Abstract
Background
Roux-en-Y gastric bypass (RYGB) is an effective treatment for diabetes. Glucagon-like peptide-1 (GLP-1) is a gut hormone that is important to glucose homeostasis.
Objective
This study aimed to assess GLP-1 level and its predictors after RYGB.
Methods
The study design was a meta-analysis. The data sources were MEDLINE, EMBASE, Web of Science, and the Cochrane Databases. The study selection composed of studies with pre- and post-RYGB levels. The main outcomes were as follows: Primary outcome was the change in postprandial GLP-1 levels after RYGB. Secondary outcomes included the changes in fasting glucose, fasting insulin, and fasting GLP-1 levels after RYGB. Meta-regression to determine predictors of changes in GLP-1 levels was performed. Outcomes were reported using Hedge’s g.
Results
Twenty-four studies with 368 patients were included. Postprandial GLP-1 levels increased after RYGB (Hedge’s g = 1.29, p < 0.0001), while fasting GLP-1 did not change (p = 0.23). Peak postprandial GLP-1 levels gave the most consistent results (I 2 = 9.11). Fasting glucose and insulin levels decreased after RYGB (p < 0.0001).
Roux limb length was a significant predictor for amount of GLP-1 increase (β = − 0.01, p = 0.02). Diabetes status, amount of weight loss, length of biliopancreatic limb, and time of measurement were not significant predictors (p > 0.05).
Conclusion
Postprandial GLP-1 levels increase after RYGB, while fasting levels remain unchanged. Shorter Roux limb length is associated with greater increase in postprandial GLP-1, which may lead to better glycemic control in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become a growing pandemic. Since 1980, worldwide obesity has doubled [1]. In the USA, more than one third of the adult population were obese (BMI of ≥ 30 kg/m2) in 2014 [2]. Parallel to this, there has been a rapid increase in obesity-related comorbidities including hypertension, hyperlipidemia, diabetes, coronary artery disease, non-alcoholic fatty liver disease (NAFLD), gallbladder disease, and certain types of cancer. In 2012, approximately 210 billion dollars were spent to treat obesity-related conditions in the USA, accounting for 21% of health care expenditures [3].
Roux-en-Y gastric bypass (RYGB) is a bariatric surgery developed by Mason and Ito in 1967 for the management of obesity [4]. It involves the creation of an upper gastric pouch. A portion of the small intestine is divided at the mid-jejunum, and the distal portion is anastomosed to the gastric pouch to become the Roux limb. The excluded portion of the stomach and the proximal small intestine, also known as the biliopancreatic limb, are anastomosed distally to the distal end of the Roux limb. Ingested nutrients flow down the Roux limb and come in contact with the biliary and pancreatic secretions below the level of the anastomosis in the segment of the small intestine known as the common channel. In the Swedish Obese Subjects (SOS) study, patients who underwent RYGB lost 27 ± 12% of their baseline weight at 15 years and had a decreased mortality rate compared to the control group [5].
In addition to inducing weight loss, RYGB has been shown to be effective at treating obesity-related comorbidities including type 2 diabetes mellitus (T2DM). In a STAMPEDE (Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently) trial, 150 obese and uncontrolled diabetic subjects were randomized to receive intensive lifestyle therapy and medications or intensive lifestyle therapy and medications and bariatric surgery (RYGB or sleeve gastrectomy). At 3 years, those who underwent RYGB had significant improvement in their glycemic control compared to those who received intensive lifestyle therapy and medications alone. Additionally, more patients in the RYGB arm were able to discontinue their anti-diabetic medications [6]. While the benefits of RYGB on glycemic control is clear, the exact mechanisms remain unknown.
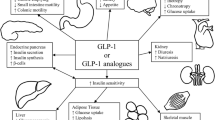
It has been hypothesized that the early delivery of nutrients to the distal gut may lead to improvement in diabetes after RYGB. Also known as the hindgut hypothesis, this theory implies an important role of the incretin hormone glucagon-like peptide-1 (GLP-1) on glycemic control after bypass surgery [7]. Secreted by L-cells in the distal ileum and colon, GLP-1 secretion is stimulated by nutrients within the lumen. It then binds to receptors in the pancreatic beta-cells to promote the release of insulin and therefore plays a key role in glycemic control. In addition, GLP-1 also promotes satiety, delays gastric emptying, increases insulin sensitivity, and decrease glucagon secretion [8].
