Abstract
Phenolic compounds have high antioxidant capacity and are effective in preventing free radical formation and even their elimination in various ways. In this research, extraction of these compounds from lemon processing waste powder (LPWP) was investigated using Electrohydrodynamic (EHD) processes. In this regard, the effects of two independent factors of EHD time (in three levels of 10, 20 and 30 min) and EHD voltage (in three levels of 0, 19 and 15 kV) as well as their mutual effect on dependent responses of total phenolic content, antioxidant activity, extraction yield as well as the color parameters of L* and b* of the extracts were investigated. The results of this study showed that change in EHD time from 10 to 30 min and EHD voltage from 0 to 19 kV resulted in a significant increase in total phenolic content and extraction yield of the extracts. The Fourier transform infrared spectroscopy showed that the EHD process did not lead to any destruction of the functional groups of the extracts. In addition, scanning electron microscopy images showed higher porosity of LPWP microstructure, when longer EHD time or higher EHD voltage were applied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout history, humans have used plants for their nutritional purposes [1]. But after discovery of the medicinal properties of some plants in treating some diseases, human have used these plants to treat diseases and improve their health [1]. The Lemon, Citrus lemon L., is a medicinal plant of the family Rutacea [2]. It is the third most important citrus fruit species, rich in phenolic compounds, vitamins, minerals, dietary fiber, essential oils and carotenoids. Research has shown that lemon is useful in the treatments of some diseases [3]. and has an antioxidant and antimicrobial properties [4]. Each year, lemon juice factories generate a high amount of waste and by-products that are an important source of phenolic compounds [5]. These compounds have a high antioxidant potency and are effective in preventing the formation of free radicals and even eliminating them through different ways [6]. Many researches has shown that the use of these compounds as a biological additive contributes to promoting human health and reducing the risk of chronic diseases such as cancer, coronary arteries, heart disease, stroke and Alzheimer [7]. It should be noted that due to the dangers of synthetic antioxidants are used in foods, pharmaceutical, health and chemical industries on human health, the tendency to use natural antioxidants, such as phenolic compounds in food, is increasing [8]. Therefore, considering the widespread use of phenolic compounds in various industries, the necessity of using appropriate methods to extract these compounds from plant materials is increasing. Traditionally, extraction of bioactive phenolic compounds from natural products are performed using different solvents [9]. But traditional extraction methods such as maceration, Soxhlet and shaker have many problems, such as large amount of solvent utilization, long extraction time and lower extraction efficiency [10]. Therefore, new methods for the extraction of bioactive compounds have been developed to overcome the limitations of traditional methods and prevent the degradation of phenolic compounds. Therefore, novel techniques for the extraction of bioactive compounds have been developed to overcome the limitations of the traditional extraction methods and prevent the degradation of phenolic compounds [11]. Recently, electrical fields that cause electrical stimulation of biological tissues have been used as a novel extraction method for the extraction of various compounds [12]. In particular, one of the electrical technologies used is high voltage electrical discharges (HVED) or Electrohydrodynamic (EHD) technology, which is a green extraction technique [13]. This process is a non-thermal technique for increasing the mass transfer from sample to solvent with less extraction impurities, at ambient temperature, in short time and with lower energy consumption, which acts based on the corona discharge phenomenon [14]. The EHD system consists of one or several discharge electrodes (shape of needle, pin or wire) and a grounded electrode in the form of a metal plate [14]. When applying a high voltage between these electrodes, the air is ionized around the tip of the discharge electrode, and these ions propelled from the discharge electrodes toward the grounded electrode at a high velocity, and this results in the creation of ion-drag flow, which is known as corona wind [15]. So far, limited studies have been employed for the recovery of bioactive compounds from plant materials including grape pomace [16], flaxseed cake [17], sesame cake [18], and papaya peel [19] by this method. The review of available resources shows that no study has investigated the effect of two important independent variables of time and voltage of EHD as a novel technique for extraction of phenolic compounds from lemon waste. Thus, in this research attempts to evaluate the effect of two independent variables of time (10, 20 and 30 min) and voltage (0, 15 and 19 kV) of EHD system, as well as their mutual effects on the total phenolic content, antioxidant activity, extraction yield and color parameters of L* and b* of the extracts were studied. Moreover, the type of functional groups of extracts obtained from selected extraction treatments and their possible changes was investigated using Fourier transform infrared (FT-IR) spectroscopy. Furthermore, scanning electron microscopy (SEM) was used to investigate the Structural changes of lemon processing waste powder the selected extraction treatments. The hypothesis of this study is that by using the EHD process for extraction of phenolic compounds from LPWP, especially in terms of the appropriate combination of EHD time and EHD voltage, while preserving the physicochemical properties of extracted phenolic compounds, the extraction yield of these compounds also increases.
