Abstract
In the present study, the extraction of β-carotene from carrot processing waste was studied using the combination of ultrasonic pretreatment and shaking incubation process. In this regard, the effects of ultrasonic time (in three levels of 0, 40, 80 min) and shaking incubation time (in three levels of 30, 75 and 120 min) as well as the mutual effects of these two variables on dependent responses of β-carotene content, antioxidant activity, and color parameters of L*, a* and b* of the extracts containing β-carotene pigment were investigated. The results of this study showed that the increase of ultrasonic time from 0 to 80 min caused the significant increase of β-carotene (P ≤ 0.001), antioxidant activity (P ≤ 0.01), a* color parameter (P ≤ 0.01), and b* color parameter (P ≤ 0.001) of the extracts. Furthermore, the increase of shaking incubation time from 30 to 120 min led to the significant increase of β-carotene content of extracts (P ≤ 0.001). Fourier Transform Infrared spectroscopy did not show any destruction of functional groups of the extracts. In addition, scanning electron microscopy images indicated more porous structure of solid residues in condition of the increase of ultrasonic and shaking incubation times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Color is one of the main features of food. It plays an important role in the initial recognition of food products, perception of consumer from the quality of food products and even the recognition of food spoilage. Production and consuming a considerable number of artificial colors with chemical origin in food products cause detrimental effects on the environment and may lead to allergy, food poisoning and carcinogenesis for human. Thus, the avoidance of consumers from foods containing artificial colors forces the food industry to replace them with natural pigments [1, 2].
Carotenoids are one of the main groups of natural pigments in the food industry [3]. These compounds play an important role in the avoidance of lung, skin, bladder and intestinal cancer, cataracts and heart diseases [4]. Among carotenoids, the yellow–red pigment of β-carotene has a special place for manufacturers and consumers of food products [5]. This pigment is the important precursor of vitamin A which cannot be made in the human body. β-carotene pigment can be widely used in various business sectors like food, pharmaceutical and chemical industries [6].
Researchers have been attracted to extract natural β-carotene pigment from various raw plant materials especially waste agricultural products [7]; because every year a large part of agricultural wastes is disposed as garbage all over the world [8]. Therefore, by using agricultural wastes to manufacture natural pigments like β-carotene, the conservation of environment from the harmful effects of wastes is possible [9]. It is worth nothing to mention that among vegetables, carrot has the highest β-carotene content. Carrot waste as an economic agricultural byproduct with abundant production all over the world can be used as a natural source for the extraction of β-carotene pigment [10].
In order to extract β-carotene from sources like waste agricultural products, different methods can be used. Shaking incubation is a conventional extraction method. In this method, after mixing the solid sample with the intended solvent, the extraction is done inside the shaker incubator with the specified temperature, time and shaking speed [11]. Shaking process causes full contact of solvent with the solid phase. Other advantages of this method are simple equipment application and extraction of various samples at the same time [12]. Despite the mentioned advantages, this method has some limitations, such as low efficiency and environmental pollution caused by the use of relatively large amounts of organic solvent. In order to overcome these problems and limitations, combining this technique with other extraction methods such as ultrasonic process has been recommended [11].
Ultrasonic waves include waves with a frequency greater than human hearing but lower than microwave frequencies from 10 kHz up to 20 MHz. The main mechanism of ultrasonic is related to the cavitation phenomenon. During the ultrasonic process, high shear forces are obtained through the collapse of cavitation bubbles which leads to break the cell walls and then the cell content is released in the solvent. Ultrasonic process can increase the speed of extraction and reduce the temperature and solvent volume. Thus, it is very useful for extraction of heat-sensitive compounds [13,14,15].
In the present study, β-carotene was extracted with a combination of ultrasonic pre-treatment and the shaking incubation process. The aim of this study was to evaluate the effect of three levels of ultrasonic time (0, 40 and 80 min) and three levels of shaking incubation time (30, 75 and 120 min), as well as their mutual effects on the dependent variables of β-carotene content, antioxidant activity and color parameters of L*, a* and b*. Fourier Transform Infrared (FT-IR) spectroscopy was used to explore the possibility of destruction of functional groups of the extracts. Furthermore, scanning electron microscopy (SEM) was used for investigation of probable microscopic changes of carrot processing waste during extracting treatments.
