Abstract
Two series of diquaternary cationic surfactants designated as E9Nm and E11Nm having two different alkyl chains in their chemical structure were synthesized. The chemical structures of these surfactants were confirmed using elemental analysis, FTIR and 1H-NMR spectra. The surface activities of the different surfactants were determined using surface and interfacial tension at 25 °C. The surface parameters including: critical micelle concentration, effectiveness, efficiency, maximum surface excess and minimum surface area were determined. The surface activities of the cationic surfactants were correlated with their chemical structure. The surface activities of the surfactants increased with increasing the hydrophobic chain length. The adsorption and micellization tendencies of the surfactants in solution were determined using the free energies of adsorption and micellization. The synthesized surfactants were evaluated as biocides against bacteria and fungi. Biocidal activity data showed that a gradual increase in the hydrophobic chain length of the surfactant molecules gradually increases the efficiency of these surfactants as biocides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of surfactants based on environmentally friendly feedstocks is a concept that is gaining momentum in the detergent and cosmetic industries. Gemini cationic surfactants containing ester groups are a response to increasing consumer demand for products that are both greener and more efficient [1]. Several families of new surfactants have been developed using environmentally friendly processes based on various products and by-products of the oleochemical industry [2, 3]. Production of these entirely natural molecules may replace the surfactants conventionally used.
Cationic surfactants, with almost 7 % of the total surfactant market, have many applications such as asphalt additives, fabric softeners, biocides, corrosion inhibitors, and textile auxiliaries. Cationic surfactants are strongly adsorbed onto surfaces through an ion exchange mechanism [4–6]. Nevertheless, the aquatic toxicity of cationic surfactants is higher than other surfactants and more irritating to the eyes and to the skin. The toxicity of these surfactants results from their tendency to adsorb onto negatively charged surfaces [6, 7].
Different tactics have been considered to overcome this problem. The main approach is the introduction of cleavable bonds in the surfactant structure. The search for novel surfactants with higher efficiency and effectiveness gave birth to the concept of Gemini surfactants. Gemini surfactants are made up of two surfactant molecules with their head groups chemically linked by a spacer group [8, 9]. It was found that the surfactant properties of Gemini-type surfactants, such as low critical micelle concentration (CMC) and efficient surface tension lowering were superior to the corresponding single molecule surfactants [10–17]. Several categories of Gemini surfactants have been synthesized, and their physicochemical properties have been investigated [18, 19].
Experimental Technique
Materials
The chemicals used in the study were: butyl amine, pentyl amine, hexyl amine, octyl amine, 3-dimethylamino-1-propanol, decanoyl chloride, dodecanoyl chloride, epichlorohydrin and acetone (purity 99 %) which were purchased from Alfa Aesar (Germany). Paraffin oil was obtained from ADWIC, Egypt.
Synthesis of 3-Dimethyl Aminopropyl Esters of Saturated Fatty Acids
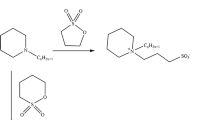
3-Dimethylamino-1-propanol (0.1 mol, 10.32 g) and sodium metal (0.1 mol, 2.31 g) were mixed under nitrogen atmosphere until the evolution of hydrogen bubbles stopped. Then an acid chloride, namely: decanoyl chloride or dodecanoyl chloride (0.1 mol) was added drop wise in the presence of acetone (100 mL) as a solvent. The reaction mixture was refluxed for 6 h, and then left to cool overnight. The reaction products were recrystallized twice from diethyl ether, and denoted as E9 for the decanoyl derivative and E11 for the dodecanoyl derivative, respectively. (melting point: E9 = 54 °C; E11: 60 °C, yield: 86, 89 %). The synthesis is shown in Scheme 1.
Reaction of Epichlorohydrin and Primary Amine
Butyl amine, pentyl amine, hexyl amine, and octyl amine (0.5 mol) and 0.1 mol of epichlorohydrin were refluxed individually in a 250-mL round-bottom flask for 6 h. The reaction mixture was left to cool overnight. The products obtained were designated as N4 for the butyl derivative, N5 for the pentyl derivative, N6 for the hexyl derivative, and N8 for the octyl derivative. The synthesis is show in Scheme 2.
