitive to the length and type of the spacer groups [1]. Gemini surfactants show very low critical micellar concentrations (CMC) compared with the corresponding monomeric (monoquaternary) surfactant [2]. Some have been found to form giant worm-like micelles [3, 4] at low concentration without any added salts or hydrophobic counterions as in the case of the classically studied systems [5, 6]. Gemini or dimeric surfactants have been generating increasing interest owing to their tunable molecular geometry [7] and their superior performance in various applications.
Several investigators [8–14] have focused on gemini surfactants with linear hydrocarbon tail groups and quaternary ammonium head groups, having the general formula:
and for simplifying the structure, it could be represented as Cn−s−m. For symmetrical gemini surfactants (n = m) [11], the effective head group area increased with increasing s (up to seven or more), owing to the repulsion between the positively charged centers (N+). Gemini surfactants are therefore important for fundamental studies of self-assembly since they offer a way to modify the “dimensionless packing parameter” (g). This parameter reflects the effective molecular geometry of a given surfactant molecule in a micelle. Thus, the effective molecular geometry is wedge like (1/3 < g < 1/2) for molecules with smaller spacers (s = 2 or 3) and cylindrical micelle whereas the geometry is cone-like (g < 1/3) for large spacers.
Experimental Procedures
Preparation of Triethanolamine Monoester
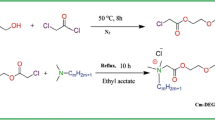
Triethanolamine (0.1 mol) and lauric, palmetic and/or stearic acid (0.1 mol) were each esterified in toluene under nitrogen gas as an inert atmosphere until the azeotropic amount of water (1.8 mL) was removed. After removal of the toluene under a vacuum using a rotary evaporator, triethanolamine monolaurate, triethanolamine monopalmitate and triethanolamine monostearate were obtained. Subsequent purification (two times) was done by means of vacuum distillation to remove the excess and unreacted materials [15]. The prepared triethanolamine monoesters had the chemical structure:
where R = alkyl chain of C11H23, C15H31 or C17H35.
Preparation of Polyoxyethylene Diester of Bromoacetic Acid
Polyoxyethylene (n = 10, 15 and 25 ethyleneoxide units) (0.1 mol) and bromoacetic acid (0.2 mol) were each esterified [16] in toluene as a solvent until 3.6 mL of water had been removed. After removal of the solvent, polyoxyethylene-di-(bromoacetate) esters having the following chemical structures were obtained as follow:
Preparation of the Cationic Gemini Amphiphiles
Triethanolamine monoesters (0.05 mol) and the polyoxyethylene-di-(bromoacetate) derivatives were refluxed in acetone for 12 h [15]. The products obtained were filtered, recrystallized twice from benzene and dried in a vacuum oven (2 mm-Hg) at 50 °C for 2 h.
Elemental analyses confirmed the purity of the synthesized surfactants and the presence of the function groups were confirmed by the FTIR-spectroscopic data as shown in Table (1).
1H-NMR spectra of the synthesized surfactants were performed using a Bruker model DRX-300 NMR spectrometer with TMS as an internal standard. The spectra showed signals at: 0.9 ppm (t, 6H, CH3), 3.6 ppm (t, 8H, –CH2OH), 4.7 ppm (S, 4H, –CH2OH) disappeared by addition of D2O, 2.1 ppm (S, 4H, –CH2COO–), 3.9 ppm (t, 8H, –COOCH2–), 3.7 ppm (t, 32H, –CH2O–) and the hydrogen atoms of the repeated methylene groups in the hydrophobic chains appeared as a quartet signal at 1.1 ppm with integration number identical to the number of hydrogen atoms. That confirmed that the chemical structure of the synthesized surfactants is as follows:

where, n = 10, 15, 25 and R = CH2CH2OH and R1 = C11H23COOCH2CH2 (lauric), C15H31COOCH2CH2 (palmetic) and C17H35COOCH2CH2 (stearic).