Materials and methods
Chemicals and reagents
In this study, the standard of β-carotene > 97%, potassium bromide (KBr), and ethanol were obtained from the Merck Company (Darmstadt, Germany). Furthermore, the powder of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) was obtained from the Sigma Chemical Company (St. Louis, MO, U.S.A).
Sample preparation
Carrots were purchased from a local grocery store in Isfahan, Iran. The prepared carrots were first washed and then were drained off to remove their surface water. Next, the carrot juice was squeezed by a domestic juicer (National, Osaka, Japan). The solid residues were dried by a convective drier (Made in Iran) at 40 °C temperature for 72 h to obtain the constant moisture content (5.83 ± 0.24%). After that, the dried residues were ground by a domestic mill (Moulinex, Paris, France). Then, the grinding particles were passed through a 20-mesh sieve to make the powder with uniform and desired particle size. Finally, the resulting homogeneous powder was packaged in polyethylene plastic zipper bags and was maintained in the freezer at temperature of − 18 °C prior to later use.
Extraction method
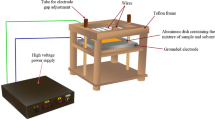
In this study, the combined extraction process of β-carotene pigment from carrot waste was first conducted by an ultrasonic bath (Model 300, Pulse, Italy) and then by a shaker incubator (Model KM C 65, Fan Azma Gostar, Tehran, Iran). For this purpose, first 1.5 g of dried carrot waste powder was mixed with 100 ml ethanol solvent in a 100 ml glass beaker. Then, the beaker containing the mixture of sample and solvent was processed within ultrasonic bath at the temperature of 50 ± 5 °C [7] and at a frequency of 20 kHz for the duration of 40 or 80 min. Obviously, treatments with 0 min ultrasonic time did not have ultrasonic pretreatment. After ultrasonic pretreatment, the sample was placed in the shaker incubator for a desired time of 30, 75 or 120 min at the rotational speed of 150 rpm and at the temperature of 30 ± 2 °C [16]. Next, the resulting mixture was filtered by a Whatman paper (No. 40, England). Finally, the following mentioned tests were carried out on the extracts obtained from all treatments.
Physicochemical analysis
Determination of β-carotene content
In order to quantify the β-carotene content of the extracted juices, the absorption of extracts were read using UV–Vis spectrophotometer (Model T70, PG Instruments, Leics, United Kingdom) in the wavelength of 450 nm [17] and in the presence of ethanol as a control. The standard curve of β-carotene (with the equation of y = 0.0263x + 0.5824 and R2 = 0.9712) was obtained through drawing of absorptions (Asample) of extract at a wavelength of 450 nm as the y-axis and the concentrations of β-carotene content (from 15 to 65 ppm with intervals of 5 ppm) as the x-axis. Therefore, the amount of β-carotene content (ppm) was calculated in accordance with Eq. 1:
Determination of antioxidant activity
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) test was used to measure antioxidant activity of the extracts. For this test, 0.1 ml of the extract and 3.9 ml of the purple-colored solution of DPPH at a concentration of 40 ppm, was poured into a test tube. Then the test tube was shaken by hand for 15 s and maintained for a period of 3 h in a dark place at room temperature (25 ± 2 °C). After this time, the absorption of extract was measured by the UV–Vis spectrophotometer (Model T70, PG Instruments, Leics, United Kingdom) at a wavelength of 515 nm. The absorption of 40 ppm DPPH solution without any extract was also measured as a control on the same wavelength. The amount of antioxidant activity of extract in terms of percentage inhibition of DPPH was calculated according to Eq. 2 [18]:
In Eq. 2, the Atest is the absorption of DPPH solution containing the examined extract and Acontrol is the absorption of DPPH solution without any extract.
Determination of color
To assess the color of the extracts, 20 ml of extract was poured into a plate glass and color parameters of L*, a* and b* were measured by a colorimeter (TES-135A, Taiwan). It should be noted that the L* color parameter represents the lightness-darkness of color and is in the range of 0 (equivalent to black) up to 100 (equivalent to black). The a* color parameter indicates the red and green color of the sample and is placed in the range of − 60 to + 60. The + a* color parameter indicates redness and the − a* color parameter indicates greenness. In addition, the b* color parameter is in the range of − 60 to + 60. The + b* color parameter is equivalent to yellowness and the − b* color parameter is equivalent to blueness [19, 20].
Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectroscopy was used to gain more information about the functional groups of the extracts and examine the possibility of their damage due to extraction processes. To perform this test, initially a potassium bromide (KBr) disk was prepared. Then, 1 up to 2 drops of extract were poured on the KBr disk by capillary tube. The disk was placed in the FT-IR spectrophotometer (Model Spectrum 65, Norwalk, Connecticut, PerkinElmer, U.S.A) and the spectrum of the extract was obtained in the region of 450–4000 cm− 1 with a spectral resolution of 4 cm− 1 [21, 22].
Scanning electron microscopy (SEM) images
To study the possibility of microscopic changes in carrot waste structure during the examined extraction treatments, the photos of the dried residues after three treatments (0 min ultrasonic-30 min shaking incubation, 80 min ultrasonic-30 min shaking incubation, and 80 min ultrasonic-120 min shaking incubation) were taken by the Scanning Electron Microscope (Model EM3200, KYKY, Beijing, China) at an accelerating voltage of 26 kV. It should be noted that to provide the SEM images with magnification of 500 times, a thin layer of gold deposited on the surface of samples using a gold deposition device (Model SBC12, KYKY, Beijing, China). The images of three mentioned treatments were compared together and were compared with the image of carrot processing waste without applying any type of extraction processes on it.
Statistical analysis
In this study, a factorial experiment in a randomized complete block design (RCBD) was used to evaluate the effects of ultrasonic time in three levels of 0, 40 and 80 min and shaking incubation time in three levels of 30, 75 and 120 min, as well as the mutual effect of these two factors on the dependent variables of β-carotene content, antioxidant activity and color parameters of L*, a*, and b*. For this purpose, the pairwise Analysis of Variance (ANOVA) and correlation of dependent variables were conducted using the SAS software version 8. Furthermore, the comparison of means was performed with the Least Significant Difference (LSD) at the probability of 5% (P ≤ 0.05). The results reported in this study are in the form of mean ± standard error (SE).
Results and discussion
β-Carotene content
According to Table 1, the ultrasonic time had significant effect on the β-carotene content of the extracts (P ≤ 0.001). According to Table 2, with increase in ultrasonic time from 0 to 80 min, the β-carotene content of extracts had a significant increase (equivalent to 73.73%) from 30.07 ± 2.08 to 52.24 ± 1.27 ppm, respectively (P ≤ 0.001). It can be concluded that the application of ultrasonic pretreatment for 40 and 80 min for β-carotene extraction from carrot processing waste led to a dramatical increase of β-carotene content of extract compared to the non-use of ultrasonic pretreatment (0 min ultrasonic time). In the present study, the growth of the extracted β-carotene pigment by increasing the ultrasonic time from 0 to 80 min can be attributed to further destruction of the cell walls of sample, increase of the contact area between the solvent and contents of cells and increase of the mass transfer of β-carotene pigment to the solvent [15]. Sun et al. studied the effect of ultrasonic extraction time on the efficiency of β-carotene extraction from citrus peels. These researchers reported that with increasing the ultrasonic time from 20 to 120 min, the efficiency of β-carotene extraction significantly increased [23]. This study is similar to results obtained in the present study.
Table 1 shows that shaking incubation time had significant effect on β-carotene content of extracts (P ≤ 0.001). According to Table 3, by increasing the shaking incubation time from 30 to 120 min, the β-carotene content of extracts had a significant increase (P ≤ 0.001) from 40.17 ± 4.47 to 47.12 ± 2.78 ppm (equivalent to 17.3%). Hejazi and Wijffels, reported that the concentration of β-carotene increased from Dunaliella salina algae by increasing the incubation time [24]. According to Table 1 and Fig. 1, the mutual effect of ultrasonic time and shaking incubation time on the β-carotene content of extracts was significant (P ≤ 0.05). In accordance with Fig. 1, the 80 min ultrasonic-120 min shaking incubation treatment had the highest β-carotene content equivalent to 53.88 ± 2.61 ppm. In addition, the 0 min ultrasonic-30 min shaking incubation treatment had the lowest β-carotene content equivalent to 22.99 ± 3.38 ppm. It should be noted that the 0 min ultrasonic-30 min shaking incubation treatment had statistically significant difference with other treatments in terms of the β-carotene content (P ≤ 0.05). However, there was no significant difference (P > 0.05) in the β-carotene content of the 80 min ultrasonic-120 min shaking incubation, 40 min ultrasonic-120 min shaking incubation, 80 min ultrasonic-75 min shaking incubation, and 80 min ultrasonic-30 min shaking incubation treatments. However, the mentioned treatments had statistically significant difference in terms of the β-carotene content with other treatments (P ≤ 0.05).
