Abstract
This meta-analysis aimed to evaluate changes in GIP after RYGB in obese patients. We searched PubMed, EMBASE, and CENTRAL for relevant studies from database inception through July 2021. Articles were eligible for inclusion if they reported pre-operative and post-operative fasting GIP levels. We found fasting GIP levels had a decreasing tendency. The decrease in fasting glucose and postprandial GIP levels was also observed. Subgroup analysis indicated diabetic subjects tended to have a more obvious fasting GIP reduction compared to non-diabetic individuals. Meta-regression showed that the amount of weight loss (% total body weight), gastric pouch volume, alimentary limb length, and biliopancreatic limb length were not related to fasting GIP decrease. Fasting GIP levels decreased significantly after RYGB in obese people, especially in diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become an increasingly serious global epidemic associated with multiple serious metabolic co-morbidities, including diabetes mellitus (DM) [1, 2]. Compared with conservative interventions (diet, exercise, and medications), bariatric surgery has been proved to be the most effective method for treating morbid obesity and DM [3, 4].
Roux-en-Y gastric bypass (RYGB), which involves connecting a small gastric pouch to the jejunum and creating a blind loop consisting of distal stomach, duodenum, and proximal jejunum that connects to the Roux limb, has been regarded as the gold standard for obesity treatment. Also, it can bring about excellent remission of obesity-related comorbidities such as insulin resistance and type 2 DM [3, 4]. Compared with sleeve gastrectomy (SG), RYGB can result in greater weight loss, higher T2DM remission rates, fewer T2DM recurrences, and better long-term glycemic control [5]. Until now, the mechanisms by which RYGB causes weight loss as well as glucose homeostasis are not fully understood. Alterations in gastrointestinal hormones (i.e., gastric inhibitory polypeptide (GIP), glucagon-like peptide (GLP)-1, peptide YY (PYY), and ghrelin) are thought to play important roles [6,7,8,9].
GIP, an incretin produced by the K cells in the small intestine, was originally called as gastric inhibitory peptide on the basis of its influence on gastric function [7, 10, 11]. Nevertheless, relevant studies have revealed that it has a negligible role in gastric motility or secretion [10]. Its insulinotropic activity and related metabolic actions are now considered of greater importance [10]. In addition to its incretin effect, GIP has been shown to promote obesity [12, 13]. The decline in GIP is more likely to occur in bariatric operations with a malabsorptive component [6, 11, 14]. GIP decrease after RYGB tends to be more pronounced compared to that after SG [6]. Most studies have reported reduced postprandial GIP levels following RYGB, but the results of fasting level changes are not constant [11]. Some reported decreased fasting GIP [6, 15], while others observed non-significant modification [16,17,18,19]. Based on these different evidences, it is hard for us to draw a conclusion about the relationship between GIP levels and RYGB. Therefore, this meta-analysis aims to assess the changes in fasting GIP levels following RYGB.
Methods
The meta-analysis was conducted based on the recommendations from the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines [20] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. The review was registered at PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) as registration number CRD42019135063.
Literature Search
A computerized search was conducted in PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from database inception to July 2021, with English language only. Free terms and medical subject headings were used together during literature search, including (gastric inhibitory peptide OR GIP OR gastric inhibitory polypeptide OR glucose-dependent insulinotropic polypeptide OR glucose-dependent insulinotropic peptide) AND (gastric bypass). The references of pertinent articles were also hand-searched to supplement the investigations.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) people with obesity (BMI > 30 kg/m2) underwent RYGB; (2) reported pre-operative and post-operative fasting GIP levels. If more than one post-RYGB GIP level was reported, the measurement value at the longest follow-up time was selected.
Exclusion criteria were as follows: (1) animal studies; (2) non-RYGB surgery; (3) data not presented as mean ± standard deviation (SD) or standard error (SE); and (4) no available data, conference abstracts, comments, reviews, and meta-analyses.