Quaternization Reaction
N,N-Dimethyl aminopropyl esters (E9 and E11) (0.05 mol) were refluxed for 12 h with N4 or N5 or N6, and N8 in the presence of acetone (100 mL) as a solvent. The reaction mixture was left to cool and the solvent was removed under vacuum at 50 °C. The diquaternary compounds obtained were recrystallized twice from acetone, then washed by diethyl ether and dried at 35 °C under vacuum. The compounds from the reaction of E9 and N4, N5, N6, N8 were designated as: E9N4, E9N5, E9N6 and E9N8, respectively. While those obtained from reaction of E11 and N4, N5, N6, N8 were designated as: E11N4, E11N5, E11N6 and E11N8. The synthesis is shown in Scheme 3.
Nomenclature
-
E9N4: bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}butylamine dichloride;
-
E9N5: bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}pentylamine dichloride;
-
E9N6: bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}hexylamine dichloride;
-
E9N8: bis{2-hydroxy-3-[3-(decanoyloxy)propyldimethylammonio]propyl}octylamine dichloride.
-
E11N4: bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}butylamine dichloride;
-
E11N5: bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}pentylamine dichloride;
-
E11N6: bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}hexylamine dichloride;
-
E11N8: bis{2-hydroxy-3-[3-(dodecanoyloxy)propyldimethylammonio]propyl}octylamine dichloride.
Surface Tension (γ) and Interfacial Tension (γ IT)
Surface and interfacial tension values were measured by using a K6 tensiometer (Krüss Instruments, Germany) with a platinum ring. Freshly prepared aqueous solutions of the synthesized gemini cationic surfactants were measured at different concentrations ranging from 5 × 10−3 to 2 × 10−8 M at 25 °C. The solution was poured into a clean 25-mL Teflon container and allowed to equilibrate for 2 h. The platinum ring was adjusted at the air–water interface of the solution, then the reading was recorded when the ring detached itself from the solution surface. The apparent surface tension values were measured a minimum of three times and the recorded values were taken as the average of these values. The platinum ring was removed after each reading, washed with dilute HCl followed by distilled water [20].
The interfacial tension measurements were obtained between aqueous solution of the synthesized surfactants at concentration of 0.1 % by weight and light paraffin oil at 25 °C. The surfactant solution (15 mL, 0.1 %) was added to a Teflon container and the platinum ring was adjusted to touch the solution surface. Light paraffin (15 mL) oil was then added slowly on the surface, and the readings were recorded at which the ring detached from the aqueous to the organic layer (paraffin oil). The ring was removed and washed with acetone followed by distilled water and the measurements were repeated three times for each surfactant. The interfacial tension values were taken as the average of the three readings obtained [20].
Emulsification Power
Emulsification power was measured by vigorously stirring a mixture of the synthesized surfactant solutions (0.1 wt%, 20 mL) and paraffin oil (20 mL) at 25 °C in a 100-mL graduated cylinder. The cylinder was shaken vigorously for 10 min and then the contents allowed to settle. The time required to separate 18 mL of pure surfactant solution was recorded and the experiment was repeated three times for each surfactant. The average time of the three experiments was taken as an indication of the emulsification power of each surfactant [21].
Antimicrobial Studies
The synthesized gemini surfactants were screened for their antimicrobial activity against bacteria and fungi using the well diffusion technique [22]. Cetyl trimethyl ammonium bromide (CTAB) was taken as a reference [23].
The bacterial and fungal strains were cultured according to the standards of the National Committee for Clinical Laboratory [22]. For bacteria, the broth media were incubated for 24 h, while for fungi the incubation was for 48 h; with subsequent filtering to remove mycelial fragments before the solution containing the spores was used for inoculation.