Materials and methods
Chemicals and reagents
2, 2-Diphenyl-1-picrylhydrazyl (DPPH) powder was bought from Sigma Chemical Company (St. Louis, MO, USA). Furthermore, sodium carbonate powder, Folin–Ciocalteu reagent, and gallic acid powder were purchased from Merck Company (Darmstadt, Germany). Also, ethanol with purity of 96% was purchased from Sina Fariman (Khorasan Razavi, Iran).
Sample preparation
Fresh lemon (variety of Citrus latifolia) with around 3.5 cm in diameter were purchased from a local grocery store in Shahreza, Isfahan, Iran. After washing the lemon with tap water, extraction of juice was performed with a semi-industrial juicer (Iran Saleh, Isfahan, Iran) and the cores in the lemon waste were removed manually. The lemon waste was dried in a hot-air convective dryer (Made in Iran) at the temperature of 40 °C and the air velocity of 1.5 m/s until the moisture content of 6.1 ± 0.2% was obtained. Then, the dried waste was powdered by a domestic mill (Model MJ-176 NR, National, Osaka, Japan). In order to achieve a uniform particle size powder, the powder was passed through a 35-mesh sieve. Finally, dried lemon powder was packaged in polyethylene bags and stored in a refrigerator (Model 81,739 Munchen, Germany) at − 18 °C until usage.
EHD set-up
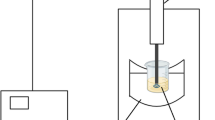
The EHD set-up, shown in Fig. 1, was employed in this study to extract phenolic compounds from lemon waste. It was consisted of a high-voltage power supply (Model HV50P OC, Fanavaran Nano-Meghyas, Tehran, Iran), a 19 cm × 23 cm stainless steel plate as the grounded electrode and 5 copper wires as the discharge electrode with a diameter of 1.2 mm. In this system, the space between the two neighboring wires was 4 cm and the electrode gap) distance between the wires and plate electrode(was adjusted at 7 cm.
EHD extraction method
In this study, phenolic compounds were extracted from lemon waste by the high voltage electric discharge process which was described in the previous section. For this purpose, 10 g of dried lemon waste powder was added to 100 ml distilled water solvent in an aluminum dish with the dimensions of 16 cm × 16.5 cm × 2 cm. The aluminum dish containing lemon waste and distilled water solvent was put in the grounded electrode of EHD system and extraction process was performed at various EHD time of 10, 20, and 30 min and various voltage of 0, 15 and 19 kV. At the end of EHD extraction process, the mixture was filtered by a sheet filter paper (Grade C75, Dorsan, Spania). Finally, the filtered extract was poured into a 50 ml falcon tube and was processed by a centrifuge (Model 2-16P, Sigma, Germany) at 7800 rpm for a period of 12 min to separate very small particles from it [6].
