Abstract
The evidence is strong that bariatric surgery is superior to medical treatment in terms of weight loss and comorbidities in patients with severe obesity. However, a considerable part of patients presents with unsatisfactory response in the long term. It remains unclear whether postoperative administration of glucagon-like peptide-1 analogues can promote additional benefits. Therefore, a systematic review of the current literature on the management of postoperative GLP-1 analogue usage after metabolic surgery was performed. From 4663 identified articles, 6 met the inclusion criteria, but only one was a randomized controlled trial. The papers reviewed revealed that GLP-1 analogues may have beneficial effects on additional weight loss and T2D remission postoperatively. Thus, the use of GLP-1 analogues in addition to surgery promises good results concerning weight loss and improvements of comorbidities and can be used in patients with unsatisfactory results after bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical Treatment of Obesity
Bariatric surgery is gaining popularity worldwide, and the total number of procedures continues to rise. The most common procedures are the laparoscopic Roux-en-Y gastric bypass (LRYGB) and the laparoscopic sleeve gastrectomy (LSG). Additionally, less common techniques are available as bariatric options, such as the biliopancreatic diversion with or without duodenal switch (BPD/BPD-DS), the adjustable gastric band (AGB), one-anastomosis gastric bypass (OAGB), and single anastomosis duodeno-ileal bypass (SADI). LRYGB provides excellent evidence-based results in terms of weight loss and a decrease in obesity-related comorbidities in the long term [1, 2]. LSG represents an alternative to LRYGB and provides comparable results concerning weight loss and comorbidities [3]. Revised guidelines from the Diabetes Surgery Summit (2015) recommend consideration of surgical treatment for patients suffering from type 2 diabetes (T2D) with a body mass index (BMI) of > 35 kg/m2. Additionally, the evaluation of patients with a BMI < 35 kg/m2 with insufficiently controlled blood sugar levels was suggested to be implemented [4, 5].
Pharmacological Alternatives for the Treatment of Obesity
Pharmaceutical methods for weight loss in the population with obesity represent an alternative to surgery [6,7,8]. Besides the long-term effects of bariatric surgery, changes in glucose metabolism and gut hormones can be measured immediately in the first postoperative days [9]. For example, the gut-derived glucagon-like peptide-1 (GLP-1) secreted by L-cells located primarily in the distal ileum and colon but also in the jejunum and duodenum [10] increases after LRYGB [9]. GLP-1 secretion is stimulated by intraluminal carbohydrates, proteins, and fat [11, 12]. In the pancreatic islets, GLP-1 acts as a glucose-dependent stimulator of insulin secretion, slows gastric emptying while increasing satiety, and reduces postprandial glucagon and food intake [13,14,15]. These functions suggest that drug-induced GLP-1 stimulation might be efficient in weight control and metabolic changes (Fig. 1). Consequently, a GLP-1 analogue named Liraglutide achieved almost 8.4 kg of weight loss and improved glycemic control compared with placebo in randomized controlled trials [6]. Additionally, GLP-1 analogues improved glucose homeostasis in patients already treated with insulin [30]. Nevertheless, bariatric surgery still provides better results concerning weight evolution and comorbidities compared with medical treatment [31].
Mechanisms by which GLP-1 analogues may have beneficial effects after metabolic surgery: GIT [16, 17], brain [14, 18, 19], cardiovascular system [20, 21], kidneys [22], skeletal muscle [23], adipose tissue [24, 25], liver [26, 27], and endocrine pancreas [15, 28, 29]. GLP-1, glucagon-like peptide-1; GIT, gastrointestinal tract
GLP-1 and Metabolic Surgery
Former literature showed that fasting levels of GLP-1 increase after LRYGB [9]. Nonetheless in comparative trials, GLP-1 analogues did not achieve the same satisfying results as in the surgical treated groups [32, 33]. Besides the single use of GLP-1 analogues in patients with impaired glucose metabolism or overweight, a combination of GLP-1 analogues with metabolic surgery might offer improved outcomes in patients fitting the criteria for a surgical approach. Due to the growing demand of more efficient surgery and management of secondary weight regain or insufficient weight loss in the long term [34, 35], therapeutic adjunctions to surgery may continue to emerge.
