Abstract
Background
This study aims to assess the clinical and physiological effects of Roux-en-Y gastric bypass (RYGBP) on type 2 diabetes associated with mild obesity (body mass index [BMI] 30–34.9 kg/m2) over 24 months postsurgery.
Methods
In this prospective trial, 36 mildly obese subjects (19 males) with type 2 diabetes using oral antidiabetic drugs with (n = 24) or without insulin (n = 12) underwent RYGBP. Follow-up was conducted at baseline and 3, 6, 12, and 24 months postsurgery. The following endpoints were considered: changes in HbA1c, fasting glucose and insulin, antidiabetic therapy, BMI, oral glucose insulin sensitivity [OGIS, from meal tolerance test (MTT)], beta-cell secretory function [ΔCP(0–30)/ΔGlu(0–30) (ΔC-peptide/Δglucose ratio, MTT 0–30 min), disposition index (DI = OGIS ⋅ ΔCP(0–30)/ΔGlu(0–30)], glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) [incremental area under the curve (AUCi)], adiponectin, C-reactive protein, and lipids.
Results
All subjects achieved normal-to-overweight BMI after 3 months. Over 24 months, 31/36 (86 %) subjects presented HbA1c <7 % [complete and partial remission of diabetes in 9/36 (22 %) and 1/36 (3 %), respectively]. Since 3 months postsurgery, improvements were observed in OGIS [290 (174) to 373 (77) ml/min/m2, P = 0.009], ΔCP(0–30)/ΔGlu(0–30) [0.24 (0.19) to 0.52 (0.34) ng/mg, P = 0.001], DI [7.16 (8.53) to 19.8 (15.4) (ng/mg) (ml/min/m2), P = 0.001], GLP-1 AUCi [0.56 (0.64) to 3.97 (3.86) ng/dl ⋅ 10 min ⋅ 103, P = 0.000], and GIP AUCi [30.2 (12.6) to 27.0 (20.2) ng/dl ⋅ 10 min ⋅ 103, P = 0.004]. At baseline and after 12 months, subjects with diabetes nonremission had longer diabetes duration, higher HbA1c, lower beta-cell secretory function, and higher first 30-min GIP AUCi, compared with those with remission.
Conclusions
RYGBP improves the glucose metabolism in subjects with type 2 diabetes and mild obesity. This effect is associated with improvement of insulin sensitivity, beta-cell secretory function, and incretin secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bariatric surgery is more effective than medical treatment for obesity, with consistent weight loss and resolution of obesity-related comorbidities. It has been well established that bariatric surgery prevents type 2 diabetes incidence and promotes durable resolution/remission of diabetes in subjects with moderate (body mass index [BMI] >35 kg/m2) to severe (BMI >40 kg/m2) obesity [1]. Since the impact of bariatric surgery on diabetes is somewhat independent of weight loss [2], the surgical treatment of diabetes has been proposed for patients with mild obesity (BMI 30–35 kg/m2) or even for overweight patients [3].
The International Diabetes Federation (IDF) [4] considers bariatric surgery as one option for the treatment of type 2 diabetes in patients with IMC >35 kg/m2 and assumes that the surgical approach may also be appropriate as a nonprimary alternative to treat inadequately controlled type 2 diabetes in candidates suitable for surgery with mild obesity (BMI 30–35 kg/m2), although further trial evidence is required in this group. Recent systematic reviews [3, 5] on the effect of bariatric surgery on type 2 diabetes in patients with mild obesity evidenced an improvement of several metabolic parameters and found a diabetes remission rate of 66–80 % of the cases, which is similar to that seen in subjects with higher degrees of obesity.
The physiopathological mechanisms underlying the effects of bariatric surgery in type 2 diabetes are not fully understood. Short-term postoperative improvement in insulin sensitivity and metabolic syndrome components, especially glucose tolerance, is greater than expected for BMI change, presumably due to caloric restriction [6]. A further improvement in insulin sensitivity accompanies weight loss [7]. The improvement of β-cell residual function is seen early after surgery and improves over time, possibly due to the enhanced incretin effect and a reduction of glucotoxicity [8], and such recovery is amplified by the progressive increase in insulin sensitivity [9]. It has been hypothesized that bypassing the proximal small intestine could reduce the production of some yet unknown factor(s) from that intestinal segment that have inhibitory action on beta-cells [10].