GLP-1 levels have been shown to increase following gastric bypass surgery in several observational studies. However, a meta-analysis investigating the effect of RYGB on changes in GLP-1 has not previously been investigated. We therefore present a systematic review and meta-analysis to assess the changes in GLP-1 levels following RYGB. In addition, a meta-regression was performed to assess predictors of changes in GLP-1 levels after gastric bypass.
Methods
Data Sources and Searches
The search strategy, study eligibility criteria, selection process, data collection process, primary and secondary outcomes, and analyses were defined a priori and are described below.
We searched four databases—MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials—from inception to August 1, 2016 without language or study design restriction. An extensive search strategy was employed to find articles that relate to changes in GLP-1 levels after RYGB. For articles related to RYGB, the following were searched by subject heading: gastric bypass; and by text words in the title and abstract: RYGB, Roux en Y gastric bypass, Roux en Y, gastric bypass, stomach bypass, bariatric surgery, obesity surgery, bypass surgery. For articles related to GLP-1, the following were searched by subject heading: glucagon like peptide 1; by text words in the title and abstract: glucagon like peptide 1, glucagon like peptide, GLP1, GLP-1, GLP 1, incretin*; and by text words in the body of the article: GLP1, GLP-1, glucagon like peptide. The final search combined the search parameters for RYGB and GLP-1.
After duplicates were removed, two of the authors (P.J and D.J) independently reviewed titles and abstracts produced by the search. Studies deemed potentially relevant were reviewed in full to determine eligibility. Disagreements regarding final study inclusion were resolved by discussion. If a consensus could not be reached, the senior author (C.T.) served as the final arbiter.
Study Selection
Study Design and Population
Randomized clinical trials, observational cohort studies, and case series were included. Reviews, editorials, case-control studies, and studies using non-human subjects were excluded, as were articles without full text availability or English translation. Only one study from the same research group was selected to preserve independence of observations. Studies which measured GLP-1 levels in the setting of GLP-1 agonist/antagonist were also excluded. Studies were included if patients were adults > 18 years of age, obese with mean body mass index (BMI) ≥ 30 kg/m2, underwent RYGB surgery for weight loss, and received serum GLP-1 measurements pre and postRYGB.
Intervention
The study intervention was defined as RYGB for weight loss. No restrictions were applied to approach (open versus laparoscopic), or technique (length of Roux limb or biliopancreatic limb). Studies were excluded if patients underwent RYGB for indications other than weight loss (e.g., malignancy) or underwent other gastrointestinal surgeries or bariatric interventions without subgroup analysis of RYGB-only patients.
Comparison
All subjects underwent GLP-1 measurement pre and postRYGB and served as their own comparison.
Outcomes
The primary outcome was defined as change in serum postprandial GLP-1 level following RYGB from baseline preRYGB level. No restrictions were applied regarding method of stimulation (mixed meal versus oral glucose tolerance test (OGTT)). Specifically, after consumption of a mixed meal or glucose drink, frequent blood sampling was performed for up to 3 h for analysis of GLP-1. The postprandial levels may then be reported using an average or area under the curve (AUC) GLP-1 level (an average of hormonal levels from different blood draws during the 3-h interval) or peak GLP-1 level (the highest hormonal level during the 3-h interval). Studies reporting one or more of the following were eligible for inclusion: average GLP-1 levels pre and postRYGB, peak GLP-1 levels pre and postRYGB, GLP-1 total area under the curve (AUC) pre and postRYGB, or difference in GLP-1 levels pre to postRYGB.
In instances when more than one postRYGB serum GLP-1 level was reported, the measurement obtained closest to 1-year postRYGB was utilized for quantitative analysis to minimize the possible effect of weight change on GLP-1 levels [5].
Secondary outcomes were defined as changes in fasting glucose, fasting insulin, and fasting GLP-1 levels after RYGB. These were defined as the serum levels of glucose, insulin, and GLP-1 in the morning after an overnight fast.
Data Extraction, Risk of Bias Assessment, and Quality Assessment
A data extraction sheet was constructed to record information on study characteristics, patient characteristics, and predefined outcomes. Risk of bias was assessed using the Cochrane Collaboration’s risk of bias in non-randomized studies of interventions (ROBINS-I) tool for observational studies and the Cochrane Collaboration’s tool for assessing risk of bias for randomized clinical trials. The domains assessed were selection bias, consideration of confounders, exposure measurement (performance bias), blinding of outcome (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and bias due to deviations from intended interventions. In this study, publications were deemed low risk of bias if more than 50% of the above domains were judged as low risk.