Physicochemical analysis
Determination of total phenolic content
Total phenolic content of lemon waste extract was measured by Folin–Ciocalteu reagent. To perform this test, 125 µl lemon extract, 500 µl distilled water, and 125 µl of Folin–Ciocalteu indicator were manually shaken for 15 s in a test tube. After that, the test tube was put in the dark for 6 min. Next, 1.25 ml sodium carbonate solution with concentration of 7% and 1 ml distilled water were added to the test tube and the solution was manually shaken. The tube was kept for 90 min in a dark place at laboratory temperature of 25 °C and finally the absorbance of the mixture was read at the wavelength of 760 nm using a UV–Vis spectrophotometer (Model T70, PG Instruments, Leics, United Kingdom). The total phenolic content is commonly stated based on gallic acid. To obtain the gallic acid standard curve, 100–600 ppm gallic acid solutions were prepared to obtain their absorbance and the standard curve. In Eq. (1), the absorption of the extract was replaced as Y parameter to measure the X parameter (total phenolic content of lemon waste extract in ppm on the basis of gallic acid) [6]:
Determination of antioxidant activity
The antioxidant activity of extracts was determined as free radical-scavenging activity of 2, 2- diphenyl-1-picrylhydrazyl (DPPH) based on the procedure proposed by [8]. For this test, 1 ml extract and 1 ml DPPH ethanol solution with concentration of 200 ppm was poured into a test tube and the mixture was manually shaken for 3 s. Next, the mixture was placed for 20 min in a dark place at temperature of 25 °C. Finally, the absorbance of the mixture was measured by the UV–Vis spectrophotometer (Model T70, PG Instruments, Leics, United Kingdom) at a wavelength of 517 nm. The absorbance of 200 ppm DPPH ethanol solution without any extract (as a control) was read on the same wavelength. The antioxidant activity in terms of inhibition percentage of DPPH was evaluated according to Eq. (2) [20].
In Eq. 2, Ac is the absorbance of control and Ae is the absorbance of DPPH solution containing the extract.
Determination of extraction yield
For this assay, the extract was poured into a round-bottom flask and the water solvent was removed from the extract by a rotary vacuum evaporator (Model RV 10B, IKA, Germany) at the temperature of 70 °C and the rotational speed of 170 rpm. Then, the sample was put in an oven (Model E.O 155, Iran) with the temperature of 90 °C to evaporate the remained solvent. The extraction yield was measured using Eq. (3) [6]:
In this equation, W0 is the initial weight of the LPWP (kg), W2 is the weight of the flax containing the remained solid after solvent evaporation (kg), and W1 is the weight of empty flask (kg).
Determination of color parameters of L* and b*
To estimate the L* and b* color parameters of the lemon extracts, 14 ml of extract was poured into a glass plate (with the diameter of 8 cm and the depth of 1.4 cm) and the color parameters were assessed by a colorimeter (Model TES-135A, Taiwan). It is necessary to mention that the L* color parameter is in the range of 0 (blackness) to 100 (whiteness). Also, the b* color parameter is in the range of + 60 to − 60. The + b* color parameter indicates yellowness and the − b* color parameter indicates blueness [21].
Fourier transform infrared (FT-IR) spectroscopy
FT-IR spectroscopy was used to identify the functional groups of the extracts and to investigate the possibility of destruction of these groups during the extraction processes. For this purpose, the solvent of the extracts was evaporated using the rotary vacuum evaporator (based on part 2.5.3) followed by using an oven (Model E.O 155, Iran) at temperature of 40 °C. After that, a pellet containing the mixture of dried LPWP extract and potassium bromide (KBr) (1:50 w/w) was prepared. Finally, the pellet was placed in a FT-IR spectrophotometer (Model spectrum 65, Norwalk, Connecticut, PerkinElmer, U.S.A) and the spectrum of the extract was recorded in the spectral region of 450–4000 cm− 1 [9].
Scanning electron microscopy (SEM) images
In order to study the LPWP microstructure changes during the extraction processes, SEM images of the LPWP without applying any extraction processes and the LPWP after three selective extraction treatment (EHD voltage of 19 kV—EHD time of 30 min, EHD voltage of 19 kV—EHD time of 10 min, and EHD voltage of 0 kV—EHD time of 10 min) were taken by a scanning electron microscope (Model LEO 435VP, LEO Electron Microscopy Ltd., Cambridge, England). It should be noted that to provide the SEM images, a fine layer of gold was put on the surface of samples using a gold sputter coating machine (Model AGAR, Sputter Coater, U.K.). In order to prepared SEM images, scanning electron microscope voltage was 18 kV and images were provided with magnification of 150 times [22].