What We Do Not Know
Instead of looking at GLP-1 agonists and metabolic surgery as two concurrent treatments, these two treatment modalities could be seen as two main pillars of one interdisciplinary approach. However, whether the combination of metabolic surgery with postoperative use of GLP1 analogues promotes additional health benefits needs to be further investigated. Those considerations prompted us to perform a review combined with a systematic literature research of possible beneficial effects of GLP-1 analogues, authorized in Switzerland, as an addition after bariatric surgery.
Materials and Methods
Literature Search and Study Selection
A systematic literature research was performed without consideration of date or language on the following MEDLINE search terms according to PRISMA guidelines [36, 37]:
“metabolic surgery[Title/Abstract]) OR bariatric surgery[Title/Abstract]) OR gastric bypass[Title/Abstract]) OR sleeve gastrectomy[Title/Abstract]) OR BPD[Title/Abstract]) OR biliopancreatic diversion[Title/Abstract]) OR bilio-pancreatic diversion[Title/Abstract]) OR vertical banded gastroplasty[Title/Abstract]) OR vertical-banded gastroplasty[Title/Abstract]) OR one anastomosis gastric bypass[Title/Abstract]) OR mini gastric bypass[Title/Abstract]) AND GLP-1 analogues[Title/Abstract]) OR GLP-1 agonists[Title/Abstract]) OR glucagon-like peptide-1 analogues[Title/Abstract]) OR glucagon-like peptide-1 agonists[Title/Abstract]) OR liraglutide [Title/Abstract]) OR lixisenatide[Title/Abstract]) OR dulaglutide[Title/Abstract]) OR exenatide[Title/Abstract]) OR semaglutide[Title/Abstract]”
Citations of relevant articles were screened as well.
Inclusion Criteria
The titles and, if available, abstracts of all retrieved articles were screened. To be included in the review, studies had to have a main group or subgroup of human individuals undergoing bariatric/metabolic surgery. Additionally, at least one group or subgroups had to have a treatment with a GLP-1 analogue after previous bariatric/metabolic surgery. Studies needed to include follow-up information on at least weight evolution. Written works containing only abstracts without further documents were excluded.
The screening for the above-mentioned inclusion criteria was carried out by two experienced researchers (RS and MK) and double-checked by a third researcher when uncertainties occurred (TD).
Data Extraction and Outcomes
Full text articles of the studies that fulfilled the search criteria were retrieved. Data on the following information was extracted: type of operation, type of drug used, number of patients, age, weight loss, comorbidities (if available, T2D [glycated hemoglobin A1C], hypertension, and dyslipidemia), and information on drug side effects and follow-up.
Assessment of Quality
Methodological quality assessment of each study was performed using a checklist of randomized and non-randomized studies of health care interventions [38].
Results
Selection of Eligible Studies and Patient Characteristics
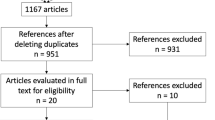
The literature search revealed 4663 potentially suitable publications (Fig. 2). After screening title and abstracts, 4636 studies were excluded and 27 assessed for further eligibility. Of these 27 assessed studies, 1 case report was excluded, 5 studies were excluded because of missing qualitative or quantitative data of interest, 5 studies were excluded because they examined the use of GLP-1 analogues prior to metabolic surgery, and 10 studies were excluded due to other reasons. Consequently, 6 studies were included for further analysis. Due to inhomogeneity of the retrieved data (different operation techniques, different time points of GLP-1 analogue administration, multiple indications) and overall the small numbers of studies, an evaluation in form of a meta-analysis as initially planned was omitted.
Studies, Patients, Type of Surgery, and GLP-1 Analogue Used in Collective
Of the 6 studies included, one was a randomized placebo controlled trial [39], one a prospective cohort trial [40], and 4 retrospective cohort studies [41,42,43,44]. Studies were performed between 2011 and 2019 and carried out in Spain, the UK, Brazil, Canada, and the United Arab Emirates. In total, 408 patients were included undergoing metabolic surgery and postoperative treatment with GLP-1 analogue. The performed surgeries included LRYGB, LSG, biliopancreatic diversion (BPD), vertical banded gastroplasty (VBG), and adjustable gastric banding (AGB). No statements can be made about actual exact numbers of each operation technique due to partially incomplete information. In all studies, the GLP-1 analogue Liraglutide was used as complementary treatment. A summary of the available baseline characteristics, surgical techniques, and drugs is presented in Table 1.