For the preparation of discs and inoculation, 1.0 mL of inocula was added to 50 mL of agar media (40 °C) and mixed. The agar was poured into 120-mm Petri dishes and allowed to cool to room temperature. Wells (6 mm in diameter) were cut in the agar plates using sterile tubes. Then, the wells were filled up to the surface of the agar with a 0.1 mL solution of the synthesized compounds consisting of 1 mg surfactants in 1 mL of DMF (DMF has negligible influence on the growth of the microorganisms). The plates were left on a level surface, incubated for 24 h at 30 °C for bacteria and then the diameter of the inhibition zones were measured. The inhibition zone formed by these compounds against the particular test microorganisms determined the biocidal activity of the synthetic compounds. The mean value of three replicates was used to calculate the zone of growth inhibition of each sample.
The antibacterial activity of the synthesized cationic compounds was tested against Streptococcus pneumonia, Escherichia coli, Bacillus subtilis and Pseudomonas aeruginosa. The anti-fungal activity was tested against Aspergillus fumigatus and Candida albicans.
Results and Discussion
Structure
The chemical structures of the synthesized surfactants (E9N8, E9N6, E9N5, E9N4, E11N8, E11N6, E11N5 and E11N4) (Scheme 3) were confirmed using elemental analyses using a Vario Elementar Analyzer (Table 1). FTIR spectroscopy was performed using Genesis Fourier transformer FTIR™ for the E11N6 surfactant as a representative sample for the synthesized surfactants. The spectrum showed the following absorption bands: 1169 cm−1 correspond to ether linkage; 1734 cm−1 correspond to the ester group; 2857 and 2927 cm−1 (CH3 and CH2), respectively, and 3387 cm−1 correspond to alcohol group (Fig. 1). 1H-NMR spectroscopy was performed using a Varian NMR-300-Mercury 300 MHz spectrometer with TMS as an internal standard. The spectrum of (E11N6 in DMSO-d6 as representative sample for the synthesized surfactants, Fig. 2) showed signals at: δ = 0.85 ppm (m, CH 3 (CH2)8), (CH 3 (CH2)4), 1.23 ppm (m, CH3 ( CH 2 ) 8 , CH3 ( CH 2 ) 4 ), 1.50 ppm (m, CH 2 CH2C=O), 1.82 ppm (m, OCH2 CH 2 CH2 +N), 2.26 ppm (m, CH2 CH 2 C=O), 2.51 ppm (m, CH 2 N(CH 2 )CH 2 ), 3.17 ppm (s, +N(CH 3 )), 3.47 ppm (m, +NCH 2 CH2CH2O, CHCH + 2 N), 3.65 ppm (m, OCH2CH2 CH + 2 N), 4.05 ppm (m, OH). The synthesized compounds were completely soluble in water.
Surface Activity
Figure 3 shows the variation of surface tension against –log C of the synthesized surfactants at 25 °C. It is clear that the surface tension values of the synthesized surfactants decrease gradually with increasing concentration. The surface tension isotherms show the characteristic inflection corresponding to the critical micelle concentration (CMC) at which surfactant micelles begins to form. The surface active properties of the synthesized surfactants were extracted and listed in Table 2. The synthesized surfactant molecules have two different hydrophobic chains. The first is attached to the ester group, and the second is attached to the tertiary nitrogen atom. The two different chains have different influence on the surface activity of the surfactants in their solutions.