Most of the current knowledge about these physiopathological mechanisms comes from patients with BMI >35 kg/m2, and it is conceivable that the role of each of these mechanisms may vary depending on the degree of obesity, among other factors. Previous studies on bariatric surgery in subjects with type 2 diabetes and mild obesity aimed at its clinical improvement [2, 11, 12], but few data related to its physiology in this specific population are available.
The aim of this study is to assess both the clinical and physiological effects of Roux-en-Y gastric bypass (RYGBP) on type 2 diabetes in subjects with mild obesity over 24 months postsurgery. We hypothesize that an improvement in type 2 diabetes in these subjects is associated with improvement in insulin sensitivity, beta-cell secretory function, and incretin secretion.
Subjects and Methods
Thirty-six subjects (19 males) with type 2 diabetes and grade I obesity (BMI 30–34.9 kg/m2) participated in a prospective trial, approved by the Ethics Review Board of the State University of Campinas, and all of them provided written informed consent before participation. Among them, 24 subjects were on insulin therapy plus oral antidiabetic drugs (OAD) and 12 were using only OAD. They underwent RYGBP between September 2007 and August 2009.
The inclusion criteria were age 18 to 60 years old, type 2 diabetes using insulin or OAD, HbA1c levels >7 %, and grade I obesity. The exclusion criteria were as follows: positive anti-GAD autoantibodies; undetectable beta-cell function, defined by C-peptide levels <1 ng/ml; insulin therapy duration >10 years; hepatic dysfunction (transaminases >2.5 times the upper limit of normal); renal failure (creatinine >1.4 mg/dl); history of neoplasia in the last 5 years; systemic corticotherapy for more than 14 days in the last 3 months; and unwillingness or inability to give informed consent.
All participants were evaluated before surgery (baseline) and 3, 6, 12, and 24 months postsurgery (100 % follow-up rate for all time points). From baseline through 12 months postsurgery, the subjects were admitted at 7:00 a.m. after a 12-h overnight fasting for a complete medical history, physical examination, assessment of anthropometrics, a standard meal tolerance test (MTT), and other blood tests. The OAD were withdrawn on the day before and on the day of the test. For subjects on insulin therapy, it was withdrawn on the day of the test (none of them used long-acting insulin). Further clinical follow-up was conducted after 12 months. Data of 24 months postsurgery (basal blood glucose and insulin, HbA1c, weight, BMI, antidiabetic therapy) were analyzed.
Surgical Procedures
All operations were performed by the same surgical team, using the same technique. The main characteristics of the open RYGBP were a 30-ml gastric pouch, a biliopancreatic limb of 100 cm, an alimentary limb of 150 cm, and a common limb consisting of the remainder of the small intestine.
Anthropometrics
The anthropometric evaluation included height, weight, BMI (weight/height2), and waist circumference (above the right iliac crest).
Meal Tolerance Test
Subjects were submitted to standard MTT based on a mixed meal containing 515 kcal (41.8 % fat, 40.7 % carbohydrate, 17.5 % protein). The meal was eaten within 10 min. Meal start was considered time 0. Blood samples were drawn at times −60, −30, 0, 15, 30, 45, 60, 90, 120, 150, and 180 min for analysis of glucose, insulin, C-peptide, glucagon-like peptide-1 (GLP-1), and glucose-dependent insulinotropic polypeptide (GIP). Blood samples were collected in tubes with EDTA.K3 and Sigma diprotin A was added in the tubes for GLP-1 and GIP. The area under the curve (AUC) of each parameter was calculated by the trapezoidal method. The incremental AUC (AUCi) was calculated as total AUC minus the area under the basal value.
Blood Analysis
Serum samples were immediately analyzed for glucose (glucose oxidase method), HbA1c (high performance liquid chromatography), total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides (routine standard methods). Serum samples were stored in a freezer at −80 °C for posterior analysis of insulin (ELISA, Bayer Corp., Tarrytown, NY), C-peptide (RIA, Linco Research, St. Charles, MO), GLP-1 and GIP (ELISA, Linco Research, St. Charles, MO), and adiponectin (ELISA, R&D Systems Inc., Minneapolis, MN).