Surface Tension Measurements
Surface tension measurements were obtained using a K–6 Du-Nouy Tensiometer (Kruss GmbH). Freshly prepared aqueous Gemini amphiphile solutions, with a concentration range 0.01–0.00001 mol/L, were poured into a clean 30 mL petri dish with a mean diameter of 28 mm. Apparent surface tensions [16] were measured a minimum of three times. The platinum ring was then removed, washed with diluted HCl followed by distilled water. The measurements were carried out at different temperatures, namely 25, 40 and 55 °C.
Solubilization
Solubilization towards octanol and paraffin oil (as polar and nonpolar solubilizate, respectively) was determined as a possible future application of these compounds. The light scattering technique was used to determine the solubilization behavior [15] of the diquaternary gemini surfactant. The instrument used was a Hatch Model 2100P Turbidimeter (the measurement unit of the instrument is the NTU, which is the Nephelometry–Turbidimitry Unit). The experiment was done by adding different quantities of paraffin oil to 100 mL of the surfactant solution (0.15%) and dispersed into droplets by hand. The system was then mixed using a rotary shaker at 150 rpm for different time intervals (5, 10, 15, 20, 30, 40, 50, 60, 70 and 80 min) at 25 °C. The solubilization behaviors of the surfactant systems were measured in NTU units.
Results and Discussion
There are two variables existing in the gemini molecules under investigation which influence their behavior — the alkyl hydrophobic chains (tails) and the nonionic polyoxyethylene hydrophilic chains (spacers). Hence the behavior of these compounds will be greatly affected by both length and nature of hydrophobic and hydrophilic groups in the molecules. Influences of the mentioned variables will be discussed separately in the following discussion.
Effect of Hydrophobic Chain Length on the Surface Active Properties of Gemini Amphiphiles
Surface Tension (γ) and Critical Micelle Concentration (CMC)
Figures 1, 2, 3, 4, 5 represent the variation of surface tension (γ) against −log concentration of the synthesized gemini amphiphile solutions at 25, 40 and 55 °C. The surface tension isotherms of the gemini amphiphiles show no minimum at the critical micelle concentration, which is a good indication for their purity. The surface tension values of the gemini amphiphiles have lower values than the corresponding monoquaternary homologues which indicates their higher surface activity. The critical micelle concentrations were determined based on the surface tension data and presented in Table 2. Analyzing the surface tension isotherms indicates that the increasing in hydrophobic chain length from 12 to 18 carbon atoms increases the surface tension at equilibrium, Figs. 1, 2, 3. Meanwhile, the critical micelle concentrations (CMC) decreased to lower values, (Table 2).
The critical micelle concentrations of quaternary surfactants containing one positive charge (N+) follow the equation [17]: \( {\text{CMC}}\;{\text{=}}\;{\text{ }}A - BN,\) where N is the number of carbon atoms along the alkyl chain of the surfactant molecule, A and B are constants. In case of doubly charged cationic surfactant molecules, large deviations from the above equation are identified, which resulted from the higher charge density on these molecules due to the presence of two (N+) groups and partially charged nonionic species [5].
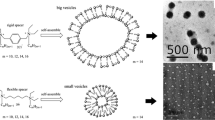
The surface tension isotherms of the synthesized gemini amphiphiles exhibit two inflection regions. The first, at lower concentrations (nearly at constant concentration) occurs between 0.0001 and 0.0002 mol/l, denoted as CMC b (secondary critical micelle concentration), while the second at relatively higher concentrations represent the critical micelle concentration CMC a . CMC a values were found to be highly sensitive towards the increasing length of the hydrophobic side chain. The secondary critical micelle concentration may be due to the multilayer aggregates formed in dilute solutions [18–19]. This aggregative structure is highly effective towards variation of concentration and temperature due to the large number of surfactant molecules participating in each structure. The latter geometrical aggregates decrease the surface tension rapidly, then on increasing the surfactant concentrations, deformation occurred and consequently spherical micelles are formed at CMC a . The multilayer structure (lamellar phase) [4] is greatly affected by temperature and dispersed by currents at higher degrees (40 and 55 °C) due to the random motion of the molecules. The aggregation of the dialkyl amphiphiles includes different geometrical structure which varied between double layer structures and multiloop structures [18]. These structures form a bilayer assembly of amphiphile molecules in the solutions [19]. Increasing the temperature increases CMC a , which can be interpreted as resulting from the presence of polyoxyethylene chains in the surfactant molecules.