The quality of observational studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale (NOS). The domains assessed were selection of cohort, ascertainment of exposure/comparator, and assessment of outcome. Quality of randomized clinical trials was assessed using the JADAD score. In this study, high quality was defined as an NOS score of ≥ 6 or a JADAD score of ≥ 3.
Two authors (P.J and D.J.) independently extracted data and assessed the risk of bias and study quality for each of the articles. Any disagreements were resolved by discussion and consensus, with the senior author (C.T.) serving as the final arbiter if consensus could not be reached.
Data Synthesis and Analysis
Our primary analysis was a mean difference in GLP-1 levels postRYGB from baseline preRYGB. Hedge’s g was used to report the pooled effect size. A pretest-posttest correlation coefficient of 0.5 was used. Traditional forest plots with two-sided 95% CIs were constructed.
Sensitivity analyses were performed. Subgroups were predefined and used to assess the influence of specific factors on the primary outcome. Factors included diabetic status preRYGB (present versus absent), method of postprandial GLP-1 measurement (peak versus area under the curve (AUC)/mean), and different levels of pretest-posttest correlation coefficient (0.1 versus 0.9).
Univariate meta-regression was performed to assess the influence of predefined clinical characteristics on the primary outcome. These included diabetic status preRYGB, length of Roux limb (cm), length of biliopancreatic limb (cm), amount of weight loss postRYGB (% total body weight), and timing of postRYGB GLP-1 measurement (months).
Heterogeneity was assessed for the individual meta-analyses using the χ 2 test and the I 2 statistic. Significant heterogeneity was defined as p < 0.05 using the χ 2 or I 2 > 50%. A random-effect model was used except for when statistical heterogeneity was not significant. Differences in subgroups were assessed using an χ 2 test for interaction with a p < 0.05 defined as statistically significant. To assess for publication bias, a funnel plot was created and visually inspected for asymmetry. The trim and fill method was used to correct for funnel plot asymmetry and provide an adjusted effect. The classic fail-safe test was also applied to assess risk of bias across studies. Analyses were performed using comprehensive meta-analysis version 3.0 (Englewood, NJ).
Results
Study Selection
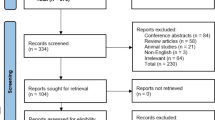
A total of 2680 studies were identified. After removal of duplicates, 1673 abstracts were reviewed. One thousand four hundred twenty-one studies were excluded leaving 252 articles for full manuscript review. After individual review of all manuscripts, 30 studies satisfied all criteria. One of the 30 studies, however, contained relevant discrepancy that could not be reconciled, and therefore was excluded. A total of 29 studies were included in the systematic review and meta-analysis (Fig. 1) [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37].
Study Characteristics and Risk of Bias
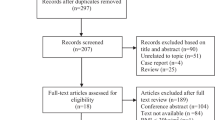
Details of the included studies are shown in Table 1. Twenty-five out of 29 studies were cohort studies. Four studies were randomized controlled trials (RCTs) with one of the study arms being an RYGB group. Only the RYGB arm of the RCTs was included in the analysis. Out of 29 studies, 14 reported postprandial GLP-1 levels only, while 10 reported both postprandial and fasting levels. Five studies only reported fasting GLP-1 levels. Only the 24 studies that reported postprandial GLP-1 levels before and after RYGB were included in the meta-analysis.
A total of 24 included studies yielded a total of 368 subjects. Mean age of the subjects included in the studies ranged from 36 to 52 years old. Mean BMI prior to RYGB ranged from 32.1 to 51.9 kg/m2. Median time of postRYGB GLP-1 measurement was 6.5 months (range 0.07 to 12).