Antioxidant activity
As can be seen in Table 1, the ultrasonic time had a significant effect on the antioxidant activity of extracts (P ≤ 0.01). According to Table 2, there was a 53.1% increase in the antioxidant activity of extracts (P ≤ 0.0) with the increase of ultrasonic time from 0 to 80 min (14.84 ± 1.14 and 22.72 ± 1.61% inhibition of DPPH, respectively). Although the average of antioxidant activity of extracts obtained by 80 min ultrasonic pretreatment is higher than that obtained by 40 min ultrasonic pretreatment, there is no significant difference in terms of the antioxidant activity between these two times (P > 0.05). The increase of the antioxidant activity of extracts from 0 to 80 min ultrasonic time (in accordance with Table 2) can be attributed to the increase of β-carotene content as a strong antioxidant in the extracts (Table 2). As Table 4 shows there is a significant positive correlation (P ≤ 0.001) between the two responses of β-carotene content and antioxidant activity and this matter confirms the significant direct relationship between these two responses.
According to Table 3, the antioxidant activity of extracts increased from 19.1 ± 1.64 to 20.43 ± 2.26% inhibition of DPPH by increasing the shaking incubation time from 30 to 120 min, respectively. However, Tables 1 and 3 show that the shaking incubation time did not have significant effect on the antioxidant activity of extracts (P > 0.05). With attention to the increase in β-carotene content of extracts from 30 to 120 min of shaking incubation time (in accordance with Table 3), the increase of the antioxidant activity of extracts by increasing the shaking incubation time during this period can be explained. Yen et al. examined the antioxidant activity of the peanut seed testa extract by using shaking incubation extraction method. They concluded that the antioxidant activity increased because of the increase of extraction of phenolic compounds [25].
According to Table 1 and Fig. 2, the mutual effect of the ultrasonic time and shaking incubation time on the antioxidant activity of extracts was not statistically significant (P > 0.05). Although the most antioxidant activity of extracts (24.28 ± 2.24% inhibition of DPPH) was related to the 40 min ultrasonic-120 min shaking incubation treatment and the lowest average of the antioxidant activity of extracts (13.32 ± 4.38% inhibition of DPPH) was related to the 0 min ultrasonic-120 min shaking incubation treatment, these two treatments did not have statistically significant difference with each other and with other treatments in terms of the antioxidant activity response (P > 0.05).
Color properties
L* color parameter
Table 1 shows that the ultrasonic time had a significant effect on the L* color parameter of extracts (P ≤ 0.01). According to Table 2, with the increase of the ultrasonic time from 0 to 40 min, the average of the L* color parameter of extracts significantly decreased (equivalent to 3.13%) from 25.56 ± 0.2 to 24.76 ± 0.13, respectively (P ≤ 0.01). In addition, by increasing the ultrasonic time from 40 to 80 min, it significantly increased (equivalent to 2.38%) from 24.76 ± 0.13 to 25.35 ± 0.21, respectively (P ≤ 0.01). According to Table 2, L* color parameter decrease of extracts from 0 to 40 min of ultrasonic time can be attributed to the severe increase of β-carotene content of extracts (60.49% increase) during this period of ultrasonic time. Ginting stated that the increase of the β-carotene content of extract led to decrease of the L* color parameter [26]. The reason of escalation of the L* color parameter of extracts from 40 to 80 min of ultrasonic time, can be caused by oxidation of compounds in extracts (except β-carotene) in this period of ultrasonic time. According to Table 2, the increase in the β-carotene content of extracts in the range of 40 up to 80 min of the ultrasonic time, is only 8.25%, and so the effect of reduction of the L* color parameter by increasing the β-carotene content became pale, but the effect of increasing the L* color parameter by the oxidation process during this period was much more intense. Table 4 also illustrates that the L* color parameter had a significant negative correlation with the antioxidant activity parameter (P ≤ 0.01). This matter shows that whatever the oxidation of the antioxidant compounds in extract was higher, the antioxidant activity of extract was less and therefore the L* color parameter increased.