Statistical analysis
In this paper, a factorial experiment in a completely randomized design (CRD) was used to investigate the effect of two factors of EHD voltage in three levels of 0, 15 and 19 kV and EHD time in three levels of 10, 20 and 30 min and their mutual effect on the dependent variables of total phenolic content, antioxidant activity, extraction yield, and color parameters of L*and b*. For this aim, Analysis of Variance (ANOVA) and correlation of dependent variables were conducted using the SPSS software version 21. The results in this study were reported as mean ± standard error (SE).
Results and discussion
Total phenolic content
As shown in Table 1, EHD time (P ≤ 0.05) and EHD voltage (P ≤ 0.01) have significant effects on total phenolic content of extracts (P ≤ 0.05) while their mutual effect does not have significant effect on total phenolic content of the extracts (P < 0.05). As shown in Fig. 2a by increasing the EHD time from 10 to 30 min, the total phenolic content of extracts has a significant increase (P ≤ 0.05) from 426.49 ± 15.71 ppm to 469.17 ± 12.88 ppm (equivalent to 10%). Appling EHD process results in the formation of corona wined which disrupt cell membranes, destruct cells, and release the phenolic compounds from cells to the extraction solvent [9]. Therefore, prolonging EHD time from 10 to 30 min intensifies the destruction of cell walls and enhances the mass transfer and extraction of phenolic compounds. Roselle-Soto et al. investigated the extraction of phenolic compounds and protein from olive kernel using the high voltage electrical discharges process. These researchers stated that using high voltage electrical discharges leads to fragmentation of olive kernel powder and facilitates the extraction of phenolic compounds [23]. This result is in good agreement with the present study. As Fig. 2b shows, with increase in EHD voltage from 0 to 19 kV, the amount of total phenolic content of extracts has a significant increase (P ≤ 0.01) from 419.69 ± 11.28 ppm to 474.62 ± 9.56 ppm (equivalent to 13.09%). This result was in acceptable agreement with that reported by Xi et al., who studied the extraction of phenolic content from pomegranate peel using high voltage electrical discharge. These researchers stated that the electric field intensity had a significant effect on the yield of extracted phenolic content [24]. Also, Boussetta et al. evaluated the extraction of phenolic compounds and antioxidants from grape pomace using the high voltage electrical discharges process. They reported that by increasing the EHD energy input into sample and solvent mixture, extraction of phenolic content increased because of the increase of the cell membrane damage [25]. Figure 2c illustrates the mutual effect of EHD voltage and EHD time on total phenolic content. As Fig. 2c shows the highest amount of total phenolic content equaled to 496.61 ± 6.92 ppm, observed at EHD time of 30 min and EHD voltage of 19 kV and the lowest amount of total phenolic content was related to 399.88 ± 35.58 ppm, observed at EHD time of 10 min and EHD voltage of 0 kV. These two treatments do not have statistically significant difference with each other and with other treatments in terms of the total phenolic content response (P > 0.05).
Antioxidant activity
Table 1 indicates that both independent variables of EHD time and EHD voltage have significant effects on the antioxidant activity of extracts (P ≤ 0.01), while their mutual effect does not have significant effect (P > 0.05) on this dependent variable. According to Fig. 3a, the antioxidant activity of the extracts has a significant decline from 88.06 ± 0.60% to 84.16 ± 0.91% with an EHD time growth from 10 to 30 min, respectively (P ≤ 0.01). In explaining this result, it can be indicated that by increasing the EHD processing time, the energy input from EHD system to the sample increases which leads to the damage and destruction of some antioxidant compounds of the extracts [26]. Roselle-Soto et al. stated that electrical discharge at high energy input produces free reactive radicals and chemical products through electrolysis, which can result in the destruction of some compounds with antioxidant activity properties [26]. Reducing antioxidant activity at EHD time of 30 min compared to EHD time of 10 min can be due to long exposure time of the extracted compounds to oxygen and other environmental oxidizing agents [6]. According to Fig. 3b, when EHD voltage increases from 0 to 19 kV, the amount of antioxidant activity of the extracts increases from 83.99 ± 0.92% to 88.29 ± 1.01% (equivalent to 5.12%), respectively (P ≤ 0.01). This result can be ascribed to an increase in the diffusion of phenolic compounds with antioxidant properties to the solvent by increasing the EHD voltage (Fig. 2b). Figure 3c indicates that the mutual effect of EHD time and EHD voltage on the antioxidant activity of extracts. As can be seen in this figure, treatment of 10 min EHD time- EHD voltage of 19 kV had the highest antioxidant activity equivalent to 89.76 ± 0.29 ppm and treatment of 30 min EHD time- EHD voltage of 0 kV had the lowest antioxidant activity equivalent to 81.69 ± 0.02 ppm. It should be noted that two mentioned treatments do not have significant difference with each other and with other treatments in terms of the antioxidant activity response (P > 0.05).