Data Collection, Risk of Bias Assessment, and Quality Assessment
The data extraction and quality assessment were conducted by three investigators independently, and disagreements were resolved by discussion. The titles and abstracts of all articles identified by literature search were screened following the inclusion criteria. Full-text was further reviewed if the information from the abstract met eligibility criteria. A standard data extraction form was used to collect the following information: study characteristics (the first author, publication year, country, study design), patient demographics (mean age, number of patients included, mean BMI before surgery, diabetes, length of follow-up), outcome (GIP levels before and after surgery), sample measuring method, and characteristics of RYGB (operative technique, gastric pouch volume, Roux limb length, and biliopancreatic limb length). If possible, we would contact the authors by email for the missing information. The Cochrane Collaboration’s risk of bias in non-randomized studies of interventions (ROBINS-I) tool was used for assessing risk of bias in observational studies. The domains assessed were confounding, selection of participants, classification of intervention, deviation from intervention, missing data, measurement of outcomes, and selection of reported results. For randomized controlled trials (RCTs), the Cochrane Collaboration’s tool was used to assess risk of bias in randomized trials. This tool evaluated risk of bias through six dimensions: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. The quality of the observational studies was assessed by the Newcastle–Ottawa Scale (NOS), which consists of three factors: patient selections, comparability of the study groups, and the assessment of outcomes. The scale’s score ranged from 0 to 9, and studies with a score equal to or higher than 5 were considered to be eligible for our meta-analysis. We used the JADAD score to evaluate the quality of RCTs. A NOS score of ≥6 or a JADAD score of ≥3 was considered high quality.
Outcome Measures
The primary outcome was defined as the change in fasting GIP levels post-RYGB. Secondary outcome included fasting glucose and postprandial GIP. All types of postprandial simulation, such as glucose tolerance test or mixed meal tolerance test, were included. When multiple postprandial values were reported, testing at the longest duration was preferred. The postprandial GIP levels reported using an area under the curve (AUC) were included.
Statistical Analysis
All statistical analyses were performed by Review Manager (RevMan version 5.3) and Stata (version 12.0). A p value <0.05 was considered statistically significant. Standardized mean difference (SMD) with a corresponding 95% confidence interval (95% CI) was calculated for continuous outcomes. For studies that only reported SE, SD values were computed using the formula: SD = SE × √ n, where n is the sample size. Heterogeneity was measured using Cochran’s Q tests and I2, with a significance threshold of p value <0.1 and I2 > 50% [22]. A fixed-effect model would be chosen to pool results except for when statistical heterogeneity was significant. Subgroup analysis was performed based on whether or not there is DM (yes vs. no vs. yes/no), different types of measuring methods (ELISA vs. RIA), and follow-up duration varies (> 6 months vs. ≤ 6 months). Sensitivity analyses were carried out to investigate whether the outcomes were stable by changing the pooling model (fixed-effects model or random-effects model) and using the one-study-out method. A meta-regression analysis was performed to assess if change of GIP levels was affected by the amount of weight loss (% total body weight), gastric pouch volume, Roux limb length, and biliopancreatic limb length. Possible publication bias was evaluated by Begg’s test and Egger’s test using Stata software.
Results
Search Process, Study Characteristics, Risk of Bias Assessment, and Quality Assessment
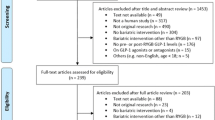
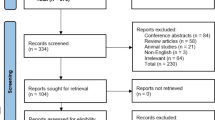
A total of 468 potential articles were identified through database searching and no additional publications were added to the search result by manual search. After removing duplicates and screening titles and abstracts, 261 records were excluded and 207 remained for full-text analysis. A total of 189 full-text articles did not meet the inclusion criteria. Hence, the remaining eighteen articles were incorporated into the final meta-analysis. Of the included studies, two studies had two arms; these arms were analyzed separately. The detailed process of study selection is shown in Fig. 1. The study characteristics are shown in Table 1. We identified eighteen cohort studies and two RCTs from the literature [19, 38]. The weighted mean age prior to RYGB of the included patients was 43.67 ± 5.30 years, and the weighted mean BMI at baseline was 43.24 ± 5.68 kg/m2. The follow-up period ranged from 4 days to 24 months.
Both RCTs were deemed to be of high quality (JADAD score ≥ 3) and moderate overall risk of bias. Eight non-randomized studies were evaluated to have a moderate overall risk of bias, eight had a low overall risk of bias, and two had a serious overall risk of bias. Risk of bias across cohort studies assessed using ROBINS-I is depicted in Table 2. The quality assessment of included cohort studies using the NOS is shown in Table 3.
Primary Outcome
Twenty trials involving 252 patients reported changes in fasting GIP levels following RYGB. Because of between-study homogeneity (p = 0.16, I2 = 24%), a fixed-effects model was used to pool result. The result showed that RYGB could significantly decrease the GIP levels of obese patients (SMD = 0.38, 95% CI 0.21 to 0.56, p < 0.0001) (Fig. 2). No significant publication bias was seen with Begg (p = 0.347) or Egger (p = 0.180) test.