It is clear from Table 2 that the critical micelle concentration values ranged between 0.028 and 0.708 mM for E9Nm and 0.018–0.447 mM for E11Nm surfactants. Analyzing the CMC data reveals two main points. First, a gradual increase in the fatty alkyl chain length linked to the ester groups and also the fatty alkyl chains linked to the tertiary nitrogen atoms decreases the CMC values gradually. This effect can be attributed to the increase in the hydrophobicity of the surfactant molecules by increasing the chain length. Increasing the hydrophobicity increases the adsorption tendency of surfactant molecules, which decreases their CMC values [23]. Second, the butyl, pentyl, hexyl and octyl chains attached to the tertiary nitrogen atom have more influence on the CMC values than the increase in the alkyl chains attached to ester groups. The critical micelle concentration of the surfactant molecules depends on the length of the alkyl chains attached to the surfactant molecules and their geometry. Increasing the alkyl chain length attached to the surfactant molecules gradually decreases the critical micelle concentration as shown in Fig. 4. The hydrophobic effect is the main reason for micellization at a low concentration. The geometry of molecules depends, as well, on the repulsion and attraction between the function groups in the molecules [24]. The alkyl chains linked to the tertiary nitrogen atom are surrounded by two hydroxyl groups, which exert a repulsive force on each other. The repulsion is increased by increasing the alkyl chain length, which forces the molecules to self-associate at low concentration. Micellization leads to hydration of molecules which decreases the repulsion by forming hydrogen bonds with water molecules [24].
The spreading pressure at the CMC (π CMC) is defined as the difference between surface tension at the CMC and that of distilled water. Generally, the spreading pressure of the synthesized surfactants increased with increasing alkyl chain length. This indicates increasing surface activity of the different surfactant molecules by increasing the length of the hydrophobic groups (either from E9 to E11 or N4 to N8). Similarly, the efficiency values (PC20) of E9Nm and E11Nm varied with increasing hydrophobic chain lengths. The three surface parameters: CMC, π CMC and PC20 showed comparatively higher surface activity of E9Nm and E11Nm than some diquaternary ammonium salts reported in the literature [25, 26].
The maximum surface excess concentration values (Γ max) of the surfactant solutions were calculated according to Gibb’s adsorption equation [27].
where dγ/dlnC is the maximum slope, n is the number of solute species whose concentration varies with surfactant concentration and R, T, C are the gas constant, temperature and concentration, respectively.
The minimum surface area at the interface occupied by each surfactant molecule at the saturation condition is calculated using the following equation [27]:
where N is Avogadro’s number. Increasing the hydrophobic chain length of the surfactants increased the maximum surface excess concentration. The increase in alkyl chain length increases the repulsion between surfactants molecules and the aqueous phase which forces the surfactant molecules to the interface. The minimum surface area (A min) is the average area occupied by each surfactant molecule at the interface. Higher surface excess concentration of surfactant molecules at the interface creates tight packing. E9N8 and E11N8 have the highest surface concentration at 1.14 × 10−10 and 1.18 × 10−10 mol/cm2 which is accompanied by the lowest average area at 146.04 and 140.18 Ǻ2.
Thermodynamic of Adsorption and Micellization
The thermodynamic functions of adsorption and micellization of surfactant molecules determine their tendency towards the two processes. The data in Table 2 show that ∆G ads and ∆G mic values of the synthesized surfactants are always negative. That indicates that the two processes occur spontaneously in the aqueous phase, i.e., adsorption and micellization are exothermic processes. ∆G ads is more negative than ∆G mic, which indicates a higher tendency towards adsorption than micellization. The adsorption tendency is reflected from the sharp decrease in surface tension values by small increase in concentration. E11Nm derivatives have more negative ∆G mic and ∆G ads values than E9Nm derivatives. This represents the role of the alkyl chain length on the micellization and adsorption of the synthesized surfactants.
The adsorption and micellization data were calculated using the thermodynamic equations as follows [2, 10]:
Interfacial Tension and Emulsification Power
The interfacial tension (γ IT) of the synthesized surfactants (Table 2) against light paraffin oil ranged between 4 and 5 mN/m, which shows higher surface activity compared to the diquaternary cationic surfactants reported in the literature [25, 26]. The low values of interfacial tension can be attributed to the presence of two alkyl chains in the surfactant molecule. This increases the hydrophobicity of the molecules and consequently increases their efficiency at the liquid–liquid interface.