Weight Loss in Patients Undergoing Bariatric Surgery and GLP-1 Analogue Treatment
All studies reported on weight loss after different time points [39,40,41,42,43,44]. The use of Liraglutide achieved an additional weight loss in all studies included. In the paper of Gorgojo et al., 15 patients undergoing either RYGB, BPD, VBG, LSG, or AGB regained 10.1% ± 8.2% of their weight loss nadir before beginning the medical treatment with Liraglutide (mean time metabolic surgery to Liraglutide 5.2 years). In the study, the mean weight of 106.0 ± 7.2 kg could be reduced to 102.6 ± 6.9 kg by using 1.6 ± 0.2 mg of Liraglutide postoperatively [41]. In a placebo RCT, Miras et al. showed that patients with prior LRYGB or LSG with primary excellent weight loss (126.8 ± 25.1 kg to 85.3 ± 16.2 kg in LRYGB and 132.1 ± 29 kg to 104.3 ± 21.7 kg in LSG) regained weight (12.0 ± 9.4 kg after LRYGB and 9.3 ± 5.7 kg after LSG, respectively). However, 1.8 mg Liraglutide induced greater weight loss compared with the placebo at two different time points (− 3.7 kg, − 4.6 to − 2.8; p = < 0.0001 in week 10 and − 5.3 kg, − 6.2 to − 4.4; p = < 0.0001 in week 26) [39]. Another paper demonstrated that patients with 16.7 ± 16.2 kg weight regain after LRYGB, LSG, VBG, or AGB achieved additional 7.1% TWL (interquartile range [IQR] 5.1–12.2%) at 16 weeks and 9.7% TWL (IQR 7.8–13.9%) at 28 weeks after implementation of 2.9 ± 0.2 mg of postoperative Liraglutide [43]. Suliman et al. demonstrated 6.1% (3.1–8.7%) additional total weight loss (TWL%) in a cohort of mainly LRYGB and LSG patients adding 3 mg of Liraglutide. In another collective of LRYGB, LSG, and AGB patients, a significant additional weight loss was reached with the use of 3.0 mg Liraglutide (6.6 ± 7.1 TWL% after LRYGB, 3.6 ± 3.0 TWL% after LSG, and 4.9 ± 5.6 TWL% after AGB) [44]. The time points of weight loss displayed range from 6 to approximatively 35 weeks. Details on weight evolution can be found in Table 2.
T2D, Hypertension, and Dyslipidemia in Patients After Bariatric Surgery and GLP-1 Analogues Treatment
Two contributions contained detailed information about obesity-related comorbidities. The study of Gorgojo et al. reported that the amount of patients with a A1C of less than 7% was increased from 66.7 to 81.8% after 1.6 ± 0.2 mg Liraglutide [41], In the randomized controlled trial, the multivariable linear regression demonstrated a decrease of A1C [%] compared with placebo (R2 = − 1.22 [− 1.80 to − 0.64]; p = 0.0001). [39] However, both contributions did not observe differences in arterial hypertension or dyslipidemia.
Reporting of Side Effects Concerning GLP-1 Analogues in Patients Who Underwent Bariatric Surgery
Information about side effects of the used additive medication was found in 5 out of 6 papers. Most commonly reported side effects were gastrointestinal symptoms like nausea (9–37%), constipation (11–36%), and diarrhea (2–31%) [39, 43, 44]. Rye et al. also showed a high prevalence of gastroesophageal reflux (GERD) in 35% of the patients treated with Liraglutide. Other side effects, reported in small numbers, included fatigue, headache, dizziness, and other general symptoms.
Discussion
This systematic review may show beneficial effect of the postoperative addition of the GLP-1 analogue Liraglutide after metabolic surgery. Although most data had relatively short follow-up and did not allow a quantitative synthesis in form of a meta-analysis nor a statement regarding macrovascular outcomes, the present assessment demonstrates promising results for additional weight loss and improvement of T2D.