Extraction yield
As can be seen in Table 1, the effect of two independent variables of EHD time (P ≤ 0.001) and EHD voltage (P ≤ 0.01), and the mutual effect of these variables on extraction yield of LPWP is significant. As Fig. 4a reveals, the extraction yield of the extracts has a significant increase from 33.50 ± 0.95% to 37.00 ± 0.51% with increase in the EHD time from 10 to 30 min (equivalent to 10.44%), respectively (P ≤ 0.05). The disruption of cell walls and the release of intracellular compounds such as phenolic compounds increase at 30 min EHD time due to the increasing effect of EHD process on the sample. This result is in good agreement with the Shahram and Dinani (2019) who found that the extraction efficiency of orange pomace significantly increased with increasing EHD time from 2 to 10 min [9]. Table 2 shows a positive correlation (P ≤ 0.001) and thus a significant direct relationship between the total phenolic content and antioxidant activity of the extracts. Figure 4b illustrates that, the extraction yield of the extracts has a significant growth from 34.00 ± 1.12% to 36.33 ± 0.66% (equivalent to 6.85%) by increasing the EHD voltage from 0 to 19 kV, respectively (P ≤ 0.01). The results indicate that increasing the EHD voltage and thus increasing electric field intensity have led to further destruction of the cell wall and increased extraction yield of the extracts [9]. Figure 4c indicates the mutual effect of EHD time × EHD voltage on the extraction yield parameter. As can be observed in this figure, the treatment of 30 min EHD time- EHD voltage of 19 kV (treatment with maximum EHD time and voltage) has the highest extraction yield equivalent to 38.00 ± 1.00% and the treatment of 30 min EHD time- EHD voltage of 0 kV had the lowest extraction yield equivalent to 36.00 ± 0.50%. These two mentioned treatments have statistically significant difference with each other.
Color parameters
Lightness-darkness color parameter (L*)
Table 1 demonstrates that the effects of EHD time (P ≤ 0.01) and EHD voltage (P ≤ 0.05) on the L* color parameter of extracts was statistically significant. However, the mutual effect of these two independent parameter on the L* color parameter of extracts was not statistically significant (P > 0.05). As can be seen in Fig. 5a, with the increase of EHD time from 10 to 30 min, the L* color parameter of the extracts declined significantly from 18.19 ± 0.26 to 16.72 ± 0.15 (equivalent to 8.08%), respectively (P ≤ 0.01). The reduction of the L* color parameter of extracts by changing EHD time from 10 to 30 min can be attributed to the oxidation of compounds in the extracts and reduction of antioxidant activity of the extracts (Fig. 3a). Table 2 confirmed positive correlation and direct relationship of the two parameters of the L* color parameter and antioxidant activity (P > 0.05). Tiwari et al. (2010) studied the effect of sonication treatment on anthocyanins and color of red grape juice. They stated that more oxidation occurs as the sonication time increases and it results in darkness of the extract and reduction of the L * color parameter [27]. This result is consistent with the results achieved in this part of our study. According to Fig. 5b with the EHD voltage increase from 0 to 15 kV, the L* color parameter of extracts was significantly improved from 16.89 ± 0.24 to 17.92 ± 0.35 (equivalent to 6.1%), respectively (P ≤ 0.05) and by increasing the EHD voltage from 15 to 19 kV, it was significantly lessened from 17.92 ± 0.23 to 17.51 ± 0.23 (P > 0.05). The increase of the L* color parameter with changing the EHD voltage from 0 to 15 kV can be ascribed to an intensification of the extracted antioxidant compounds during the EHD extraction process (Fig. 3b). Figure 5c illustrates the mutual effect of EHD time and EHD voltage on the L* color parameter of the extracts. In this figure, the lowest datum of the L* color parameter (16.35 ± 0.24) is related to 30 min EHD time—EHD voltage of 0 kV and the highest datum of that (18.84 ± 0.28) is related to 10 min EHD time—EHD voltage of 15. These two mentioned treatments do not show significant difference with each other and with other treatments in terms of L* color parameter (P > 0.05).