As can be seen in Table 1, the effect of the shaking incubation time on the L* color parameter of extracts was very significant (P ≤ 0.01). Table 3 shows that by increasing the shaking incubation time from 30 to 75 min, the average of the L* color parameter of extracts had significant decrease (equivalent to 2.93%) from 25.55 ± 0.19 to 24.8 ± 0.19, respectively (P ≤ 0.01). However, with increasing the shaking incubation time from 75 to 120 min, the average of the L* color parameter of extracts had significant increase (equivalent to 2.06%) from 24.8 ± 0.19 to 25.31 ± 0.19, respectively (P ≤ 0.01). The reason of reduction in the L* color parameter of extracts by increasing the shaking incubation time from 30 to 75 min can be attributed to increase of the β-carotene content of extracts by increasing the shaking incubation time from 30 to 75 min (in accordance with Table 3). According to Table 3, with the increase of the shaking incubation time from 30 to 75 min and from 75 to 120 min, the averages of the β-carotene content of extracts increased 7.72 and 8.9%, respectively. Thus, it can be concluded that the increase in the β-carotene content of extracts in ranges of 30 up to 75 min and 75 up to 120 min was fairly analogous. However, the stronger oxidation of compounds which were effective on the L* color parameter of extracts was expected to happen at a longer shaking incubation time from 75 up to 120 min and it led to increase the L* color parameter of the extracts.
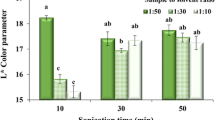
According to Table 1 and Fig. 3, the mutual effect of the ultrasonic time and shaking incubation time on the L* color parameter of extracts was not statistically significant (P > 0.05). As can be seen in Fig. 3, the highest average of the L* color parameter related to the 0 min ultrasonic-30 min shaking incubation treatment equaled to 25.9 ± 0.41. According to Fig. 1, this treatment had the lowest average of the β-carotene content. In Fig. 3, also the lowest average of the L* color parameter related to 40 min ultrasonic-120 min shaking incubation treatment equaled to 24.61 ± 0.15. According to Fig. 1, this treatment had no significant difference (P > 0.05) with the 80 min ultrasonic-120 min shaking incubation treatment that had the highest average of the β-carotene content. Therefore, the argument considered in this section related to the inverse relationship of the β-carotene content of extracts and L* color parameter was justified another time.
a* color parameter
In this study, the measured values for the a* color parameter were negative for all treatments. As can be seen in Table 1, the ultrasonic time had a significant effect on the a* color parameter of extracts (P ≤ 0.01). Table 2 shows that with the increase of ultrasonic time from 0 to 80 min, the average of the a* color parameter increased significantly (equivalent to 50.51%) from − 0.97 ± 0.1 to − 0.48 ± 0.07, respectively (P ≤ 0.01). However, there was no significant difference in the mean values of the a* color parameter between the ultrasonic times of 40 and 80 min (P > 0.05). As already mentioned in Table 2, with the increase of the ultrasonic time from 0 to 80 min, the β-carotene content of extracts increased. Thus, the color of extracts tended to the more red color and the a* color parameter of extracts increased. It is interesting to note that Table 4 confirmed the significant positive correlation (P ≤ 0.01) of the two parameters of the a* color parameter and β-carotene content, as well. Okuno et al., also examined the extraction of antioxidants from sweet potato waste powder using supercritical carbon dioxide (CO2) and then reported the increase of the a* color parameter with increase of the β-carotene content [27]. The results of this research have a desirable correspondence with the results obtained in this part of the present study.
Table 1 shows that the shaking incubation time had no significant effect on the a* color parameter of extracts (P > 0.05). In other words, even though according to Table 3, by increasing the shaking incubation time from 30 to 120 min, the redness of extracts or the a * color parameter increased from − 0.73 ± 0.08 to − 0.64 ± 0.11, respectively, in general the 30, 75 and 120 min shaking incubation times had statistically no significant difference in the a* color parameter (P > 0.05). According to Table 1 and Fig. 4, the mutual effect of the ultrasonic time and shaking incubation time on the a* color parameter of extracts had no significant difference (P > 0.05). Hence, all presented treatments shown in Fig. 4 had no significant difference compared to each other in terms of the a* color parameter (P > 0.05).