All included studies were deemed to be of high quality and low risk of bias (Fig. 2). Risk of bias across studies was assessed using a funnel plot. Visual inspection demonstrated that smaller and statistically insignificant studies appeared to be missing likely due to publication bias (Fig. 3a). Duval and Tweedie’s trim and fill method resulted in a smaller increase in postprandial GLP-1 levels after RYGB. However, the increase in postprandial GLP-1 levels after RYGB remained significant (Fig. 3b). Specifically, Hedge’s g decreased from 1.29 [1.15, 1.43] to 1.17 [1.04, 1.30]. The classic fail-safe method yielded 2124 studies that would be required to show no difference in preRYGB and postRYGB postprandial GLP-1 levels.
a Funnel plot of changes in postprandial GLP-1 levels after RYGB. b Funnel plot with Duval and Tweedie’s trim and fill method. A white circle represents a study, while a black circle represents an unpublished study. Visual inspection demonstrates a publication bias with small, non-significant studies missing. With the Duval and Tweedie’s trim and fill method, the Hedge’s g of postprandial GLP-1 levels decreases from 1.29 [1.15, 1.43] to 1.17 [1.04, 1.30], although overall, the Hedge’s g remains positive suggesting a significant increase in postprandial GLP-1 levels after RYGB
Primary Outcome
All of the 24 studies that reported postprandial results demonstrated an increase in postprandial GLP-1 levels after RYGB with Hedge’s g of 1.29 [1.15, 1.43] (p < 0.0001) (Fig. 4). Heterogeneity across studies was low with I 2 of 32.27 (p < 0.067) and Q-value of 33.96.
A sensitivity analysis showed that postprandial GLP-1 levels after RYGB were significantly higher than the preRYGB levels regardless of diabetic status prior to RYGB. Specifically, Hedge’s g’s were 1.76 [1.36, 2.17], 1.39 [1.17, 1.61], and 1.25 [0.99, 1.49] in the non-diabetic, mixed, and diabetic groups, respectively (p = 0.106).
Similarly, the primary outcome remained the same regardless of how postprandial GLP-1 levels were reported (peak versus mean versus AUC) (p = 0.162). However, the heterogeneity across studies was the lowest when peak postprandial levels were used (I 2 of 9.11 versus 48.95 for peak versus AUC levels, respectively). In studies that assessed both peak and AUC postprandial GLP-1 levels, peak levels occurred at a median of 30 (range 20–60) min after glucose or meal stimulation. When the pre-post correlation coefficient was changed from 0.5 to 0.1 and 0.9, postRYGB GLP-1 levels remained higher than the preRYGB levels (Hedge’s g = 1.66 [1.42, 1.89] (p < 0.0001) and Hedge’s g = 0.82 [0.71, 0.93] (p < 0.0001), respectively).
Secondary Outcomes
Fifteen out of 24 studies reported pre and postRYGB fasting glucose and insulin levels. Fasting glucose levels decreased after RYGB (Hedge’s g = − 0.85 [− 0.98, − 0.71] (p < 0.0001)). Heterogeneity across studies was low with I 2 of 22.68 (p = 0.20) and Q-value of 18.11. Similarly, fasting insulin levels were lower after RYGB compared to the preRYGB levels (Hedge’s g = − 0.97 [−1.31, − 0.63] (p < 0.0001)). Heterogeneity across studies was however high with I 2 of 78.54 (p < 0.0001) and Q-value of 65.22.
Analysis of the 15 studies that reported fasting GLP-1 showed that there was no significant change in fasting GLP-1 levels before and after RYGB (Fig. 5). Specifically, Hedge’s g was 0.10 [− 0.06, 0.27] (p = 0.23). Heterogeneity across studies was low with I 2 of 46.16 (p = 0.03) and Q-value of 26.00.
Meta-Regression Analysis
Meta-regression analysis showed that length of Roux limb was a significant predictor of the amount of change in postprandial GLP-1 levels after RYGB (β = − 0.01, R 2 = 0.69, p = 0.02) (Table 2). Diabetes status prior to RYGB, the amount of weight loss, the length of biliopancreatic limb, and time from RYGB were not significant predictors of the amount of GLP-1 increase after RYGB (p > 0.05 in all) (Table 2).
Discussion
This systematic review and meta-analysis demonstrate that postprandial GLP-1 levels increase after RYGB, while fasting levels do not change. In addition, measurement of peak postprandial GLP-1 levels, which occurs at 30 min after meal or glucose stimulation, appears to give the most consistent results and should be used as a standard measurement. Meta-regression analysis shows that shorter Roux limb length appears to be associated with higher postprandial GLP-1 levels, which may lead to better glycemic control. Diabetic status prior to gastric bypass, amount of weight loss, length of biliopancreatic limb, and time from RYGB are not associated with increased GLP-1 levels after RYGB. These results suggest that bariatric surgical techniques may be individualized by adjusting Roux limb length based on diabetic status and monitored using postprandial GLP-1 to achieve optimal glycemic results.