Blueness–yellowness color parameter (b*)
Table 1 shows that EHD time and EHD voltage had significant effects on the b* color parameter of the extracts (P ≤ 0.05). However, the mutual effect of them on the b* color parameter of the extracts was not statistically significant (P > 0.05). According to Fig. 6a, by changing the EHD time from 10 to 20 min, the average value of b* color parameter had a significant decrease (P ≤ 0.05) from 1.50 ± 1.44 to 0.96 ± 0.14 (equivalent to 36%), respectively and by changing the EHD time from 20 to 30 min, the average value of b* color parameter had an insignificant increase (P > 0.05. According to Fig. 6b, with the increase of the EHD voltage from 0 to 19 kV, the average value of the b* color parameter of the extracts significantly increased from 0.86 ± 0.14 to 1.52 ± 0.19 (equivalent to 76.74%), respectively (P ≤ 0.05). Totally, the results indicate that with the increase of the EHD voltage from 0 to 19 kV, the trend of color of the extracts increased to the yellow color. This is probably due to the increased extraction yield (Fig. 4b) and specially increased extraction of carotenoid pigments with a strong antioxidant property (Fig. 3b) with the increase of the EHD voltage [28]. According to Table 2, the highly significant positive correlation (P ≤ 0.001) between the b* color parameter and antioxidant activity of the extracts can confirm this hypothesis. Figure 6 C illustrates the mutual effect of EHD time and EHD voltage on the b* color parameter of the extracts. According to this figure, the lowest average of the b* color parameter is associated with 30 min EHD time without applying EHD process (0.58 ± 0.275) and the highest average of that (1.72 ± 0.369) is associated with 10 min EHD time- EHD voltage of 19 kV. These two treatments do not have statistically significant difference with each other and with other treatments in terms of b* color parameter response (P > 0.05). It can be interpreted that with increasing voltage from 0 to 19 kV, the amount of released polyphenol and antioxidant compounds increased, thus the b* color parameter is increased.
FT-IR spectroscopy
FT-IR spectroscopy was carried out to identify the characteristics of the functional groups of three selective treatments of EHD time of 30 min—EHD voltage of 19 kV, EHD time of 10 min—EHD voltage of 19 kV, and EHD time of 10 min—EHD voltage of 0 kV. It was also used to investigate the possible changes of these groups during extraction of EHD process with different times and voltages. As can be seen in Fig. 7, the FT-IR spectra obtained from all three treatments overlapped each other. Therefore, by comparing the FT-IR spectra, it can be concluded that application of the EHD process at two times of 10 min and 30 min and at voltage level of 19 kV in order to extract the phenolic compounds from lemon waste compared to maceration treatment for 10 min without EHD process (voltage of 0 kV) did not lead to any alteration in the functional groups of the extracts. It is necessary to mention that in this figure, the peak of 610.62 cm−1 represents CH=CH bending [20], the peak of 779.16 cm−1 can be related to C=C cis out-of-plan in the carotenoids [29], and the peak of 816.40 cm−1 is the sign of R-CH=CH-R [30]. In addition, the peak of 1053.11 cm−1 can be related to PH2 band in phosphines [9], the peak of 1231.27 cm−1 represents C–C ether stretching [31], and the peak of 1408.12 cm−1 can be attributed to the C–H bending [32]. The peak of 1619.79 cm−1 represents C=O bending [33], the clear peak of 1723.56 cm−1 shows the carbonyl C=O stretching of protonated carboxylic acids [6], the peak of 2937.20 cm−1 indicates CH2 symmetric stretch of methyl group [9], and the peak of 3422.57 cm−1 represents O–H bending [6].
