Abstract
Background
The surgical treatment of obesity ameliorates metabolic abnormalities in patients with type 2 diabetes. The objective of this study was to evaluate the early effects of Roux-en-Y gastric bypass (RYGB) on metabolic and hormonal parameters in patients with type 2 diabetes (T2DM).
Methods
Ten patients with T2DM (BMI, 39.7 ± 1.9) were evaluated before and 7, 30, and 90 days after RYGB. A meal test was performed, and plasma insulin, glucose, glucagon, and glucagon-like-peptide 1 (GLP-1) levels were measured at fasting and postprandially.
Results
Seven days after RYGB, a significant reduction was observed in HOMA-IR index from 7.8 ± 5.5 to 2.6 ± 1.7; p < 0.05 was associated with a nonsignificant reduction in body weight. The insulin and GLP-1 curves began to show a peak at 30 min after food ingestion, while there was a progressive decrease in glucagon and blood glucose levels throughout the meal test. Thirty and 90 days after RYGB, along with progressive weight loss, blood glucose and hormonal changes remained in the same direction and became more expressive with the post-meal insulin curve suggesting recovery of the first phase of insulin secretion and with the increase in insulinogenic index, denoting improvement in β-cell function. Furthermore, a positive correlation was found between changes in GLP-1 and insulin levels measured at 30 min after meal (r = 0.6; p = 0.000).
Conclusion
Our data suggest that the RYGB surgery, beyond weight loss, induces early beneficial hormonal changes which favor glycemic control in type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a chronic, progressive, and multifactorial disease that has reached epidemic proportions globally in adults and children. Lifestyle changes that are associated with the pharmacological treatment of obesity may not always be effective in patients with severe obesity. These changes promote weight loss between 5% and 10% of the initial weight, and approximately 100% of the patients regain in 5 years. Surgical treatment is the most effective treatment for weight loss and maintenance, and it reduces obesity-related complications, particularly type 2 diabetes [1].
Surgeries involving intestinal bypass have beneficial effects for diabetics. Approximately 84% of diabetic patients who undergo Roux-en-Y gastric bypass (RYGB) experience complete remission of the disease [2]. Important decreases in glucose levels appear earlier, and these effects seem to be independent of weight loss, which is a secondary effect. Many studies have demonstrated that RYGB surgery promotes changes in intestinal hormones, such as increasing glucagon-like peptide 1 (GLP-1) levels in response to food intake, increasing insulin secretion, reducing glucagon secretion, improving glycemic control, and reducing appetite [3–7].
There are a few prospective studies that have evaluated the early effects of bariatric surgery by analyzing glycemic control a few days after surgery and before a massive weight loss occurs. The objective of this study was to evaluate the early hormonal influence after bariatric surgery on metabolic profile in patients with type 2 diabetes and obesity at levels II and III.
Patients and Methods
The following criteria were required for patients to be recruited in this study from Obesity and Hypertension Outpatient Clinic at the Hospital do Rim e Hipertensão, Universidade Federal de São Paulo: diagnosed with type 2 diabetes, 25 to 65 years of age, obesity at levels II and III, and the use of oral anti-diabetic medication. The diabetes diagnosis was confirmed according to American Diabetes Association criteria. Patients were excluded from the study for the following reasons: using a dipeptidyl peptidase-4 inhibitor or a GLP-1 agonist therapy; with a severe psychiatric disease; cancer, renal, cardiac or hepatic failures; alcohol or drug abuse or severe pulmonary disease. Patients taking insulin therapy and with uncontrolled blood pressure (systolic BP ≥160 mmHg) were also excluded.
The Ethics Committee of the Federal University of Sao Paulo approved this study, and all participants signed an informed consent form.
A multidisciplinary team of nutritionists, psychologists, and endocrinologists evaluated all patients who were selected for the study during six visits before surgery. The nutritional intervention was performed to check and encourage the patients to adhere to the proposed diet at every visit. The psychological evaluation was important to diagnose cases of depression and severe eating disorders, both of which were exclusion criteria for the study. A physical examination was performed at every visit, and waist circumference and BMI determinations were made. The waist circumference was measured at the midpoint between the last rib and the iliac crest.