GLP-1 is a gut hormone that is secreted by L-cells in the distal ileum and colon. It belongs to a group of hormones called incretins, which play an important role in glycemic control [38]. Also known as the incretin effect, oral glucose administration stimulates incretin secretion, which leads to greater insulin release from the pancreas compared to an equal amount of glucose administered intravenously. The postprandial elevation in GLP-1, along with the decrease in fasting insulin and glucose levels, demonstrated in this meta-analysis highlights the importance of postprandial GLP-1 amplification as a mechanism of T2DM treatment following RYGB.
To date, the exact mechanism by which GLP-1 levels increase after RYGB remains unclear. In the hindgut hypothesis, secretions and nutrients reach the distal ileum and colon more quickly following gastric bypass, which may lead to more stimulation of L-cells and therefore a higher level of GLP-1 [39]. Alternatively, the foregut hypothesis proposes that an exclusion of the duodenum and proximal jejunum after RYGB may prevent the secretion of a yet unidentified inhibitory product (i.e., anti-incretin factor) [40]. Therefore, a higher level of incretin is observed as a result of less inhibition by the proximal small bowel. More recently, it has also been observed that RYGB alters both the levels and composition of bile acids, which may play a key role in glucose metabolism. Specifically, RYGB bypasses the foregut and therefore increase digestate-free bile acids delivered to the distal gut. This leaves more bile acids free for ileal reuptake, which leads to increased serum bile acids after gastric bypass. Increased free ileal bile acids activate TGR-5 and FXR leading to increased GLP-1 production and equilibration of glucose homeostasis [41, 42]. Additionally, a higher conjugated to unconjugated bile acid ratio has been observed after RYGB in some studies, which are thought to directly induce more GLP-1 secretion [43, 44].
In our study, the length of Roux limb is a significant negative predictor of the amount of GLP-1 increase after RYGB. This finding suggests that RYGB patients with a shorter Roux limb may have a better glycemic control compared to those with a longer Roux limb (as supported by a higher increase in GLP-1 levels in the former group). While there are some studies assessing the effect of limb lengths on the amount of weight loss (most of which showed varied results) [45,46,47,48], there have been only a few small studies that evaluated the effect of limb lengths on T2DM remission [49, 50]. Gupta et al. showed that a shorter common channel limb (< 600 cm) appeared to be associated with a higher chance of T2DM remission. In contrary, Kaska et al. suggested that a longer biliopancreatic limb (> 100 cm) improves diabetic effect of RYGB. As far as we know, no studies have previously reported an association between the Roux limb length and glycemic effect after gastric bypass. These results may be due to small sample size.
Possible explanations for the beneficial effects of a shorter Roux limb on glycemic control include changes in bile acid metabolisms and intestinal transit time. By adjusting the length of Roux limb, the location of where bile acids (which travel down the biliopancreatic limb) meet the nutrients (which travel down the Roux limb) is affected. This may lead to changes in bile acid metabolism and an increase in GLP-1 secretion. Furthermore, different Roux limb lengths may have varied intestinal transit time.
In previous rat models, it has been demonstrated that a shorter Roux limb is associated with faster transit time [51, 52]. Therefore, RYGB patients with a shorter Roux limb may have a shorter bowel transit time than those with a longer Roux limb, which likely cause the nutrients to reach the ileum sooner and therefore a higher spike in GLP-1. Further studies on the effect of Roux limb length on GLP-1 levels and glycemic control after RYGB are warranted.