Subgroup and Sensitivity Analysis
We performed subgroup analyses by DM (yes, no, or yes/no), type of measuring method, and follow-up duration. The diabetic subgroup consisted of 9 studies and the analysis showed that fasting GIP levels decreased significantly after RYGB as compared with that before surgery (SMD = 0.59, 95% CI 0.31 to 0.86, p < 0.0001), but a slight decrease (SMD = 0.38, 95% CI 0.01 to 0.75, p = 0.04) was observed in the non-diabetic groups. What’s more, GIP levels in the mixed groups were not significantly changed postoperatively (SMD = 0.13, 95% CI −0.18 to 0.44, p = 0.40) (Fig. 3). In the subsequent analysis, the outcomes showed that GIP levels after RYGB were remarkedly lower than the pre-RYGB levels regardless of the length of follow-up (> 6 months or ≤ 6 months) and which method of GIP measurement was used.
In sensitivity analysis, a random-effects model yielded a similar result (SMD = 0.40, 95% CI, 0.19 to 0.61, p = 0.0002) with the fixed-effect analysis. When removing any one study in turn, the pooled results were not markedly changed.
Meta-regression Analysis
Meta-regression analysis showed that the amount of weight loss (% total body weight), gastric pouch volume (range, 20 to 40 ml), Roux limb length (range, 100 to 150 cm), and biliopancreatic limb length (range, 30 to 150 cm) were not significant predictors of fasting GIP decreased after RYGB (p > 0.05 in all) (Table 4).
Secondary Outcomes
Eleven trials involving 126 patients documented the postprandial GIP levels before and after RYGB. Significant heterogeneity was identified between these studies (p < 0.00001, I2 = 77%), and the random-effects model showed that postprandial GIP levels after RYGB were significantly lower than pre-surgery levels (SMD = 0.66; 95% CI 0.09 to 1.23, p = 0.02). Eighteen studies reported pre- and post-RYGB fasting glucose levels. Due to significant heterogeneity, the random-effects model was selected for analysis. The pooled result showed fasting glucose levels decreased significantly after RYGB (SMD = 0.96, 95% CI 0.67 to 1.26, p < 0.00001).
Discussion
Many of the beneficial metabolic effects of RYGB have been attributed to changes in related gastrointestinal hormones [39, 40]. A previous meta-analysis by Jirapinyo et al. [41] showed postprandial GLP-1 levels increased following RYGB but fasting levels remained unchanged and the former was negatively connected with alimentary limb length. Through a systematic review and meta-analysis, Xu et al. [42] found that fasting ghrelin levels increased significantly after RYGB and this variation was related to the time course of RYGB but not to surgical technical characteristics (including gastric pouch volume, biliopancreatic limb length, and alimentary limb length). To our knowledge, this is the first meta-analysis exploring changes in GIP levels after RYGB in people with obesity. We found that fasting GIP levels decreased postoperatively and these changes appeared to be particularly marked in diabetic subjects. In addition, postprandial GIP levels decreased were observed. All the included studies did not report the association between GIP change and pouch size and the length of the Roux limb and biliopancreatic limb. This is also the first to probe into this association in obese subjects. Our meta-regression analysis indicated that the amount of weight loss and RYGB surgical technical characteristics were not associated with fasting GIP altered.
GIP is a hormone mainly secreted by the K cells in the duodenum and upper jejunum. Compared with lean people, obese and diabetic subjects had higher basal and stimulated GIP levels [43, 44]. But so far, its role in the development of obesity and diabetes is still unclear. GIP has been shown to promote insulin resistance and the conversion of glucose to fatty acids and their storage in adipose [7, 45, 46]. The reduction in GIP demonstrated in this meta-analysis highlighted the importance of GIP antagonism as a mechanism of obesity and DM treatment following RYGB. Nevertheless, the exact mechanism by which GIP decreases postoperatively remains unclear. This could be partly explained by the exclusion of the upper small intestine, which would lead to less stimulation of K cells and therefore a lower level of GIP. Also, it was found that the presence of food and bile was necessary for the secretion of GIP [11]. In RYGB, the food in the alimentary limb is not exposed to bile, thus possibly preventing its release.