After withdrawal of anti-diabetic medication for 12 h, patients were made to fast overnight for 12 h, and blood samples were collected for glucose, insulin, and glycated hemoglobin (HbA1c) determinations. A standard liquid meal of 353 kcal (46.8% carbohydrates, 32.2% proteins, and 12.5% lipids) was then given to the patients for blood glucose, insulin, glucagon, and GLP-1 measurements at 0, 30, 60, 90, and 120 min. This procedure was performed before and at 7, 30, and 90 days after the surgery.
Bioelectrical impedance measurements were collected from all patients to evaluate body composition (body water and lean and fat masses) before and after the surgery (Quantump BIA 101Q Akern RJL Systems, Clinton Township, MI, EUA). The bioelectrical impedance analysis was performed in the morning after fasting overnight and after the first urine.
Surgical Technique
The technique used in this study was RYGB, which combines restrictive and malabsorptive mechanisms. A vertical gastric pouch (20–30 ml) was constructed with surgical staples in the lesser curvature of the stomach. Gastrojejunostomy adjustment was performed using a 32-French tube. Reconstruction was performed by RYGB with an alimentary limb measuring 100 cm and a biliopancreatic limb of 50 cm from the ligament of Treitz.
Metabolic Study
The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated from fasting glucose and insulin levels. The insulinogenic index was calculated as the ratio between the changes in insulin and glucose levels 30 min after the meal test to evaluate the first phase of insulin secretion; according to the formula (∆I30/∆G30).
Analytical Procedure
Plasma insulin levels were determined by an automatic immunoassay system (Auto DELFIA insulin kit, PerkinElmer Life and Analytical Sciences, Waltham, Massachusetts, USA). Glucose levels were determined by the glucose oxidase method, and HbA1c was determined by high-performance liquid chromatography.
Active GLP-1, which is an indicator of GLP potential action, was measured by enzyme-linked immunosorbent assay immunofluorescence (Linco Research, specific for humans). The assay cross-reactivity with GLP-1 7–36 and GLP-1 7–37 is 100%, but it is not with GLP-1 9–36, glucagon, and GLP-2.
Glucagon concentrations were measured by radioimmunoassay (Linco Research), and the assay cross-reactivity is 100% with glucagon, and its cross-reactivity with oxyntomodulin is less than 0.1%.
Statistical Analyses
All data were analyzed using the software Statistical Package for the Social Sciences (SPSS), version 18.0 (SPSS Inc, Chicago, IL). The Friedman test and post hoc analysis were used to compare the values of all parameters studied, which were obtained during the meal test before and at 7, 30, and 90 days after surgery. Spearman coefficient was used to determine the correlations between the different variables before and after surgery. Data are expressed as mean±standard deviation, and p < 0.05 was considered statistically significant.
Results
Clinical and laboratory profiles of the patients before and at 7, 30, and 90 days after RYGB are shown in Table 1. High blood glucose values were observed both at fasting and after a standard meal test (Fig. 1). This was accompanied by a mild increase in postprandial insulin secretion, with values describing a flat curve. No changes in GLP-1 and glucagon levels were observed during the meal test.
No significant reductions in BMI and fat mass were observed 7 days after RYGB; however, a significant decrease in free fat mass was observed, which is presumably linked to fluid loss (Table 1). By this time, reductions in both fasting blood glucose and insulin levels lead to marked reduction in HOMA-IR index. Also, changes occurred in the shape of the blood glucose, insulin, GLP-1, and glucagon curves, delineated by the levels of these variables determined during the meal test (Fig. 1). These curves began to show peaks in GLP-1 and insulin levels at 30 min after food ingestion and a progressive decrease in glucagon and blood glucose levels throughout the meal test. A positive correlation was observed between changes in the areas under the curves of glucagon and insulin values (r = 0.8; p = 0.015). However, the alterations noted in the areas under the curves delineated by the postprandial hormonal and glucose values did not reach statistical significance, and no changes in the insulinogenic index were observed after 7 days of RYGB. Also, no correlations were found between these initial changes and the changes in BMI or fat mass.
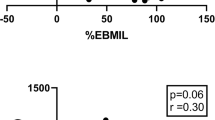
Thirty and 90 days after RYGB, blood glucose and hormonal parameters changes continued in the same direction, becoming more expressive and statistically significant. Particularly, an expressive peak of GLP-1 and insulin was observed at 30 min after the meal test Considering all the observations made during the post-surgical 3-month period, a positive correlation was found between changes in GLP-1 and insulin levels measured at 30 min after meal ingestion (r = 0.6, p = 0.000; Fig. 2). As a consequence, the insulinogenic index showed values higher than those observed before surgery, which reflects a recovery in the first phase of insulin, contrasting with a pronounced suppression of the glucagon levels and reductions in blood glucose values observed (Fig. 1). This improvement in glycemic control resulted in progressive reductions in HbA1c while HOMA-IR remained low (Table 1).