This study is the first systematic review and meta-analysis that explores the change in GLP-1 levels after RYGB. In addition to showing that postprandial GLP-1 levels increase after RYGB, the study also demonstrates that fasting glucose and fasting insulin levels decrease, likely as a result of improved insulin sensitivity. The meta-analysis shows that fasting GLP-1 levels do not change after gastric bypass. A likely explanation for this is that RYGB affects the rate that nutrients and bile acids reach L-cells in the distal ileum and colon rather than affecting L-cells directly, only changes in postprandial and not fasting basal GLP-1 levels are observed. In addition, our study also reveals that method of GLP-1 measurement remains relatively varied from one study to another. Specifically, to measure postprandial GLP-1, patients may be fed with a standardized meal or oral glucose administration. The GLP-1 levels may be measured using a peak level, a mean level, or an area under the curve (AUC) over different time periods (ranging from 90 to 180 min in the included studies). Nevertheless, regardless of the methods of stimulation or measurement, postRYGB GLP-1 levels remain significantly higher compared to preRYGB levels. In our analysis, measurement of peak GLP-1 levels postprandially appears to give the most consistent results as supported by the smallest heterogeneity score (I 2 and Q-value). Based on the studies that measured both peak and AUC postprandial levels, the peak levels occur at a median of 30 min after glucose or meal stimulation. Therefore, moving forward, standardizing GLP-1 measurement using a peak level at 30 min may be considered instead of an AUC level, which requires blood draw every 15 to 30 min for at least 1.5 to 3 h.
This study has a few limitations. First, the included studies remain quite heterogenous. This is likely explained by different GLP-1 assays used across studies. To control for this heterogeneity, Hedge’s g and random-effect models are therefore used to report the pooled effect. Additionally, our analysis only includes the studies with high quality and low risk of bias. The criteria used to assess the quality and risk of bias are relatively objective with an Ottawa score of ≥ 6 or a JADAD score of ≥ 3 and low risks of bias in more than 50% of the Cochrane criteria. Additionally, not all of the included studies report diabetes status prior to RYGB, amount of weight loss, length of Roux limb, or biliopancreatic limb of the included subjects. Therefore, this may limit our power at detecting the effect of these other factors on change in GLP-1 after gastric bypass. In our study, a shorter Roux limb length is associated with a greater increase in postprandial GLP-1, which may lead to better glycemic control in this population. Further prospective studies to confirm this finding should be considered before any specific recommendation on the optimal length of the Roux limb can be made for patients with concomitant T2DM and obesity.
Conclusion
This systematic review and meta-analysis demonstrate that RYGB is associated with an increase in postprandial GLP-1 level. This change appears to parallel a decrease in fasting insulin and fasting glucose levels. Additionally, the study also reveals that measuring postprandial GLP-1 using a peak level appears to result in a more consistent result than an average or AUC level, and that a shorter Roux limb length may predict a greater increase in postprandial GLP-1. Moving forward, postprandial GLP-1 levels may serve several useful purposes. They may be useful in assessing patient response to therapy, may serve as a guide to modifying or adding pharmacologic therapy, and may help in the development of new procedures. To date, bariatric procedures have been designed primarily to treat obesity, with T2DM efficacy being a fortunate secondary effect. We now have an opportunity to fine-tune existing surgeries and develop new devices to emphasize T2DM treatment outcomes.
Abbreviations
- RYGB:
-
Roux-En-Y Gastric Bypass
- GLP-1:
-
Glucagon-Like Peptide-1
- BMI:
-
Body Mass Index
- NAFLD:
-
Non-alcoholic Fatty Liver Disease
- US:
-
United States
- SOS:
-
Swedish Obese Subjects
- T2DM:
-
Type 2 Diabetes Mellitus
- STAMPEDE:
-
Surgical Therapy and Medications Potentially Eradicate Diabetes Efficiently
- AUC:
-
Area Under The Curve
- OGTT:
-
Oral Glucose Tolerance Test
- ROBINS-I:
-
Risk Of Bias In Non-Randomized Studies—Of Interventions
- NOS:
-
Newcastle-Ottawa Quality Assessment Scale
- CI:
-
Confidence Interval
- RCT:
-
Randomized Controlled Trial
- FXR:
-
Farnesoid X Receptor
References
WHO | Obesity and overweight. WHO n.d. http://www.who.int/mediacentre/factsheets/fs311/en/ Accessed 16 Aug 2016.
Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015:1–8.
Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–30. https://doi.org/10.1016/j.jhealeco.2011.10.003.
Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am. 1967;47:1345–51.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. https://doi.org/10.1056/NEJMoa066254.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370:2002–13. https://doi.org/10.1056/NEJMoa1401329.
Mason EE. The mechanisms of surgical treatment of type 2 diabetes. Obes Surg. 2005;15:459–61. https://doi.org/10.1381/0960892053723330.
Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. https://doi.org/10.1016/j.peptides.2015.08.013.