Since the implementation of bariatric and metabolic surgery, the trend towards safer and more efficient procedures is essential to the field. The surgical treatment of obesity has been a milestone not only in treating overweight itself but also in treating obesity-related comorbidities. Nevertheless, poor primary response or relapse of obesity and related comorbidities is not uncommon, which is usually addressed by additional nutrition counseling or revisional surgery in highly selected cases. For instance, the present literature shows a relapse of cured diabetes in 35–50% after a median disease-free period of approximatively 8 years [5, 45]. Behavioral and pharmacotherapeutic interventions to address postsurgical weight regain and maintenance of reduced comorbidities are subjects for further investigations [46]. A patient with weight regain, insufficient weight loss, or relapse of comorbidities after surgery needs to be evaluated by an interdisciplinary team. Besides dietary counseling and revisional surgery (e.g., banding or distalization of RYGB, re-sleeve, conversion to RYGB, BPD-DS, or SADI), new medical agents might offer an alternative that has not been considered so far.
Literature search showed an additional weight loss in all studies included; however, different parameters were used for weight loss measurement which may impede the interpretation of the findings. Additionally, there is only one RCT included in the reviewed papers, which influence the quality of the evidence considerably. The lack of a longer follow-up in the present data allows only an assessment of the GLP-1 analogue effect up to maximally 2 years [39,40,41,42,43,44]. The choice of operation technique had no influence of the response on GLP-1 analogues; however, not all studies included the data on different procedures. Besides weight loss, reduction of obesity-related comorbidities might be achieved by the new medical treatment addition. For instance, Liraglutide seems to have beneficial effects on A1C after metabolic surgery [39, 41]. In contrast, hypertension and dyslipidemia were not affected by the additive medical treatment, although weight reduction should have a positive effect on these comorbidities as well. To allow us a statement on hypertension and dyslipidemia, and more importantly on macrovascular outcomes as a consequence of additional weight loss, more reliable data is needed. Since literature only provides data on the specific agent Liraglutide and metabolic surgery, the use of other GLP-1 analogues may be of interest for future investigations. Long-lasting GLP-1 analogues such as Liraglutide have no influence on gastric emptying [47]. Comparatively, short-term agents like lixisenatide or exenatide slow down gastric emptying significantly [48, 49]. Nevertheless, the long-lasting agents were superior concerning glucose metabolism in comparative trials [50] [Table 3].
Besides good results in weight loss and comorbidities, high prevalence of gastrointestinal side effects can be found in the corresponding study collectives [39, 43, 44]. In general, GLP-1 analogues showed several typical side effects on the gastrointestinal tract [62]. In contrast, the prevalence of unspecific or specific gastrointestinal symptoms after bariatric surgery is rather high [63]. Abdominal symptoms might be caused by early or late surgical complications [64]. After LRYGB, potential life-threatening internal hernias may cause unspecific gastrointestinal symptoms [65]. Concerning the emerging possible long-term complication of GERD and Barrett after LSG [66, 67], the slowed gastric emptying caused by GLP-1 could be responsible for a deterioration of symptomatic or asymptomatic acid reflux into the lower esophagus [68]. Another decisive question will be the cost and reimbursement of the corresponding medical agents. For instance, insurances in Switzerland do only take over the costs for GLP-1 in a few selected cases (at the moment BMI > 35 or > 28 with comorbidities).
Synoptically, obesity and its comorbidities are chronic diseases that despite an efficient treatment, such as metabolic surgery, can recur. As in any other chronic diseases involving various organ systems, a multidisciplinary treatment approach, similar to oncological patients, may offer the most beneficial outcome in the long-term. Despite the effectiveness of metabolic surgery in the vast majority of patients, poor long-term weight loss and relapse of comorbidities present a commonplace in the daily practice. Addition of promising medical treatments such as GLP-1 analogues may offer improved outcomes concerning weight loss plus resolution of comorbidities in patients with poor response to surgery alone in the future.
References
Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA - J Am Med Assoc [Internet]. 2014 Jun 11 [cited 2018 Oct 17];311(22):2297–304. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2014.5988
Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med [Internet]. 2017 Sep 21 [cited 2018 Oct 17];377(12):1143–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28930514
Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity. JAMA [Internet]. 2018 Jan 16 [cited 2018 Dec 3];319(3):255. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29340679
Cefalu WT, Rubino F, Cummings DE. Metabolic surgery for type 2 diabetes: changing the landscape of diabetes care. Diabetes Care. 2016;39(6):857–60.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Obes Surg [Internet]. 2017 Jan 12 [cited 2018 Dec 3];27(1):2–21. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-016-2457-9
Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med [Internet]. 2015 Jul 2 [cited 2018 Oct 17];373(1):11–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26132939
Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–16.
Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–61.
Falkén Y, Hellström PM, Holst JJ, et al. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96(7):2227–35.
Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Ross SA, Dupre J. Effects of ingestion of triglyceride or galactose on secretion of gastric inhibitory polypeptide and on responses to intravenous glucose in normal and diabetic subjects. Diabetes [Internet]. 1978 Mar [cited 2019 Dec 20];27(3):327–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/640238
Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol [Internet]. 2012 [cited 2019 Dec 20];209(209):309–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22249821
Vilsbøll T. On the role of the incretin hormones GIP and GLP-1 in the pathogenesis of type 2 diabetes mellitus. Dan Med Bull [Internet]. 2004 Nov [cited 2019 Dec 20];51(4):364–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16009062
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Hare KJ, Vilsbøll T, Asmar M, et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59(7):1765–70.
Meier | December ; J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Publ Gr [Internet]. 2012 [cited 2020 Apr 28];8:728–42. Available from: www.nature.com/nrendo
Muscogiuri G, DeFronzo RA, Gastaldelli A, et al. Glucagon-like peptide-1 and the central/peripheral nervous system: crosstalk in diabetes. Trends Endocrinol Metab. Elsevier Inc. 2017;28:88–103.
Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72.
Rüttimann EB, Arnold M, Hillebrand JJ, et al.. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology [Internet]. 2009 Mar 1 [cited 2020 Apr 29];150(3):1174–81. Available from: https://academic.oup.com/endo/article-lookup/doi/10.1210/en.2008-1221
Petrie JR. The cardiovascular safety of incretin-based therapies: a review of the evidence [Internet]. Cardiovasc Diabetol. 2013;12 [cited 2020 Apr 29]. Available from: http://www.cardiab.com/content/12/1/130
Boyle JG, Livingstone R, Petrie JR. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: s comparative review. Clin Sci [Internet]. 2018 [cited 2020 Apr 29];132(15):1699–709. https://doi.org/10.1042/CS20171299.
Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. Nat Publ Group. 2017;13:605–28.
Luque MA, González N, Márquez L, et al. Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human myocytes. J Endocrinol. 2002;173(3):465–73.
Ruiz-Grande C, Alarcón C, Mérida E, et al. Lipolytic action of glucagon-like peptides in isolated rat adipocytes. Peptides. 1992;13(1):13–6.
Villanueva-Peñcarrillo ML, Márquez L, González N, et al. Effect of GLP-1 on lipid metabolism in human adipocytes. Horm Metab Res. 2001;33(2):73–7.
Bifari F, Manfrini R, Dei Cas M, et al. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Pharmacol Res. Academic Press. 2018;137:219–29.
Jun LS, Millican RL, Hawkins ED, et al. Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes. 2015;64(3):819–27.
Zhou JY, Poudel A, Welchko R, et al. Liraglutide improves insulin sensitivity in high fat diet induced diabetic mice through multiple pathways. Eur J Pharmacol. 2019;15:861.
DeFronzo RA, Okerson T, Viswanathan P, et al.. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: A randomized, cross-over study. Curr Med Res Opin [Internet]. 2008 [cited 2020 Apr 29];24(10):2943–52. Available from: https://www.tandfonline.com/doi/full/10.1185/03007990802418851
Tack CJ, Jacob S, Desouza C, et al. Long-term efficacy and safety of combined insulin and glucagon-like peptide-1 therapy: evidence from the LEADER trial. Diabetes Obes Metab. 2019;21(11):2450–8.
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med [Internet]. 2017 Feb 16 [cited 2018 Oct 17];376(7):641–51. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1600869
Cotugno M, Nosso G, Saldalamacchia G, et al. Clinical efficacy of bariatric surgery versus liraglutide in patients with type 2 diabetes and severe obesity: a 12-month retrospective evaluation. Acta Diabetol [Internet]. 2014 Apr 14 [cited 2018 Oct 17];52(2):331–6. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00592-014-0644-5
Yong W, Shibo W, Jingang L. Remission of insulin resistance in type 2 diabetic patients after gastric bypass surgery or exenatide therapy. Obes Surg. 2012 Jul;22(7):1060–7.
Shimizu H, Annaberdyev S, Motamarry I, et al.. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes Surg [Internet]. 2013 Nov 5 [cited 2018 Jun 19];23(11):1766–73. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-013-1012-1
Qiu J, Lundberg PW, Javier Birriel T, et al.. Revisional bariatric surgery for weight regain and refractory complications in a single MBSAQIP accredited center: what are we dealing with? Obes Surg [Internet]. 2018 Apr 20 [cited 2018 May 29]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29679337
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Miras AD, Pérez-Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–59.