Discussion
The present study demonstrated a progressive improvement in glycemic control, along with gastrointestinal hormonal changes and improvement in both insulin sensitivity and production after bariatric surgery, in obese patients with diabetes.
Initially, 7 days after surgery, we noticed a decrease in both fasting plasma insulin and glucose with a consequent reduction in HOMA-IR index, which characterizes an improvement in insulin sensitivity. Although only marginally significant by this time, the reductions in the areas under the curves of insulin and blood glucose levels after meal also indicate a decrease in insulin resistance.
The marked reduction in HOMA-IR of 67% was associated with 7.7% reduction in fat mass, suggesting that other factors than body weight reduction could be influencing the early changes in glucose homeostasis. One mechanism to be considered could be dependent on caloric restriction [8, 9]. Isbell et al. [10] investigated the influence of caloric restriction on insulin resistance and production, comparing a group of obese individuals undergoing RYGB surgery to a control group of obese patients, 4 days after an equivalent post-bariatric surgery diet. They observed similar decreases in insulin production and improved insulin sensitivity in both groups, while there were increases in GLP-1 secretion, which took place only in the group submitted to bariatric surgery. The authors, thus, concluded that the changes observed in insulin production and sensitivity were independent both on the surgical procedure and GLP-1 production and proposed the caloric restriction as the mechanism responsible for the changes observed. In fact, after RYGB, starvation can activate enzymes involved in gluconeogenesis. The mechanisms that involve the portal vein sensors promote a decrease in hepatic glucose production and decrease insulin resistance. These effects are independent of GLP-1 [11–14].
Seven days after RYGB surgery, a peak in GLP-1 serum levels occurred 30 min after the test meal diverging from what was observed before surgery. This was also associated with alterations in the shape of the postprandial glucagon and insulin curves. Despite the decrease in the area under the curve of insulin secretion after meal, a peak in insulin levels was observed 30 min after food ingestion, similar to that observed in the GLP-1 curve. In addition, a positive correlation was observed between the decrease in the areas under curves of glucagon and insulin after meal, suggesting the occurrence of two related phenomena or the presence of a third factor interfering with the secretion of these two hormones. The early improvement in GLP-1 secretion after RYGB could be explained by these early hormonal changes. Some authors have suggested that RYGB surgery might affect the enteroinsular axis by delivering incomplete digested food to the ileum, leading to increases in GLP-1 secretion [2, 15–18]. The higher levels of GLP-1 could contribute indirectly to reductions in blood glucose and insulin levels after meal and the suppression of glucagon levels. Comparing the acute effects of RYGB versus gastric restrictive surgery in obese patients with T2DM, Kashyap et al. [19] observed, after 7 days of surgery, similar changes in BMI in both groups. Insulin sensitivity increased only after RYGB, suggesting that some other factors than caloric restriction could be involved in the amelioration of glucose homeostasis. These observations contrast with those reported by Isbell et al. and us, which indicate that during the first days following RYGB surgery, an early increase in insulin sensitivity, but not in insulin production, contributes for the early improvement in blood glucose control. Although an increase in GLP-1 has been observed after a meal, no changes in the insulinogenic index were observed after 7 days of RYGB, suggesting that the response of insulin secretion to blood glucose levels was not altered during this short period.
Thirty days after RYGB surgery, we observed a substantial reduction in body weight and fat mass and a further decrease in both fasting and postprandial blood glucose levels. These changes were associated with remarkable increases in GLP-1 levels and with additional decreases in serum glucagon after meal. A marked increase in insulonogenic index was noted, suggesting increased β cell sensitivity to glucose with recovery in the first phase of insulin secretion with maintenance of insulin sensitivity. Our results are in accordance to those reported by Kashyap et al. [19] who also observed robust increases in GLP-1 secretion after meal and improvement in β cell function only 4 weeks after RYGB, which did not occur after restrictive surgery. The significant positive correlation observed between changes in GLP-1 and insulin, from the basal levels to those observed at 30 min after meal, before and all time after surgery, strongly suggests an important role of GLP-1 in the improvement of insulin secretion. The mechanisms by which the GLP-1 response to food ingestion is blunted and improves after RYGB are not fully understood. It has been accepted that, after RYGB, the early exposition of the lower gut to the ingested nutrients would anticipate the physiological release of gut incretins [20–23]. However, other mechanisms could contribute to increases in GLP-1 levels or action. It has been shown recently that there was a decrease in dipeptidyl peptidase-4 activity [24] and an improvement in incretin effect on insulin secretion [25] after RYGB in type 2 diabetic patients by mechanisms independent of weight loss. Other determinants of impaired insulin secretion in type 2 diabetes, such as glucose toxicity and lipotoxicity [26–28], which probably are reduced after surgery, may contribute to improve β cell function. Thus, changes in the incretin levels are probably not the only factor responsible for the improvement in insulin secretion early after RYGB [29–33].