In the current study, subgroups were predefined and used to assess the effect of specific factors on the change of GIP. Preoperative diabetic status was assumed to be an important factor, since previous studies had found this problem but failed to provide a definite conclusion [11]. Rubino et al. [32] showed that fasting GIP levels decreased in obese diabetic patients but not in obese nondiabetics. There are also some studies pointing out no change in fasting GIP after RYGB regardless of whether there is DM [27, 30]. Our subgroup analysis suggested that the decline in GIP levels was correlated with diabetic status prior to RYGB. The forest plot is shown in Fig. 3. GIP decreased markedly in the diabetic group, whereas it decreased slightly in the non-diabetic group. In addition, no significant change was observed in the mixed group. Studying the change in GIP after RYGB without grouping the populations by preoperative diabetic status could lead to untrue and unreliable results [32]. An interesting finding of our study is the significant effect of RYGB on GIP levels in diabetic patients. Whether RYGB can cause the variation of GIP in non-diabetic patients still needs further study. Decreased GIP after RYGB is more likely to occur in diabetic patients. This may be ascribed to glycemic control. The improvement of GIP resistance caused by upregulating GIP receptors could lead to GIP decrease through negative feedback regulation [11].
As mentioned earlier, GIP is elevated in obese patients with DM and contributes to insulin resistance and obesity. From another point of view, a more obvious GIP reduction in diabetic patients (SMD = 0.59, 95% CI 0.31 to 0.86) compared to normal individuals (SMD = 0.38, 95% CI 0.01 to 0.75) suggested that GIP played a crucial role in glucose homeostasis. However, the precise mechanism is not well understood and may be explained by the following reasons. On the one hand, the ameliorations in GIP resistance after RYGB may also make a contribution. Previous studies have suggested that patients with DM are resistant to the action of GIP [8, 47]. The cause of this GIP resistance is the downregulation of GIP receptors [48]. Hyperglycemia is thought to directly downregulate GIP receptors in pancreatic beta-cells, and reversal of hyperglycemia has been regarded as a contributor to GIP receptor upregulation [49]. The decreased GIP levels could improve the GIP-resistant state through upregulating GIP receptor, thus resulting in glucose control [11]. On the other hand, GIP has been reported to promote glucose absorption in the small intestine by increasing the number of GLUT1 receptors [11, 50, 51], so the reduction in GIP succeeding RYGB can be responsible for the improvement in glucose metabolism. Consequently, GIP antagonists may be beneficial in the treatment of obese diabetic patients. In order to explore the relationship between changes in GIP and glycemic control, we performed an additional meta-regression analysis. Regrettably, we failed to find any association between GIP decrease and the decline in fasting blood glucose (β = 0.03, p = 0.91, data not shown). Considering small patient sizes, the pooled results should be cautiously treated. Additionally, glycemic control after RYGB is likely to be the result of a combination of multiple gastrointestinal hormones changes such as GLP-1, PYY, and ghrelin. Their effect on blood glucose control can interfere with our meta-regression results.
This meta-analysis provides a quantifiable measure of GIP change after RYGB. However, several limitations should be pointed out. First, twenty studies with 252 patients only were enrolled in our study. The relatively small samples recommend caution in extrapolating firm conclusions from our observation. Furthermore, most of the included studies, but not all, reported gastric pouch volume, Roux limb length, or biliopancreatic limb length of the included patients. These may weaken our strength to explore the real association between changes in GIP levels and surgical technical characteristics. Second, dietary changes and the amount of time following surgery may play an important role in GIP level changes. Due to the limitations of the included studies, these data points were not available. Both impacts could be studied in randomized control trials with a non-surgical arm and followed over time. Another limitation is that most of the included studies were observational in nature. They are of suboptimal quality relative to experimental study. Therefore, randomized controlled study of RYGB versus medical treatment or placebo with long time follow-up and large sample sizes are also warranted to further examine the impact of RYGB on GIP levels.
Conclusions
Based on the available evidence, fasting GIP levels significantly decreased following RYGB. These changes were more pronounced in diabetic subjects. Changes in fasting GIP levels post-RYGB need to be reported separately for diabetic and non-diabetic patients. Randomized prospective studies with larger sample sizes and longer follow-up are needed to validate these findings.
References
Di Cesare M, Bentham J, Stevens GA, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–392. https://doi.org/10.1016/S2213-8587(21)00045-0. Erratum in: Lancet Diabetes Endocrinol. 2021;9(7):e2.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73
Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397(10271):293–304. https://doi.org/10.1016/S0140-6736(20)32649-0.
McTigue KM, Wellman R, Nauman E, et al. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass: The National Patient-Centered Clinical Research Network (PCORNet) bariatric study. JAMA Surg. 2020;155(5):e200087. https://doi.org/10.1001/jamasurg.2020.0087.