b* color parameter
It should be noted that in this study, the b* color parameter values of extracts were positive for all treatments. Table 1 shows that the ultrasonic time had a significantly profound impact on the b* color parameter of extracts (P ≤ 0.001). According to Table 2, with the increase of the ultrasonic time from 0 to 80 min, the values of the b* color parameter had a significant increase (equivalent to 12.62%) from 29.07 ± 0.53 to 32.74 ± 0.44, respectively (P ≤ 0.001). These results indicate that with the increase of the ultrasonic time from 0 to 80 min, the trend of color of extracts increased to the yellow color. The reason for this probably was relevant to the increase of the β-carotene content of extracts with the increase of the ultrasonic time from 0 to 80 min (Table 2). The highly significant positive correlation (P ≤ 0.001) presented in Table 4 between the β-carotene content and b* color parameter of extracts also proved this statement. Ginting reported that with the increase of the β-carotene content of the extract from orange-fleshed sweet potato, the b* color parameter increased [26]. This result is consistent with the results obtained in this part of the present research.
Table 1 shows that the shaking incubation time had a significant effect on the b* color parameter of extracts (P ≤ 0.05). Table 3 indicates that by increasing the shaking incubation time from 30 to 120 min, especially in the period of 75 up to 120 min, the trend of color of extracts increased to the yellow color. According to Table 1 and Fig. 5, the mutual effect of the ultrasonic time and shaking incubation time on the b* color parameter of extracts were significant (P ≤ 0.05). As shown in Fig. 5, the 0 min ultrasonic-30 min shaking incubation treatment with the average of the b* color parameter equivalent to 27.7 ± 1.2, had the lowest and the 80 min ultrasonic-30 min shaking incubation treatment with the average of the b* color parameter equivalent to 33.55 ± 0.16, had the highest average of this dependent response. These two treatments had a statistically significant difference in terms of the b* color parameter compared to each other (P ≤ 0.05). According to Fig. 1, the 0 min ultrasonic-30 min shaking incubation treatment, had the least amount of the β-carotene content. Thus, the lowest amount of the b* color parameter of this treatment was predictable. According to Fig. 1, the 80 min ultrasonic-120 min shaking incubation treatment had the highest amount of the β-carotene content which had no significant differences in terms of the β-carotene content with 80 min ultrasonic-30 min shaking incubation treatment (P > 0.05). Accordingly, the result of the highest amount of the b* color parameter of 80 min ultrasonic-30 min shaking incubation treatment corresponded with the presented argument related to the direct relationship of the β-carotene content and b* color parameter.
FT-IR
FT-IR spectroscopy was performed to identify the characteristics of the functional groups of extracts obtained from nine extraction treatments presented in Fig. 6. In addition, the FT-IR spectroscopy was used to examine the possible changes of these functional groups during the mentioned extraction treatments. As can be seen in Fig. 6, the FT-IR spectra resulted from the nine treatments overlapped each other. The entire spectra of FT-IR in this figure, had the absorption peaks at the specified wave numbers, which the peaks of 3658 and 3343 cm− 1 represent an hydroxyl group (O–H stretch) [28,29,30], peaks of 2974 and 2897 cm− 1 represent asymmetrical stretching of C–H groups [31, 32], the presence of peaks at 1454 and 1381 cm− 1 indicate C-H2 bending [31], the peak of 1263 cm− 1 confirms a C–O stretching [33], the peak of 1085 cm− 1 represents vibrational C–C bond [29], the peak of 1050 cm− 1 can be ascribed to CH3 stretching [29], the peak of 876 cm− 1 represents R–CH=CH–R [29], the peak of 803 cm− 1 confirms wagging vibration of RH–C=C–RH [34], and the peak of 662 cm− 1 can be ascribed to CH=CH stretching vibration [35]. Thus, by comparing the spectra of FT-IR, it can be concluded that the use of the ultrasonic pretreatment (40 and 80 min) to extract the β-carotene pigment from carrot processing waste compared to non-use of this pretreatment (0 min), did not lead to any destruction of the mentioned functional groups in the extracts. Furthermore, the increase of the ultrasonic time from 0 to 80 min or shaking incubation time from 30 to 120 min did not lead to the destruction of the functional groups in the extracts, as well.