SEM images
To investigate the microstructure changes of LPWP during the extraction process, the SEM images of the initial and untreated LPWP and the dried residues after three selected treatments of EHD time of 10 min—EHD voltage of 0 kV, EHD time of 10 min—EHD voltage of 19 kV, and EHD time of 30 min—EHD voltage of 19 kV were prepared and presented in Fig. 8a–d, respectively. It can be seen in this figure that the LPWP without applying any extraction process (Fig. 8a) compared to the EHD time of 10 min—EHD voltage of 0 kV (Fig. 8b), EHD time of 10 min—EHD voltage of 19 kV (Fig. 8c), and EHD time of 30 min—EHD voltage of 19 kV (Fig. 8d) has a smooth surface without any porosity. Comparison of two treatments of the EHD time of 10 min—EHD voltage of 0 kV (Fig. 8b) and the EHD time of 10 min- EHD voltage of 19 kV (Fig. 8c) shows that using the EHD process (Fig. 8c) resulted in considerable damage in the cell walls of the sample and deep cavities in the sample. Comparison of two treatments of the EHD time of 10 min—EHD voltage of 19 kV (Fig. 8c) with the EHD time of 30 min—EHD voltage of 19 kV (Fig. 8d) indicates that longer EHD processing time led to further disintegration and deep cavities in the sample (Fig. 8d). Based on the images presented in Fig. 8, it can be concluded that the use of the EHD process and application of longer EHD extraction time leads to further destruction and collapse of the cell walls of sample and also increase porosity of the LPWP. Similar result was reported by Shahram and Taghian Dinani (2019) who stated that increasing the EHD time from 2 to 10 min led to more pores and rupture in the cell structure of the remaining air-dried solids of orange pomace powder [9].
Scanning electron microscopy images of a untreated lemon waste powder, b the remained solids after treatment with Extraction time of 10 min—EHD voltage of 0 kV, c the remained solids after treatment with EHD time of 10 min—EHD voltage of 19 kV, and d the remained solids after treatment with EHD time of 30 min—EHD voltage of 19 kV
Conclusions
In this research, EHD process was used to extract phenolic compounds from lemon processing waste powder (LPWP) and the effects of the EHD time (in three levels of 10, 20 and 30 min) and EHD voltage (in three levels of 0, 15 and 19 kV) as well as their mutual effect were studied. The most important conclusions obtained in this research include:
Increasing the EHD voltage from 0 to 19 kV resulted in a significant growth of the phenolic content (P ≤ 0.01), antioxidant activity (P ≤ 0.01), extraction yield (P ≤ 0.01) and color parameters of L* and b* (P ≤ 0.05) of the extracts.
Changes the EHD time from 10 to 30 min resulted in a significant intensification of phenolic content (P ≤ 0.05) and extraction yield (P ≤ 0.001) and significant fall of the antioxidant activity (P ≤ 0.01), extraction yield (P ≤ 0.01) and color parameters of L* (P ≤ 0.01) and b* (P ≤ 0.05) of the extracts.
The increase of the EHD voltage from 0 to 19 kV and the increase of the EHD time from 10 to 30 min did not disturb the functional groups of FT-IR spectra of the extracts, but they led to greater destruction and porosity in microscopic structure of the LPWP.
Considering the results of our research, it can be proposed that the EHD extraction process is capable to extract phenolic compounds from agricultural wastes such as lemon waste to produce products with great economic value and also lessen agricultural waste and environmental problems.