Effectiveness (π cmc) and Efficiency (pC20)
The effectiveness (π cmc) is defined as the difference between the surface tension value of distilled water (γ 0) and that of surfactant solution (γ) at the critical micelle concentration:
While, the efficiency (pC20) is the −log concentration of the surfactant solution, which lowers the surface tension 20 units. The most efficient surfactant produces a greater depression of the surface tension at CMC. Gemini amphiphiles have good surface activity, Table 2, which shows in general the decrease in surface tension values at the CMC compared with the monoquaternary homologue series [20, 21].
The effectiveness values ranged between 30 and 43 m Nm−1. The increase in hydrophobic chain length has a reducing effect on π cmc values. The same behavior could also be observed in the efficiency values, pC20 (Table 2). Increasing the number of methylene groups (–CH2–) along the hydrophobic chains increases the hydrophobicity of the molecules, hence water–hydrophobe interactions increase which decreases the surface tension, followed by a decrease in the effectiveness, as well as the efficiency.
Minimum Surface Area (A min)
The minimum surface area (A min ) of surfactant molecules is defined as the average area occupied by each surfactant molecule on the air–water interface. A min values increase with decreases in the angle between surfactant molecule (tail) and the interface, which describes the angular position of surfactant molecules at the interface.
The minimum surface area (A min) of the synthesized gemini amphiphiles has been calculated and listed in Table 2 based on Gibb’s adsorption model of 2:1 electrolytes [15]:
where N A is Avogadro’s number; γ C and R denote surface tension in m Nm−1, bulk concentration in (mol/l) and the universal gas constant (8.31 × 107 J mol−1 K−1), respectively.
Table 2 shows that the minimum surface area (A min) values of the synthesized gemini amphiphiles in their solutions are proportionally influenced as the hydrophobic side chains increase. It is important to mention that the A min values are much higher than 20 A 2, which indicates the surfactant molecules are not normal to the air–water interface, but at different angles that depend mainly on the number of methylene groups (–CH2–) of the hydrophobic tail.
The gemini amphiphiles have twice the surface area at the interface as their single-headed counter parts [15], which refers to the two firmly anchored quaternary nitrogen centers (N+) at the interface [22]. The area of the lauryl diquaternary derivative is the lowest of the synthesized series of alkyl diquaternary compounds. Upon increasing the alkyl chain length, the molecules occupy a larger area at the air–water interface. Thus, the stearate diquaternary derivative has the largest area at the interface, (Table 2).
Effect of Hydrophilic Chain Length on Surface Active Parameters of Gemini Amphiphiles
The presence of polyoxyethylene (POE) chains in the synthesized lauryl-, palmetyl-, and stearyl polyoxyethylene gemini amphiphiles affected their surface active properties. Hence, the effect of POE chains will be discussed in the following sections.
Surface Tension and Critical Micelle Concentration (CMC)
Figures 3, 4, 5 represent the effect of various oxyethylene units on the surface tension of lauryl polyoxyethylene diquaternary amphiphiles.
It was found that the variation of surface tension values upon increasing oxyethylene repeat units is insignificant at higher concentrations especially the shorter POE chains (γ = 33 and 30 m Nm−1 for lauric diquaternary, 42 and 41 m Nm−1 for palmetyl diquaternary at n = 10 and 15, respectively) at 25 °C.
Increasing the temperature up to 40 and 55 °C decreases the surface tension values significantly (Figs. 3, 4, 5). This behavior can be explained as being a result of the combination between the cationic and nonionic characters of the diquaternary amphiphiles [17]. The nonionic property in the synthesized gemini amphiphiles reflects mainly on the values of the critical micelle concentrations (CMC) at higher temperature. Increasing the spacer chain length (POE) separates the two positively charged centers (N+). As a result, the average CH2/charge (N+) ratio decreased, which encourages the micellization at higher concentrations [7, 9–11]. The results of the critical micelle concentration (CMC) and the influence of temperature on polyoxyethylene chains showed better agreement with the explanations of several investigators [23–25].