Insulin Sensitivity
Insulin sensitivity (IS) was estimated by the oral glucose insulin sensitivity (OGIS), the result of a mathematical model based on the dynamic relationship between glucose and insulin during the MTT. For this index, higher values represent higher IS [13].
Beta-Cell Insulin Secretory Function
The beta-cell insulin secretory function was estimated by the index ΔCP(0–30)/ΔGlu(0–30), which was calculated as (CP30–CP0)/(Glu30–Glu0), in which CP0 and Glu0 represent the fasting (time 0) values of C-peptide and glucose and CP30 and Glu30 represent the values of C-peptide and glucose at time 30 min of the MTT. This is a variation of the classic insulinogenic index, in which values of insulin are used instead of the C-peptide [14]. The early insulin response to the meal (first 30 min) relates closely to the first-phase insulin secretion [14]. C-peptide and insulin are secreted in equimolar concentrations, but insulin is subject to hepatic first pass extraction, as opposed to C-peptide. Thus, peripheral serum insulin underestimates insulin secretion, which is more reliably represented by serum C-peptide.
The disposition index (DI), a measure of beta-cell function relative to the prevailing IS, was defined as the product of ΔCP(0–30)/ΔGlu(0–30) and OGIS (an IS index).
Definition of Diabetes Remission
Diabetes remission was defined according to the recommendation of the American Diabetes Association [15]. Complete remission was defined by normoglycemia (HbA1c <6 % and fasting glucose <100 mg/dl) of at least 1 year duration in the absence of active pharmacologic therapy. Partial remission was defined by subdiabetic hyperglycemia (HbA1c 6.0–6.4 % and fasting glucose 100–125 mg/dl) of at least 1 year duration in the absence of active pharmacologic therapy. These criteria were evaluated 24 months postsurgery.
Statistics
SPSS v16.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. Data are presented as mean and standard deviation (mean ± SD). Comparisons between baseline and postsurgery data were obtained by Wilcoxon test. Comparisons between subgroups (with or without insulin therapy at baseline; diabetes remission or nonremission) were obtained by Mann-Whitney test. Bivariate correlations among the occurrence of diabetes remission and other variables were tested by Spearman’s rho test. Binary logistic regression was performed to find predictors of diabetes remission. Statistical significance was assumed if P < 0.05.
Results
At screening, there were no significant differences between subjects with or without insulin therapy in relation to age, gender distribution, time from diabetes diagnosis, BMI, HbA1c, fasting glucose, insulin, and C-peptide. The results of the metabolic tests at baseline were not different between these subgroups, except for beta-cell secretory function (DI), which was lower in subjects on insulin therapy [4.74 (5.86) vs. 10.79 (10.89) (ng/mg) [ml/min/m2], P = 0.025].
There were no differences in any variables between these subgroups over the postsurgery follow-up and they were analyzed as a whole group. The subjects’ age was 47.4 (8.4) years (range 31 to 54 years) and the time from diabetes diagnosis was 9.7 (4.8) years (range 1 to 17 years) The body mass and metabolic parameters are presented in Table 1 and glucose metabolism parameters are presented in Table 2.
Surgical Outcome and Adverse Events
Per hospital protocol, the total length of inpatient stay was 7 days, including stay in an intensive care unit over 24 h after surgery. One subject required prolonged stay (total of 40 days) due to intestinal obstruction, which required exploratory laparotomy (recovered without sequelae). Within 30 days postsurgery, another subject had abdominal wall infection in the surgical site (recovered after outpatient antibiotic therapy, without sequelae).
After 30 days postsurgery, the following mild adverse events, related to bariatric surgery, were observed: mild-to-moderate iron deficiency anemia in 5/36 subjects (all recovered after iron supplementation and nutritional therapy) and incisional hernia in 3/36 subjects (none required surgical repair and there were no complications). Two late, severe adverse events were observed, both of them unlikely to be related to the bariatric surgery. One subject had a nonfatal acute myocardium infarction 18 months postsurgery (recovered after inpatient medical therapy and coronary artery angioplasty). Another subject had diagnosis of esophageal adenocarcinoma, 15 months postsurgery (partially recovered after surgery, radiotherapy, and chemotherapy). No suspected signs or symptoms of the carcinoma were observed before the diagnosis.