In summary, our data showed that RYGB induces significant hormonal changes that influence glucose metabolism and begins soon after the RYGB postoperative period. As early as 7 days after surgery, we observed reductions in glucose, insulin, and HOMA-IR associated with increases in GLP-1 and decreases in glucagon levels. Improvement in beta cell function and a more expressive decrease in glucagon secretion can be observed only later on, and they are associated with further increases in GLP-1 levels and greater weight loss. Our data suggest that the RYGB surgery induces early beneficial hormonal changes and is a very efficient surgical therapy for rapid glycemic control in obese patients with type 2 diabetes.
References
Sjostrom L, Lindroos A, Peltonen M, et al. Lifestyle, diabetes and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–93.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be most effective therapy for adult onset diabetes mellitus. Ann Surg. 1995;222(3):339–50. Discussion 350–52.
Pories WJ, MacDonald KG, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Nutr. 1992;55(2 Suppl):582S–5S.
Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84.
Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239(1):1–11.
Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. 2004;292(14):1724–37.
Jazet IM, Pijl H, Frölich M, et al. Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism. 2005;54(6):705–12.
Jazet IM, Ouwens DM, Schaart G, et al. Effect of a 2-day very low-energy diet on skeletal muscle insulin sensitivity in obese type 2 diabetic patients on insulin therapy. Metabolism. 2005;54(12):1669–78.
Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after roux-en-y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–42.
Mithieux G, Misery P, Magnan C, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2:321–9.
Mithieux G, Bady I, Gautier A, et al. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab. 2004;286:E370–5.
Mithieux G, Andrelli F, Magnan C. Intestinal gluconeogenesis: key signal of central control of energy and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2009;12:419–23.
Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–11.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9.
Hickey MS, Pories WJ, MacDonald Jr KG, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the of foregut? Ann Surg. 1998;227:637–43. discussion 43–4.
Naslund E, Backman L, Holst JJ, et al. Importance of small bowel peptide for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg. 1998;8:253–60.
Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150(6):2518–25.
Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond). 2009;34(3):462–71.
Le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243(1):108–14.
Holdstock C, Zethelius B, Sundbom M, et al. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes. 2008;32(11):1640–6.
Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–85.
Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg. 2008;247(6):968–75.
Alam ML, Van der Schueren BJ, Ahren B, et al. Gastric bypass surgery, but not caloric restriction, decreases dipeptidyl peptidase-4 activity in obese patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:378–81.
Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16.
Boden G. Free fatty acids—the link between obesity and insulin resistance. Endocr Pract. 2001;7(1):44–51.
Ruan H, Lodish HF. Regulation of insulin sensitivity by adipose tissue-derived hormones and inflammatory cytokines. Curr Opin Lipidol. 2004;15(3):297–302.
Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28(2):187–218.
Manfredini G, Ermini M, Scops L, et al. Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol Gastrointest Liver Physiol. 1985;249:G519–27.
Patti ME et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–7.
Wang PYT, Caspi L, Lam CKL, et al. Upper intestinal lipids trigger a gut–brain–liver axis to regulate glucose production. Nature. 2008;452:1012–6.
Lima MMO, Pareja JC, Alegre SM, et al. Acute effect of Roux-en-y gastric bypass on whole-body insulin sensitivity: a study with euglicemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(8):3871–5.
Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–5.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umeda, L.M., Silva, E.A., Carneiro, G. et al. Early Improvement in Glycemic Control After Bariatric Surgery and Its Relationships with Insulin, GLP-1, and Glucagon Secretion in Type 2 Diabetic Patients. OBES SURG 21, 896–901 (2011). https://doi.org/10.1007/s11695-011-0412-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0412-3