Emulsion formation is an important property of surfactants used in several applications, including: foaming agents, paints and cosmetics. On the contrarily, formation of stable emulsions is an undesirable property is a few surfactant applications such as corrosion and scale inhibitors. The synthesized diquaternary surfactants showed relatively low oil–water emulsion stability as shown in Fig. 5. The stabilities of the oil-in-water emulsions formed using the tested surfactants ranged between 460 and 178 s. This shows the low emulsification tendency of these surfactants, and consequently their safe applicability in the field of petroleum applications including biocides and prevention of acidic dissolution of metals.
Biological Activity of the Synthesized Compounds
The bacterial cell membrane is formed of phospholipids and specific amino acids. The function of the cellular membrane is mainly to control diffusion of the materials necessary for biological reactions and excretion of wastes produced. Control of the two processes is determined by selective permeability. The factor controlling selectivity of polar species to enter or exit the cell is the charged amino acids (teichoic acid) while control of nonpolar materials is the phospholipids and peptidoglycans. When the selective permeability of the cellular membrane is disturbed for any reason, the biological reactions and activities in the cell are disturbed which leads to the death of the microorganism. The role of biocides is to disturb and/or destroy the selective permeability of these membranes in order to kill the microorganisms. This typically happens in the case of the cationic surfactants [28].
Table 3 shows the biological activities of the synthesized diquaternary cationic surfactants against Gram-positive and Gram-negative bacteria. No effect was observed against fungi. It is clear that increasing CMC values of these surfactants increases their biological activity. The highest CMC value at 0.71 mM for E9N4 corresponded to the highest inhibition zone diameter at 19.6 ± 0.63, 21.3 ± 0.32, 15.9 ± 0.46 and 18.6 ± 0.36 mm for S. pneumonia, B. subtilis, E. coli and A. Fumigatus species.
Similarly, biological activity increased with increasing A min values of the surfactant molecules at the interface. Increasing A min increases the area of interaction between surfactant molecule and the cell membrane. This increases the biocidal activity of the surfactant molecules. Smaller areas of interaction between surfactant molecules and the cell membrane decrease their influence on that membrane, and consequently the influence on membrane hydration and selective permeability decreased. This effect can be observed in case of E9N8 and E11N8, which have the lowest inhibition zone diameter. Meanwhile, the highest inhibition zone diameter was observed in case of E9N4 and E11N4. The influence of alkyl chain length (n = 4, 5, 6, 8) on the inhibition zone diameter is graphically represented in Figs. 6 and 7. It is clear that the gradual increase in the alkyl chain from 4 to 8 carbon atoms decreases the inhibition zone diameter gradually. The biological activity data of the synthesized compounds shows moderate activity compound to the control (CTAB) as shown in Table 3. The moderate biocidal activities of the synthesized compounds, determined in terms of minimum inhibitory concentration, are given in Table 4. All of the tested compounds showed relatively high MIC values compared to the control. The relatively high MIC values of the synthesized compounds show low toxicity and consequently applicability as biocides in preventing bacterial growth in different applications.