Anderwald C-H, Tura A, Promintzer-Schifferl M, et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care. 2012;35:2580–7. https://doi.org/10.2337/dc12-0197.
Borg C, le Roux C, Ghatei M, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–5. https://doi.org/10.1002/bjs.5227.
Bose M, Teixeira J, Olivan B, et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2010;2:47–55. https://doi.org/10.1111/j.1753-0407.2009.00064.x.
Breitman I, Saraf N, Kakade M, et al. The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass. J Am Coll Surg. 2011;212:617–25. https://doi.org/10.1016/j.jamcollsurg.2010.12.040.
Bryant EJ, King NA, Falken Y, et al. Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery. Surg Obes Relat Dis. 2013;9:802–8. https://doi.org/10.1016/j.soard.2012.09.011.
Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–22. https://doi.org/10.1007/s11605-009-1060-y.
Chronaiou A, Tsoli M, Kehagias I, et al. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg. 2012;22:1761–70. https://doi.org/10.1007/s11695-012-0738-5.
Evans S, Pamuklar Z, Rosko J, et al. Gastric bypass surgery restores meal stimulation of the anorexigenic gut hormones glucagon-like peptide-1 and peptide YY independently of caloric restriction. Surg Endosc Interv Tech. 2012;26:1086–94. https://doi.org/10.1007/s00464-011-2004-7.
Falken Y, Hellstrom PM, Holst JJ, et al. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–35. https://doi.org/10.1210/jc.2010-2876.
Fellici AC, Lambert G, Lima MMO, et al. Surgical treatment of type 2 diabetes in subjects with mild obesity: mechanisms underlying metabolic improvements. Obes Surg. 2015;25:36–44. https://doi.org/10.1007/s11695-014-1377-9.
Gandolfini M-P, Coupaye M, Bouaziz E, et al. Cardiovascular changes after gastric bypass surgery: involvement of increased secretions of glucagon-like peptide-1 and brain natriuretic peptide. Obes Surg. 2015;25:1933–9. https://doi.org/10.1007/s11695-015-1643-5.
Hansen EN, Tamboli RA, Isbell JM, et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol-Gastrointest LIVER Physiol. 2011;300:G795–802. https://doi.org/10.1152/ajpgi.00019.2011.
Jorgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol-Endocrinol Metab. 2012;303:E122–31. https://doi.org/10.1152/ajpendo.00073.2012.
Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33:786–95. https://doi.org/10.1038/ijo.2009.79.
Lips MA, de Groot GH, van Klinken JB, et al. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol. 2014;80:834–42. https://doi.org/10.1111/cen.12254.
Morinigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–601. https://doi.org/10.1381/096089206779319338.
Nosso G, Griffo E, Cotugno M, et al. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48:312–7. https://doi.org/10.1055/s-0041-111505.
O’Brien CS, Wang G, McGinty J, et al. Effects of gastrogastric fistula repair on weight loss and gut hormone levels. Obes Surg. 2013;23:1294–301. https://doi.org/10.1007/s11695-013-0917-z.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740–8. https://doi.org/10.1007/s11695-012-0622-3.
Promintzer-Schifferl M, Prager G, Anderwald C, et al. Effects of gastric bypass surgery on insulin resistance and insulin secretion in nondiabetic obese patients. Obesity. 2011;19:1420–6. https://doi.org/10.1038/oby.2011.92.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42. https://doi.org/10.1097/01.sla.0000133117.12646.48.
Salinari S, Bertuzzi A, Guidone C, et al. Insulin sensitivity and secretion changes after gastric bypass in normotolerant and diabetic obese subjects. Ann Surg. 2013;257:462–8. https://doi.org/10.1097/SLA.0b013e318269cf5c.
Samat A, Malin SK, Huang H, et al. Ghrelin suppression is associated with weight loss and insulin action following gastric bypass surgery at 12 months in obese adults with type 2 diabetes. Diabetes Obes Metab. 2013;15:963–6. https://doi.org/10.1111/dom.12118.
Steven S, Hollingsworth KG, Small PK, et al. Weight loss decreases excess pancreatic triacylglycerol specifically in type 2 diabetes. Diabetes Care. 2016;39:158–65. https://doi.org/10.2337/dc15-0750.
Umeda LM, Silva EA, Carneiro G, et al. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21:896–901. https://doi.org/10.1007/s11695-011-0412-3.