Effectiveness (π cmc) and Efficiency (pC20)
The presence of nonionic spacer chains influences the effectiveness (π cmc) of the diquaternary amphiphile solutions at their CMC. Lower POE spacer groups posses lower π cmc values at CMC, while increasing the spacer lengths increases the π cmc values for palmetyl- and stearyl diquaternary amphiphiles with some deviation for lauryl diquaternary amphiphiles (POE = 25 units), Table 2.
Increasing the temperature increases the effectiveness (π cmc), while the efficiency (pC20) of the synthesized amphiphiles increased as an absolute value Table 2.
Effect of Structure on Thermodynamic Parameters of Micellization and Adsorption of Gemini Amphiphiles
According to the Gibb’s equations of thermodynamics, the thermodynamic functions of micellization and adsorption including, free energy, entropy and enthalpy change (ΔG, ΔS, ΔH, respectively) were calculated from the surface parameter data listed in Table 3 according to the following equations [15]:
Table 3 represents the micellization thermodynamic functions for the different synthesized gemini amphiphiles at different temperatures. The standard free energy of micellization (ΔG mic) was found always in negative values indicating that the process of micellization is a spontaneous one. Also, the standard free energy decreases by increasing the hydrophobic chain length. Meanwhile, a slight decrease in (ΔG mic) occurred on increasing the spacer chains (oxyethylene units). This is due to the hydrophobic chains and their repulsion by water molecules which is the motivating factor in micelle formation. In spite of the formation of hydrogen bonds and the attraction of head groups by water molecules, the alkyl chain-water molecules repulsion forcing the amphiphile molecules into a less energetic state which is the micelle form to reduce the mentioned repulsion.
The standard enthalpy change of micellization (ΔH mic) was found to be positive at some times and negative at others enforcing the idea that the micellization process is governed by the total entropy change (ΔS mic) and the main factor affecting the micellization is the transfer of methylene group from the interior of the micelle to its bulk. That transfer decreases the entropy change to lower values due to the highly ordered chains in the bulk of the micelles. Table 4 shows the variation of adsorption parameters of the synthesized diquaternary gemini amphiphiles at different temperatures. The standard free energy change of adsorption (ΔG ads) was found to be more negative than that for the micellization process (ΔG mic), which refers to the higher tendency of these amphiphiles to adsorption at air–water interface rather than micellization. The preference of adsorption is governed by the thermodynamic stability of the molecules at the air–water interface. The free energy change of adsorption per methylene group for the diquaternary amphiphiles was found to be −0.50 Kcal [26]. It is clear from analyzing the data of adsorption in Table 4 that (ΔG ads) per methylene group of gemini amphiphiles is higher than the values predicted by other investigators [26]. This increase in ΔG ads/(–CH2–) can be traced to the presence of the nonionic spacer chains within the amphiphiles, which increases the adsorption process at the air–water interface. The hydrogen bonds occurring between the water and surfactant molecules provide good stability for the adsorbed amphiphiles at the interface, which is considered a good agreement with the data obtained from A min values. Decreasing the polyoxyethylene spacer length shifts ΔG ads/(–CH2–) to lower values (<−0.50 Kcal) indicates that the POE chain length is the predominant factor influencing the amphiphile adsorption at the air–water interface.
Effect of Structure on Solubilization Behaviors of the Synthesized Gemini Amphiphiles
The mechanism of solubilization has been of interest and clarified in several publications [27, 28]. The nature of surfactant molecules (hydrophobic and hydrophilic parts) has a great influence on the solubilization process. Micellar volume also was found to influence the solubilization process. The origin of solubilization (oil-removal) via the micellar system is based on two factors:
-
1.
Adsorption of the solubilizate on the micelles, which depends on, the polar sites in the micelles and the solubilizate (first stage solubilization).
-
2.
Diffusion of the solubilizate into the micelles, which depends mainly on the effective volume of solubilization (later solubilization stage).