Anthropometrics
Most of the body mass reduction was observed 3 months postsurgery, and all subjects achieved a BMI within the normal or overweight ranges over 24 months postsurgery (Table 1). Excessive weight loss was not observed. The BMI ranges after 12 and 24 months postsurgery were, respectively, 20.2 to 28.4 kg/m2 and 20.9 to 29.7 kg/m2. The weight loss 12 and 24 months postsurgery were, respectively, −19.3 (6.7) kg and −16.9 (6.0) kg.
Lipids, C-reactive Protein, and Adiponectin
Adiponectin levels increased and ultrasensitive C-reactive protein (usCRP) decreased over 1 year postsurgery (Table 1). Total cholesterol levels did not decrease significantly, the triglycerides levels decreased, and HDL-cholesterol levels increased over 24 months postsurgery (Table 1).
Glucose Homeostasis and Changes in Antidiabetic Therapy
All patients showed clinically and statistically significant improvements in fasting plasma glucose and HbA1c (Table 2). The reduction in HbA1c 12 and 24 months postsurgery were, respectively, −3.0 (1.6) % and −2.9 (1.8) %. HbA1c <7 % was observed 12 and 24 months postsurgery, respectively, in 31/36 (86 %) and 26/36 (72 %) subjects.
From 24 subjects on insulin therapy at baseline, one still required OAD and insulin therapy, and 15 required OAD (13 on monotherapy) 12 and 24 months postsurgery. From 12 subjects without insulin therapy at baseline, six still required OAD (two of them on monotherapy) 12 and 24 months postsurgery.
A profile of diabetes remission was achieved in 10/36 subjects (25 %) 12 months postsurgery and was sustained up to 24 months postsurgery (to meet criteria of at least 1 year duration for remission). Complete diabetes remission was achieved in 9/36 subjects (22 %), six of them from the subgroup without insulin therapy at baseline. Partial diabetes remission was achieved by one subject (3 %), who was from the insulin therapy subgroup.
Insulin Sensitivity and Secretion
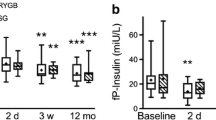
Improvements of IS, as measured by OGIS, and of beta-cell insulin secretory function, as measured by ΔCP(0–30)/ΔGlu(0–30) and DI, were observed since 3 months postsurgery (Table 2 and Fig. 2).
Incretin Secretion
GLP-1 secretion, as measured by its incremental AUC during the MTT, improved since 3 months postsurgery. The incremental AUC of GIP decreased postsurgery (Table 1 and Fig. 1).
Curves of a glucose, b insulin, c C-peptide, d GLP-1, and e GIP during a meal tolerance test in subjects with type 2 diabetes and mild obesity, performed at baseline and 3, 6, and 12 months after Roux-en-Y gastric bypass (mean values). The corresponding incremental areas under the curve (AUCi) are presented in bars (mean values)
Comparisons Between Diabetes Remission and Nonremission
We identified differences in some variables between the subjects that achieved diabetes remission (n = 10, including one with partial remission) or did not achieve it (n = 26) (nonremission) (Table 3). At baseline, subjects with nonremission had longer time from diabetes diagnosis, higher levels of HbA1c, lower beta-cell secretory function (ΔCP(0–30)/ΔGlu(0–30) and DI), and higher incremental AUC of GIP in the first 30 min of the MTT (AUCi(30 min)-GIP). There was no difference in the total AUC of GIP. Twelve and 24 months postsurgery, as expected, HbA1c was higher in subjects with nonremission. Despite of an increase in the beta-cell secretory function in both of these subgroups, the postsurgery values of ΔCP(0–30)/ΔGlu(0–30) and DI in subjects with nonremission were lower and similar to the baseline values of subjects with remission. Twelve months postsurgery, the AUCi(30 min)-GIP increased in both subgroups and was still higher in subjects with nonremission, although the percent increase was similar between subgroups.