References
Benvegnu T, Plusquellec D, Lemiegre L, Belgacem MN, Gandini A (2008) Monomers, polymers and composites from renewable resources. Elsevier, Amsterdam
El-Sukkary MMA, Shaker NO, Ismail DA, Zaki MF, Ahmed SM (2012) Surface parameters, biodegradability and antimicrobial activity of some amide ether carboxylates surfactants. Egypt J Pet 21:37–43
El-Sukkary MMA, Shaker NO, Ismail DA, Ahmed SM, Awad AI (2012) Preparation and evaluation of some amide ether carboxylate surfactants. Egypt J Pet 21:11–17
Steichen DS, Holmberg K (2001) Handbook of applied surface and colloid chemistry, vol 1. Wiley, New York, pp 309–314
Steichen DS, Gadberry JF, Karsa DR (1998) New products and applications in surface technology. Sheffield Academic Press, Sheffield, p 59
Cross J, Singer EJ (1994) Cationic surfactants. Marcel Dekker, New York, p 3
Huber L, Nitschke L, Holmberg K (2002) Handbook of applied surface and colloid chemistry, vol 1. Wiley, Chichester, pp 509–516
Stjerndahl M, Landberg D, Holmberg K (2003) Novel surfactants, preparation, application and biodegradability. Marcel Dekker, New York, pp 317–321
Tehrani-Bagha AR, Holmberg K (2007) Cleavable surfactants. Curr Opin J Colloid Interface Sci 12:81–84
Rosen MJ (1993) Geminis: a new generation of surfactants. Chem Tech 23:30–33
Menger FM, Littau CA (1993) Gemini surfactants a new class of self-assembling molecule. J Am Chem Soc 115:10083–10090
Zana R (2002) Dimeric gemini surfactants effect of the spacer group on the associate behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution a review. Adv Colloid Interface Sci 97:205–253
Aisaka T, Oida T, Kawase T (2007) A novel synthesis of succinic acid type Gemini surfactant by the function group interconversion of corynomicolic acid. J Oleo Sci 56:633
Bunton CA, Robinson L, Schaak J, Stam MF (1971) Catalysis of nucleophilic substitution by micelles of dicationic detergents. J Org Chem 36:2346–2350
Negm NA, Badr EA, Aiad IA, Zaki MF, Said MM (2012) Investigation the inhibitory action of novel diquaternary di Schiff bases on the acid dissolution of carbon steel in 1 M hydrochloric acid solution. Corros Sci 65:77–86
EL-Sukkary MMA, Ismail DA, El Rayes SM, Saad MA (2014) Synthesis and evaluation of some derivatives of polysiloxanes. Egypt J Pet 23:361–366
Negm NA, El Farargy AFM, Mohammed DE, Mohamad HN (2012) Environmentally friendly nonionic surfactants derived from tannic acid: synthesis, characterization and surface activity. J Surfactants Deterg 15:433–443
Negm NA (2007) Solubilization, surface active and thermodynamic parameters of gemini amphiphiles bearing nonionic hydrophilic spacer. J Surfactants Deterg 8:71–80
Negm NA, El Farargy AF, Al Sabagh AM, Abdelrahman NR (2011) New Schiff base cationic surfactants: surface and thermodynamic properties and applicability in bacterial growth and metal corrosion prevention. J Surfactants Deterg 14:505–514
Negm NA, Mohamed AA, El-Awady MY (2005) Influence of structure of the cationic polytriethanol ammonium bromide derivatives II. Corrosion inhibition. Egypt J Pet 14:201–210
National Committee for Clinical Laboratory Standards; methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. National Committee for Clinical Laboratory standards, Wayne (1997)
Negm NA, Mohamed AS (2008) Synthesis, characterization and biological activity of sugar-based gemini cationic amphiphiles. J Surfactants Deterg 11:215–221
Negm NA, Said MM, Morsy SMI (2010) Pyrazole derived cationic surfactants and their tin and copper complexes: synthesis, surface activity, antibacterial and antifungal efficacy. J Surfactants Deterg 13:521–528
Wegrzynska J, Chlebicki J (2006) Surface-active antielectrostatic properties of multiple quaternary ammonium salts. J Surfactants Deterg 9:221
Chlebicki J, Wegrzynska M, Oswiecimska J, Maliszewska I (2005) Preparation, surface-active properties, and antimicrobial activities of bis-quaternary ammonium salts from amines and epichlorohydrin. J Surfactants Deterg 8:227
Rosen MJ (2001) Surface and interfacial phenomena, 2nd edn. John Wiley, New York, p 151
El-Sukkary MMA, Ghuiba FM, Sayed GH, Abdou MI, Badr EA, Tawfik SM, Negm NA (2014) Evaluation of some vanillin-modified polyoxyethylene surfactants as additives for water based mud. Egypt J Pet 23:7–14
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Migahed, M.A., Negm, N.A., Shaban, M.M. et al. Synthesis, Characterization, Surface and Biological Activity of Diquaternary Cationic Surfactants Containing Ester Linkage. J Surfact Deterg 19, 119–128 (2016). https://doi.org/10.1007/s11743-015-1749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1749-8