The effective micellar volume (V eff) concerning the solubilization may be proportional to the geometrical size of the aggregate. It depends on the packing of the molecules in the aggregate and the mutual compatibility of the surfactant and oil molecules. In most cases, V eff is proportional to the size of the micelle (or aggregate). When the aggregate size is too large as in the case of gemini amphiphiles, the packing of surfactant molecules becomes too tight. Increasing the hydrophobic chains from C12 up to C18 increases the host core of the giant diquaternary aggregates [10]. Increasing the aggregate volume damages these aggregates leading to smaller spherical micelles and hence decreasing their solubilizing power [29, 30].
Accordingly, the solubilization process can be divided into three stages throughout Figs. 6, 7, 8, 9. The first stage includes the adsorption of solubilizate molecules at the micellar interface, which is indicated at t = 0. The second stage of solubilization takes place when adsorbed solubilizate molecules penetrate into the micellar core. This stage required varied from a few minutes for smaller micelles to several hours in the case of giant aggregates. Turbidity of the system during this stage gradually increased as indicated in Figs. 6, 7, 8 and 9. In smaller micelles, rapid increases in turbidity could be observed in less time as in the case of (12-15-12) surfactants. The last stage of the solubilization process is the steady state solubilization, which appears in solubilization profiles as a straight line with an approximate slope = zero.
In the case of nonpolar solubilizate (paraffin oil) (Figs. 6, 7), the first stage turbidity is found at very low values, which indicates low adsorption of nonpolar molecules at the micellar surface due to its polar nature (polyethylene oxide layer). Penetration occurred into the micellar cores as the time of the second stage of the experiment was increased. It was found that increasing the HLB values of the synthesized surfactants increases the solubilization extent of their micelles. Generally, the smaller hydrophobic chains appeared to have a higher efficiency in solubilizing nonpolar substrates and vice versa.
Meanwhile, in the case of polar solubilizate (octanol) (Figs. 8, 9), the initial stage solubilization is found at high values indicating the good adsorption of octanol molecules to the polar surface of the micelles. At the second stage of solubilization, the turbidity of the system is gradually increased at a lower rate, Tables 5, 6. This is due to the hydrogen bonds between surfactant and octanol molecules. These hydrogen bonds decrease the octanol penetration into the micellar core. Hence, the turbidity of the system stays mostly at relatively lower values.
References
Manne S, Cleveland JP, Gaub HE, Stucky GD, Hansma PK (1994) Direct visualization of surfactants hemimicelles by force microscopy of the electrical double layer. Langmuir 10:4409
Sugihara G, Miyazono A, Nagadome S, Oida T, Hayashi Y, Jeong-Soo KO (2003) Sorption and micelle formation of mixed surfactant systems in water II: a combination of cationic gemini-type surfactant with mega-10. J Oleo Sci 52(9):449
Kern F, Lequeux F, Zana R, Candau J (1994) Dynamic properties of salt-free viscoelastic solutions. Langmuir 10:1714
Rist O, Rike A, Ljonesm L, Carlsen HJ (2001) Synthesis of novel diammonium gemini surfactants. Molecules 6:979
Tanaka M, Ishida T, Araki T, Masuyama A, Nakatsuji Y, Okahara M, Terabe S (1993) Double-chain surfactant as a new and useful micelle-forming reagent for micellar electrokinetic chromatography. J Chromatogr 648(2):469
Ono D, Yamamura S, Nakamura M, Takeda T (2005) Preparation and properties of bis-(sodium sulfate) types of cleavable surfactants derived from diethyl tartrate. J Oleo Sci 54(1):51
ZanaR, Talmon Y (1993) Dependence of aggregate morphology on structure of dimeric surfactants. Nature 362:228
Zana R, Benrraou M, Rueff R (1991) Alkanediyl-, alph-, omega-, -bis (dimethyl-ammonium bromide) surfactants 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 7:1072