Diabetes remission was correlated with the baseline (preoperative) values of the following variables: negatively with HbA1c (−0.37, P = 0.028), time from diabetes diagnosis (−0.45, P = 0.006), and AUCi(30 min)-GIP (−0.43, P = 0.024); and positively with ΔCP(0–30)/ΔGlu(0–30) (0.53, P = 0.011) and DI (0.51, P = 0.021). Diabetes remission was correlated with the 12-month postsurgery values of the following variables: negatively with AUCi(30 min)-GIP (−0.38, P = 0.051, partial correlation) and positively with ΔCP(0–30)/ΔGlu(0–30) (0.43, P = 0.039) and DI (0.47, P = 0.029). It did not correlate with the postsurgery changes in any of these variables. Diabetes remission did not correlate to other variables, including baseline BMI, weight loss, number or type of the preoperative antidiabetic drugs (including insulin use), IS (OGIS), and other metabolic characteristics (Fig. 2).
A binary logistic regression was performed to predict diabetes remission using baseline (preoperative) characteristics that correlated with it as predictors: HbA1c, time from diabetes diagnosis, AUCi(30 min)-GIP, and either ΔCP(0–30)/ΔGlu(0–30) or DI. Other possible variables that did not correlate with remission (cited above) also entered the model. Entered as single variables, baseline HbA1c and time from diabetes diagnosis were predictors of diabetes remission, respectively, with odds ratio (OR) 0.44 [95 % confidence interval (CI) 0.22–0.88], P = 0.019, and OR 0.71 (95 % CI 0.55–0.91), P = 0.006. When both variables were entered together in the model, only time from diabetes diagnosis contributed to the prediction. Baseline ΔCP(0–30)/ΔGlu(0–30) was excluded due to a large standard error when entered either alone (42,950) or with the other variables (58,585) and did not contribute to the prediction model. The other variables did not contribute to the prediction too.
Another binary logistic regression was performed to predict diabetes remission replacing the baseline values by 12-month postsurgery values of the modifiable characteristics (except for HbA1c). No further variables contributed to the prediction.
Discussion
Subjects with type 2 diabetes and mild obesity submitted to RYGBP had improvements in body mass, IS, beta-cell insulin secretory function, incretin secretion, and other metabolic parameters 3 months postsurgery, sustained over 12 months. Over 24 months, all of them sustained improvements in body mass and glycemic control. Most of them reached the goal of HbA1c <7 %, even though a minority had criteria for diabetes remission.
All the patients in our study experienced a clinical improvement. However, diabetes remission was not achieved by most of them. There are some possible explanations for this. Most of our patients were on insulin therapy at baseline or had long-standing diabetes (31/36 subjects had diabetes duration >5 years). According to previous studies, these two factors are associated with a lower rate of diabetes remission after bariatric surgery [16], and both presume a lower beta-cell secretory function.
Although this study was not powered for determination of factors that influence or predict diabetes remission, some insight was obtained by comparing the subjects who achieved remission or those who did not. Among “nonremitters,” a longer time from diabetes diagnosis may relate to the progressive loss of beta-cell function over time [16, 17], and the poorer glycemic control (higher HbA1c) prior to surgery may relate to a lower beta-cell function too (either as a consequence of glucotoxicity or as a factor that limits the response to medical treatment) [18]. Indeed, the indexes of beta-cell insulin secretory function were lower in nonremitters at baseline and postsurgery. Both remitters and nonremitters had an increase in beta-cell insulin secretory function postsurgery, but nonremitters still had it at a level not higher than the baseline values of the remitters. Lower HbA1c and shorter time from diabetes diagnosis predicted diabetes remission in the binary logistic regression analysis of this study, but the beta-cell indexes did not. It is possible that these or additional beta-cell indexes might perform better in a larger sample.
The GLP-1 secretion did increase postsurgery but it does not seem to be a determinant factor for diabetes remission, as GLP-1 curve (at baseline or postsurgery) was similar in remitters and nonremitters, as described in previous studies [16]. Although the RYGBP-promoted boost in the incretin secretion is likely to contribute to beta-cell recovery [8, 19], the “incretin effect” depends on the capacity of beta-cells to respond to such stimuli.
The GIP secretion decreased in the whole MTT, with a change in its curve shape, so that its early-phase (first 30 min) secretion actually increased. This change resembles what happens to the MTT insulin curve. We hypothesize that its meaning for GIP is the same as for insulin: an increase in the early-phase secretion, meaning recovery of the secretory function, and a decrease in the late-phase secretion, meaning a recovery of sensitivity to the hormone action. According to this hypothesis and to some previous studies [20, 21], GIP resistance improved in our subjects. However, a higher GIP early-phase secretion characterized nonremitters, compared to remitters, at baseline, and despite of a postsurgery increase in both subgroups, it was even higher in nonremitters. It is possible to be either a primary defect in the latter subjects or maybe secondary to poorer glycemic control. Whether it contributed to diabetes nonremission is not clear.