Jingge Y, Zhiguang G, Brandon W D, et al. Effect of laparoscopic Roux-en-Y gastric bypass, versus, laparoscopic sleeve gastrectomy on fasting gastrointestinal and pancreatic peptide hormones: a prospective nonrandomized trial [J]. Surgery Obes Relat Dis. 2018:S1550728918303034-.
Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2015;77:28–37
Pucci A, Batterham RL. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Investig. 2019;42(2):117–28. https://doi.org/10.1007/s40618-018-0892-2.
Dimitriadis GK, Randeva MS, Miras AD. Potential hormone mechanisms of bariatric surgery. Curr Obes Rep. 2017;6(3):253–65. https://doi.org/10.1007/s13679-017-0276-5.
Mcintosh CH, Widenmaier S, Kim SJ. Glucose-dependent insulinotropic polypeptide (Gastric Inhibitory Polypeptide; GIP)[J]. Vitamins & Hormones. 2009;80(08):409–71
Rao RS, Kini S. GIP and bariatric surgery. [J]. Obes Surg. 2011;21(2):244–52
Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity [J]. Nat Med. 2002;8(7):738–42
McClean PL, Irwin N, Cassidy RS, et al. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab. 2007;293(6):E1746–55
Farey JE, Preda TC, Fisher OM, et al. Effect of laparoscopic sleeve gastrectomy on fasting gastrointestinal, pancreatic, and adipose-derived hormones and on non-esterified fatty acids [J]. Obes Surg. 2016;27(2):1–9
Abrahamsson N, Börjesson JL, Sundbom M, et al. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667–75. https://doi.org/10.2337/db16-0341.
Alexiadou K, Cuenco J, Howard J, et al. Proglucagon peptide secretion profiles in type 2 diabetes before and after bariatric surgery: 1-year prospective study. BMJ Open Diabetes Res Care. 2020;8(1):e001076. https://doi.org/10.1136/bmjdrc-2019-001076.
Purnell JQ, Johnson GS, Wahed AS, et al. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia. 2018;61(5):1142–54. https://doi.org/10.1007/s00125-018-4553-y.
Romero F, Nicolau J. Flores, Lílliam, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects[J]. Surg Endosc. 2012;26(8):2231–9
Breitman I, Saraf N, Kakade M, et al. The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass[J]. J Am Coll Surg. 2011;212(4):617–25
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41
Higgins JP, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med. 2002;21(11):1539–58
Clements RH, Gonzalez QH, Long CI, et al. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus[J]. Am Surg, 2004, 70(1):1–4; discussion 4–5.
Dirksen C, Bojsen-Møller KN, Jørgensen NB, et al. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass[J]. Diabetologia. 2013;56(12):2679–87
Hansen EN , Tamboli RA , Isbell JM, et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery[J]. American Journal of Physiology Gastrointestinal & Liver Physiology, 2011, 300(5):0–0.
Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery[J]. Diabetes Care, 2010, 33(12):e176; author reply e177.
Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects[J]. Obes Surg. 2012;22(7):1084–96
Jørgensen NB, Jacobsen SH, Dirksen C, et al. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with type 2 diabetes and normal glucose tolerance[J]. American Journal of Physiology Endocrinology & Metabolism. 2012;303(1):E122
Egger RJ, Lee H, Kovack B, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes.[J]. J Clin Endocrinol Metab. 2008;93(7):2479–85
Lips MA, De Groot GH, Van Klinken JB, et al. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients[J]. Clin Endocrinol. 2014;80(6):834–42
Nosso G, Griffo E, Cotugno M, et al. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48(5):312–7. https://doi.org/10.1055/s-0041-111505.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism.[J]. Ann Surg. 2004;240(2):236–42
Schrumpf E, Bergan A, Djøseland O, et al. The effect of gastric bypass operation on glucose tolerance in obesity[J]. Scand J Gastroenterol. 1985;20(sup107):24–31
Vetter ML, Wadden TA, Teff KL, et al. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification[J]. Diabetes. 2015;64(2):434–46
Wu Q, Xiao Z, Cheng Z, et al. Changes of blood glucose and gastrointestinal hormones 4 months after Roux-en-Y gastric bypass surgery in Chinese obese type 2 diabetes patients with lower body mass index. J Diabetes Investig. 2013;4(2):214–21. https://doi.org/10.1111/jdi.12005.