Yan W, Polidori D, Yieh L, et al. Effects of meal size on the release of GLP-1 and PYY after Roux-en-Y gastric bypass surgery in obese subjects with or without type 2 diabetes. Obes Surg. 2014;24:1969–74. https://doi.org/10.1007/s11695-014-1316-9.
Yip S, Signal M, Smith G, et al. Lower glycemic fluctuations early after bariatric surgery partially explained by caloric restriction. Obes Surg. 2014;24:62–70. https://doi.org/10.1007/s11695-013-1043-7.
Yousseif A, Emmanuel J, Karra E, et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24:241–52. https://doi.org/10.1007/s11695-013-1066-0.
Zhang X, Cheng Z, Xiao Z, et al. Comparison of short- and mid-term efficacy and the mechanisms of gastric bypass surgeries on managing obese and nonobese type 2 diabetes mellitus: a prospective study. Arch Med Res. 2015;46:303–9. https://doi.org/10.1016/j.arcmed.2015.06.003.
Laferrere B. Effect of gastric bypass surgery on the incretins. Diabetes Metab. 2009;35:513–7.
Nausheen S, Shah IH, Pezeshki A, et al. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2013;305:E507–18. https://doi.org/10.1152/ajpendo.00130.2013.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9. https://doi.org/10.1097/01.sla.0000224726.61448.1b.
Penney NC, Kinross J, Newton RC, et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes. 2015;39:1565–74. https://doi.org/10.1038/ijo.2015.115.
Kohli R, Bradley D, Setchell KD, et al. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–12. https://doi.org/10.1210/jc.2012-3736.
Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes. 2013;37:1553–9. https://doi.org/10.1038/ijo.2013.38.
Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–64. https://doi.org/10.2337/dc12-2255.
Gleysteen JJ. Five-year outcome with gastric bypass: Roux limb length makes a difference. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2009;5:242–247; discussion 247-249. https://doi.org/10.1016/j.soard.2008.08.005.
Stefanidis D, Kuwada TS, Gersin KS. The importance of the length of the limbs for gastric bypass patients—an evidence-based review. Obes Surg. 2011;21:119–24. https://doi.org/10.1007/s11695-010-0239-3.
Orci L, Chilcott M, Huber O. Short versus long Roux-limb length in Roux-en-Y gastric bypass surgery for the treatment of morbid and super obesity: a systematic review of the literature. Obes Surg. 2011;21:797–804. https://doi.org/10.1007/s11695-011-0409-y.
Dogan K, Homan J, Aarts EO, et al. A short or a long Roux limb in gastric bypass surgery: does it matter? Surg Endosc. 2016; https://doi.org/10.1007/s00464-016-5188-z.
Kaska L, Kobiela J, Proczko M, et al. Does the length of the biliary limb influence medium-term laboratory remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass in morbidly obese patients? Wideochirurgia Inne Tech Małoinwazyjne Videosurgery Miniinvasive Tech Kwart Pod Patronatem Sekc Wideochirurgii TChP Oraz Sekc Chir Bariatrycznej TChP. 2014;9:31–9. https://doi.org/10.5114/wiitm.2014.40383.
Gupta RVN, Chamany T, Makam R. Does length of common limb influence remission of diabetes? Short-term results. J Minimal Access Surg. 2016;12:54–7. https://doi.org/10.4103/0972-9941.152104.
Dutra RA, Araújo WM, de Andrade JI. The effects of Roux-en-Y limb length on gastric emptying and enterogastric reflux in rats. Acta Cirúrgica Bras Soc Bras Para Desenvolv Pesqui Em Cir. 2008;23:179–83.
Le Blanc-Louvry I, Ducrotté P, Lemeland JF, et al. Motility in the Roux-Y limb after distal gastrectomy: relation to the length of the limb and the afferent duodenojejunal segment—an experimental study. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 1999;11:365–74.
Funding
No financial or material support was received for this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent Statement
Does not apply.
Rights and permissions
About this article
Cite this article
Jirapinyo, P., Jin, D.X., Qazi, T. et al. A Meta-Analysis of GLP-1 After Roux-En-Y Gastric Bypass: Impact of Surgical Technique and Measurement Strategy. OBES SURG 28, 615–626 (2018). https://doi.org/10.1007/s11695-017-2913-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2913-1