The initial excitement on surgically induced “diabetes cure” in the scientific community is evolving to a more moderate speech about how bariatric surgery can improve glycemic control and diabetes comorbidities. High rates of diabetes remission described in the literature are possibly overestimated due to the inclusion of patients with relatively better glycemic control or more recent diagnosis of diabetes.
RYGBP improves the glucose metabolism in patients with type 2 diabetes and mild obesity. This effect is associated with improvement of insulin sensitivity, beta-cell secretory function, and incretin secretion. The identification of predictive factors for surgically induced remission of diabetes is challenging. However, in real life, a great proportion of patients submitted to bariatric surgery are not likely to achieve remission, even though most of them will achieve the diabetes treatment goals that eventually would not be reachable through the current medical therapy.
References
Eldar S, Heneghan HM, Brethauer AS, et al. Bariatric surgery for treatment of obesity. Int J Obes. 2011;35:S16–21.
Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes. 2011;33:S28–32.
Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309:2250–61.
Dixon JB, Zimmet P, Alberti KG, et al. International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese type 2 diabetes. Arq Bras Endocrinol Metabol. 2011;5:367–82.
Reis CE, Alvarez-Leite JI, Bressan J, et al. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index <35 kg/m2: a literature review. Diabetes Technol Ther. 2012;14:365–72.
Ionut V, Bergman RN. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol. 2011;5:1263–82.
Lima MM, Pareja JC, Alegre SM, et al. Acute effect of Roux-en-Y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3871–5.
Umeda LM, Silva EA, Carneiro G, et al. Early improvement in glycemic control after bariatric surgery and its relationships with insulin, GLP-1, and glucagon secretion in type 2 diabetic patients. Obes Surg. 2011;21:896–901.
Camastra S, Muscelli E, Gastaldelli A, Holst JJ, Astiarraga B, Baldi S et al. Long-term effects of bariatric surgery on meal disposal and ss-cell function in diabetic and nondiabetic patients. Diabetes 2013 Jul 8. [Epub ahead of print].
Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–7.
Shah SS, Todkar JS, Shah PS, et al. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m2. Surg Obes Relat Dis. 2010;6:332–8.
Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–8.
Mari A, Pacini G, Murphy E, et al. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–48.
Utzschneider KM, Prigeon RL, Tong J, et al. Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia. 2007;50:2516–25.
Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–5.
Hirsch FF, Pareja JC, Geloneze SR, et al. Comparison of metabolic effects of surgical-induced massive weight loss in patients with long-term remission versus non-remission of type 2 diabetes. Obes Surg. 2012;22:910–7.
Adams S, Salhab M, Hussain Z, Miller G, Leveson S. Preoperatively determinable factors predictive of diabetes mellitus remission following Roux-en-Y gastric bypass: a review of the literature. Acta Diabetol 2013 Mar 7. [Epub ahead of print].
Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol. 2012;364:1–27.
Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122–31.
Hansen EN, Tamboli RA, Isbell JM, et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol. 2011;300:G795–802.
Geloneze B, Geloneze SR, Chaim E, et al. Metabolic surgery for non-obese type 2 diabetes: incretins, adipocytokines, and insulin secretion/resistance changes in a 1-year interventional clinical controlled study. Ann Surg. 2012;256:72–8.
Acknowledgments
This work was supported by Ethicon Endo-Surgery.
Conflict of Interest
The authors disclose no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ana Cláudia Fellici and Giselle Lambert contributed equally to this work.
This study is registered at clinicaltrials.gov (NCT00566189).
Rights and permissions
About this article
Cite this article
Fellici, A.C., Lambert, G., Lima, M.M.O. et al. Surgical Treatment of Type 2 Diabetes in Subjects with Mild Obesity: Mechanisms Underlying Metabolic Improvements. OBES SURG 25, 36–44 (2015). https://doi.org/10.1007/s11695-014-1377-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-014-1377-9