Suliman M, Buckley A, Al Tikriti A, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab. 2019;21(6):1498–501.
Gorgojo-Martínez JJ, Feo-Ortega G, Serrano-Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis [Internet]. 2016 Dec [cited 2018 Sep 3];12(10):1856–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27256860
Pajecki D, Halpern A, Cercato C, et al. Tratamento de curto prazo com liraglutide no reganho de peso após cirurgia bariátrica. Rev Col Bras Cir [Internet]. 2013 [cited 2018 Oct 17];40(3):191–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23912365
Rye P, Modi R, Cawsey S, et al.. Efficacy of high-fose liraglutide as an adjunct for weight loss in patients with prior bariatric surgery. Obes Surg [Internet]. 2018 Jul 19 [cited 2018 Sep 3];28(11):3553–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30022424
Wharton S, Kuk JL, Luszczynski M, et al. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9(4):1–6.
Yu J, Zhou X, Li L, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg. 2015;25(1):143–58.
Shukla AP, He D, Saunders KH, et al. Current concepts in management of weight regain following bariatric surgery. Expert Rev Endocrinol Metab [Internet]. 2018 Mar 4 [cited 2018 Aug 25];13(2):67–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30058859
Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and-and-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53
Werner U, Haschke G, Herling AW, et al. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes [Internet]. Vol. 164, Regulatory Peptides. 2010 [cited 2020 Apr 28]. p. 58–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20570597
Linnebjerg H, Park S, Kothare PA, et al. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008;151(1–3):123–9.
Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39–47.
Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26(8):2370–7.
Christensen M, Knop FK, Holst JJ, et al. Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs. 2009;12:503–13.
Matthews JE, Stewart MW, De Boever EH, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab [Internet]. 2008 Dec [cited 2020 Apr 28];93(12):4810–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18812476
Madsen K, Knudsen LB, Agersoe H, et al. Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem. 2007;50(24):6126–32.
Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type2diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36(10):2945–51.
Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–50.
Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–91.
Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med [Internet]. 2009 Mar [cited 2020 Apr 28];26(3):268–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19317822
Blevins T, Pullman J, Malloy J, et al. DURATION-5: Exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–10.
Umpierrez GE, Blevins T, Rosenstock J, et al. The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 2011;13(5):418–25.
DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2. Diabetes Care. 2005;28(5):1092–100.
Sharma D, Verma S, Vaidya S, et al. Recent updates on GLP-1 agonists: Current advancements & challenges. Biomed Pharmacother. Elsevier Masson SAS. 108, 2018:952–62.
Lewis KD, Takenaka KY, Luber SD. Acute abdominal pain in the bariatric surgery patient. Emerg Med Clin North Am. W.B. Saunders. 2016;34:387–407.
Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg. Elsevier Ltd. 2016;27:77–81.
Aghajani E, Nergaard BJ, Leifson BG, et al.. The mesenteric defects in laparoscopic Roux-en-Y gastric bypass: 5 years follow-up of non-closure versus closure using the stapler technique. Surg Endosc [Internet]. 2017 [cited 2019 Dec 23];31(9):3743–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28205037
Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–74.
Soricelli E, Casella G, Baglio G, et al. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surg Obes Relat Dis. 2018;14(6):751–6.
Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. Elsevier GmbH. 2019;30:72–130.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Schneider reports grants from the University of Basel, grants from Department of Surgery, University Hospital Basel, grants from SFCS, grants from Freiwillige Akademische Gesellschaft Basel, and grants from Gebauer Stiftung, outside the submitted work. Dr. Peterli reports grants and other from the Johnson & Johnson, outside the submitted work. Marko Kraljević, Theresa V. Rohm, Jennifer M. Klasen, Claudia Cavelti-Weder, and Tarik Delko have no conflicts of interest or financial ties to disclose. All authors have no ties to GLP-1 analogues producing pharmaceutical companies.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent does not apply.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schneider, R., Kraljević, M., Peterli, R. et al. GLP-1 Analogues as a Complementary Therapy in Patients after Metabolic Surgery: a Systematic Review and Qualitative Synthesis. OBES SURG 30, 3561–3569 (2020). https://doi.org/10.1007/s11695-020-04750-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04750-7