Alami E, Levy H, Zana R, Skoulios A (1993) Alkanediyl-, alpha.-, omega.-, -bis-(dimethylammonium bromide) surfactants. 2. Structure of the lyotropic mesophases in the presence of water. Langmuir 9:940
Hagslatt H, Soderman O, Johnson B (1994) Divalent surfactants. Experimental results and theoretical modeling of surfactant/water phase equilibria. Langmuir 10:2177
Danino D, Talmon Y, Zana R (1995) Alkanediyl-, alpha-, omega-, -bis (dimethyl-ammonium bromide) surfactants (dimeric surfactants). 5. Aggregation and microstructure in aqueous solutions. Langmuir 11:1448
Oda R, Hucb I, Sauveur J (1997) Gemini surfactants, the effect of hydrophobic chain length and dissymmetry. Chem Commun 2105
Alami E, Beinert G, Marie P, Zana R (1993) Alkanediyl-, alpha-, omega-, -bis-(dimethylammonium bromide) surfactants 3. behaviour at the air/water interface. Langmuir 9:1465
Manne S, Schaffer TE, Huo Q, Hansma PK, Morse DE, Stucky GD, Aksay IA (1997) Gemini surfactants at solid liquid interfaces: control of interfacial aggregation geometry. Langmuir 13:6382
Negm NA (2002) Surface activities and electrical properties of long chain diquaternary bola-form amphiphiles. Egypt J Chem 45(3):483–499
Negm NA, Mahmoud SA (2003) Effect of Structure on the Physicochemical Properties of Nonionic Phosphate Amphiphiles. Egypt J Petroleum 1(13):95
Jonsson B, Lindman B, Holmberg K, Kronberg B (1998) Surfactants and polymers in aqueous solutions. Wiley, New York, pp 92
Haldar J, Aswal VK, Goyal PS, Bhattacharya S (2004) Aggregation properties of novel cationic surfactants with multiple pyridinium headgroups. small-angle neutron scattering and conductivity studies. J Phys Chem B 108(31):11406–11411
Brinkmann U, Neumann E, Robinson BH (1998) Thermodynamics and kinetics of vesicle to mixed micelle transitions of sodium tridecyl -6-benzene-sulfonate/ sodium dodecyl sulfate surfactant systems. J Chem Soc Faraday Trans 94(9):1281
Zana R (1996) Progress in synthesis in gemini surfactants. Curr Opin Colloid Interface Sci 1:566
Alami E, Holmberg K, Eastoe J (2002) Adsorption properties of novel gemini surfactants with nonidentical head groups. J Colloid Interface Sci 247:447
Lu JR, Su TJ, Li ZX, Thomas RK, Staples EJ, Tuker I, Penfold J (1997) The structure of monolayers of monododecyl dodeca-ethylene glycol at the air/water interface studied by neutron reflection. J Phys Chem B 101:10332
Ballet F, Debeauvais F, Candau F (1980) The effect of temperature and alcohol on the solubilization of water in nonaqueous solutions of polymeric emulsifiers. Colloid Polym Sci 258(11):1253
Hamid SM, Sherrington DC (1987) Novel quaternary ammonium amphiphile (meth) acrylates. Polymer 28:325, 332
Blois DW (1971) Interaction of polysilicic acid with monolayers of substances containing quaternary ammonium groups. J Colloid Interface Sci 36:226
Bazito RC, El-Seoud OA (2001) Sugar-based cationic surfactants: synthesis and aggregation of methyl-2-acylamido-6-trimethylammonio-2,6-dodeoxy-d-gluco-pyranoside chloride. J Surfactants Detergents 4(4):395
Shen D, Zhang R, Han B, Dong Y, Wu W, Zhang J, Li J, Jiang T, Liu Z (2004) Enhancement of the solubilization capacity of water in triton X-100/cyclohexane/water system by compressed gases. Chemistry 10(20):5123
Nagarajan R, Rucken Sten E (2000) Molecular theory of microemulsions. Langmuir 16(16):6400
Zana R (2005) Dynamics of surfactant self-assemblies: micelles, microemulsions, vesicles and lyotropic phases. CRC Press, Boca Raton
Jobe DJ, Reinsborough VC, White PJ (1982) Solubilization sites in micellar sodium octylsulphate solutions by ultrasonic spectroscopy. Can J Chem Rev Can Chim 60(3):279
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negm, N.A. Solubilization, Surface Active and Thermodynamic Parameters of Gemini Amphiphiles Bearing Nonionic Hydrophilic Spacers. J Surfact Deterg 10, 71–80 (2007). https://doi.org/10.1007/s11743-007-1014-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-007-1014-x