References
J. Azmir, I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, A.K. Omar, J. Food Eng. 117, 426–436 (2013)
M. Mohanapriya, L. Ramaswamy, R. Rajendran, Int. J. Ayurvedic Herb. Med. 1, 1095–1100 (2013)
G. Oboh, T.A. Olasehinde, A.O. Ademosun, J. Oleo Sci. 63, 373–381 (2014)
C.B. Jalkute, S. Shivaji, Pharmacol. Toxicol. 3, 119–122 (2011)
S. Sadat, F. Janati, H.R. Beheshti, Gida. 37, 267–271 (2012)
H. Shahram, S.T. Dinani, M. Amouheydari, J. Food Meas. Charact. 13, 487–498 (2019)
E. De Marco, M. Savarese, A. Paduano, R. Sacchi, Food Chem. 104, 858–867 (2007)
H. Shahram, S. Taghian Dinani, J. Food Process Eng. 42, e13042 (2019)
H. Shahram, S.Taghian Dinani, LWT. 111, 23–30 (2019)
S. Dey, V.K. Rathod, Ultrason. Sonochem. 20, 271–276 (2013)
N. M’hiri, I. Ioannou, M. Ghoul, N.M. Boudhrioua, Food Rev. Int. 30, 265–290 (2014)
N. Boussetta, E. Vorobiev, T. Reess, A. De Ferron, L. Pecastaing, R. Ruscassié, J.L. Lanoisellé, Innov. Food Sci. Emerg. Technol. 16, 129–136 (2012)
N. Boussetta, E. Vorobiev, Comptes Rendus Chim. 17, 197–203 (2014)
S. Taghian Dinani, M. Havet, Ind. Crops Prod.70, 417–426 (2015)
S. Taghian Dinani, N. Hamdami, M. Shahedi, M. Havet, D. Queveau, Food Bioprod. Process. 95, 83–95 (2015)
N. Boussetta, J.-L. Lanoisellé, C.V.E. Bedel-Clotour, J. Food Eng. 95, 192–198 (2009)
N. Boussetta, M. Turk, C. De Taeye, Y. Larondelle, J.L. Lanoisellé, E. Vorobiev, Ind. Crops Prod. 49, 690–696 (2013)
J.R. Sarkis, N. Boussetta, C. Blouet, I.C. Tessaro, L.D.F. Marczak, E. Vorobiev, Innov. Food Sci. Emerg. Technol. 29, 170–177 (2015)
O. Parniakov, F.J. Barba, N. Grimi, N. Lebovka, E. Vorobiev, Food Res. Int. 65, 337–343 (2014)
F. Khalili and S. Taghian Dinani, J. Food Meas. Charact.12, 974–981 (2018)
M. Mirheli and S. Taghian Dinani, J. Food Meas. Charact.12, 1818–1828 (2018).
S. Rahimpour, S. Taghian, J. Food Meas. Charact. 12, 2394–2403 (2018)
E. Roselló-Soto, F.J. Barba, O. Parniakov, C.M. Galanakis, N. Lebovka, N. Grimi, E. Vorobiev, Food Bioprocess Technol. 8, 885–894 (2015)
J. Xi, L. He, L. Yan, Food Chem. 230, 354–361 (2017)
N. Boussetta, E. Vorobiev, V. Deloison, F. Pochez, A. Falcimaigne-Cordin, J.L. Lanoisellé, Food Chem. 128, 364–370 (2011)
E. Roselló-Soto, C.M. Galanakis, M. Brnčić, V. Orlien, F.J. Trujillo, R. Mawson, K. Knoerzer, B.K. Tiwari, F.J. Barba, Trends Food Sci. Technol. 42, 134–149 (2015)
B.K. Tiwari, A. Patras, N. Brunton, P.J. Cullen, C.P.O. Donnell, Ultrason. Sonochemistry. 17, 598–604 (2010)
A.L. Curl, J. Food Sci. 27, 171–176 (1962)
J. Ye, L. Feng, J. Xiong, Y. Xiong, J. Food Sci. Technol. 46, 2131–2136 (2011)
R. Konwarh, S. Pramanik, D. Kalita, C.L. Mahanta, N. Karak, Ultrason. Sonochem. 19, 292–299 (2012)
J.J. Chavan, D.M. Ghadage, P.R. Kshirsagar, S.S. Kudale, J. Lip. Chromatogr. Relat. Technol. 38, 963–969 (2015)
R.M. Bunghez, I.R. Raduly, M. Doncea, S. Aksahin, I. Ion, Dig. J. Nanomater. Biostruct. 6, 1349–1356 (2011)
T. Rajan, S. Muthukrishnana, Asian J. Pharm Clin. Res. 6, 271–273 (2013)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maher, M., Taghian Dinani, S. & Shahram, H. Extraction of phenolic compounds from lemon processing waste using electrohydrodynamic process. Food Measure 14, 749–760 (2020). https://doi.org/10.1007/s11694-019-00323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00323-0