Zhang X, Cheng Z, Xiao Z, et al. Comparison of short- and mid-term efficacy and the mechanisms of gastric bypass surgeries on managing obese and nonobese type 2 diabetes mellitus: a prospective study. Arch Med Res. 2015;46(4):303–9. https://doi.org/10.1016/j.arcmed.2015.06.003.
Braga TG, Graças Coelho de Souza MD, Menezes M, et al. Dipeptidyl peptidase-4 activity, lipopolysaccharide, C-reactive protein, glucose metabolism, and gut peptides 3 months after bariatric surgery. Surg Obes Relat Dis 2021;17(1):113–120. doi: https://doi.org/10.1016/j.soard.2020.08.030.
Katsogiannos P, Kamble PG, Wiklund U, et al. Rapid changes in neuroendocrine regulation may contribute to reversal of type 2 diabetes after gastric bypass surgery. Endocrine. 2020;67(2):344–53. https://doi.org/10.1007/s12020-020-02203-w.
Bueter M, Ashrafian H, Roux CWL. Mechanisms of weight loss after gastric bypass and gastric banding[J]. Obesity Facts. 2009;2(5):325–31
Dirksen C, Jørgensen NB, Bojsen-Møller KN, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55(7):1890–901. https://doi.org/10.1007/s00125-012-2556-7.
Jirapinyo P, Jin DX, Qazi T, Mishra N, Thompson CC. A meta-analysis of GLP-1 after roux-en-y gastric bypass: impact of surgical technique and measurement strategy. Obes Surg. 2018;28(3):615–26. https://doi.org/10.1007/s11695-017-2913-1.
Xu HC, Pang YC, Chen JW, Cao JY, Sheng Z, Yuan JH, Wang R, Zhang CS, Wang LX, Dong J. Systematic review and meta-analysis of the change in ghrelin levels after roux-en-y gastric bypass. Obes Surg. 2019;29(4):1343–51. https://doi.org/10.1007/s11695-018-03686-3. Erratum. In: Obes Surg. 2019 Apr 10
Elahi D, Andersen DK, Muller DC, et al. The enteric enhancement of glucose-stimulated insulin release. The role of GIP in aging, obesity, and non-insulin-dependent diabetes mellitus. Diabetes. 1984;33:950–7
Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13
Oben J, Morgan L, Fletcher J, et al. Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1(7-36) amide, on fatty acid synthesis in explants of rat adipose tissue[J]. J Endocrinol. 1991;130(2):267–72
Hauner H, Glatting G, Kaminska D, et al. Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann Nutr Metab. 1988;32:282–8
Knop FK, Vilsboll T, Hojberg PV, et al. The insulinotropic effect of GIP is impaired in patients with chronic pancreatitis and secondary diabetes mellitus as compared to patients with chronic pancreatitis and normal glucose tolerance. Regul Pept. 2007;144:123–30
Lynn FC, Pamir N, Ng EH, et al. Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes. 2001;50(5):1004–11. https://doi.org/10.2337/diabetes.50.5.1004.
Piteau S, Olver A, Kim SJ, et al. Reversal of islet GIP receptor down-regulation and resistance to GIP by reducing hyperglycemia in the Zucker rat[J]. Biochem Biophys Res Commun, 2007, 362(4):0–1012.
Cheeseman CI, O'Neill D. Basolateral D-glucose transport activity along the crypt-villus axis in rat jejunum and upregulation induced by gastric inhibitory peptide and glucagon-like peptide−2[J]. Exp Physiol. 1998;83
Creutzfeldt W. The entero-insular axis in type 2 diabetes--incretins as therapeutic agents.[J]. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S288–303
Funding
This work received financial support from the Natural Science Foundation of Guangdong Province (No. 2017A030313855) and Dongguan Science and Technology of Social Development Program (No. 20211800902451 and 202050715024111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
For this type of study, formal consent is not required.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. Fasting GIP levels significantly decreased following RYGB.
2. Fasting glucose and postprandial GIP levels decreased were observed after RYGB.
3. The decline in fasting GIP after RYGB was more pronounced in diabetic subjects.
Rights and permissions
About this article
Cite this article
Gao, Z., Yang, J., Liang, Y. et al. Changes in Gastric Inhibitory Polypeptide (GIP) After Roux-en-Y Gastric Bypass in Obese Patients: a Meta-analysis. OBES SURG 32, 2706–2716 (2022). https://doi.org/10.1007/s11695-022-05959-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-05959-4