Scanning electron microscopy (SEM) images
In order to study the microscopic changes of the carrot waste powder structure during the extractive treatments, the SEM images of the carrot processing waste powder were prepared after applying three extractive treatments of 0 min ultrasonic-30 min shaking incubation, 80 min ultrasonic-30 min shaking incubation, and 80 min ultrasonic-120 min shaking incubation, along with the carrot processing waste powder without applying any extraction (as control sample). SEM images presented in Fig. 7 shows that by applying the extractive treatments, the structure of carrot waste powders became more disintegrated and porous (Fig. 7b–d) compared to the carrot waste powder structure without applying any extractive treatment (Fig. 7a). This is because the samples were exposed to the extractive processes of the ultrasonic and shaking incubation. Comparison of the carrot waste powder structure after 0 min ultrasonic-30 min shaking incubation treatment (Fig. 7b) with the carrot waste powder after 80 min ultrasonic-30 min shaking incubation treatment (Fig. 7c), showed that the use of the ultrasound waves for 80 min caused damage of cell structure, particularly the cell walls, more disintegration and more porous tissue in the structure of sample. This phenomenon resulted in the increase of the dependent variables of the β-carotene content, antioxidant activity, a* color parameter (redness) and b* color parameter (yellowness) of extracts (Table 2).
Scanning electron microscopy images of a untreated carrot waste powder, b the remained solids air-dried after 0 minultrasonic-30 min shaking incubation treatment, c the remained solids air-dried after the 80 minultrasonic-30 min shaking incubation treatment, and d the remained solids air-dried after the 80 minultrasonic-120 min shaking incubation treatment
Comparison of the carrot waste powder structure after the 80 min ultrasonic-30 min shaking incubation treatment (Fig. 7c) with the carrot waste powder structure after the 80 min ultrasonic-120 min shaking incubation treatment (Fig. 7d) indicated that the increase of the shaking incubation time from 30 to 120 min also led to further disintegration and porosity in the structure of the carrot waste powder. Based on the earlier results listed in Table 3, by increasing the shaking incubation time from 30 to 75 and 120 min, the β-carotene content of extracts increased.
Conclusions
In this study, for the first time, the combination of the ultrasonic and shaking incubation processes was investigated in order to extract the β-carotene pigment from the carrot processing waste powder. The impact of the ultrasonic pretreatment time, shaking incubation time and their mutual effect on the β-carotene content, antioxidant activity, color parameters of L*, a* and b* were studied on the extracts. Furthermore, to identify the characteristic functional groups of the extracts and to study the possible microscopic changes in the structure of the carrot waste powder, FT-IR and SEM were used, respectively. The main results obtained in this study include:
-
Increase of the ultrasonic time from 0 to 80 min, significantly increased the dependent variables of β-carotene content (P ≤ 0.001), antioxidant activity (P ≤ 0.01), a* color parameter (P ≤ 0.01) and b* color parameter (P ≤ 0.001) of extracts.
-
The increase of the shaking incubation time from 30 to 120 min resulted in a significant increase of the β-carotene content (P ≤ 0.001) of extracts.
-
The results of this study also indicated existence of the significant positive correlation between the β-carotene content and dependent variables of the antioxidant activity (P ≤ 0.01), a* color parameter (P ≤ 0.01) and b* color parameter (P ≤ 0.001).
-
Considering the FT-IR spectra of extracts, the increase of the ultrasonic time from 0 to 80 min and the increase of the shaking incubation time from 30 to 120 min did not damage the functional groups of extracts.
-
According to the results of the SEM images, the ultrasonic and shaking incubation processes especially at longer times led to considerable disintegration of the microscopic structure and led to more porous structure.
Finally, according to the results presented in this study, the use of the ultrasonic-shaking incubation method to extract the β-carotene pigment from the carrot processing waste, increased the extracted β-carotene content. Therefore, the proposed method is an efficient and effective method in order to convert the agricultural waste into natural pigments with high economic value and to reduce the environmental problems caused by agricultural waste.
References
A. Aberoumand, World J. Dairy Food Sci. 6, 71–78 (2011)
M. Shahid, F. Shahid-ul-Islam, Mohammad, J. Clean. Prod. 53, 310–331 (2013)
S.M. Rivera, R. Canela-Garayoa, J. Chromatogr. A 1224, 1–10 (2012)
T. Baysal, S. Ersus, D.A.J. Starmans, J. Agric. Food Chem. 48, 5507–5511 (2000)
P. Karnjanawipagul, W. Nittayanuntawech, P. Rojsanga, L. Suntornsuk, Mahidol Univ. J. Pharm. Sci. 37, 8–16 (2010)
J. Azmir, I.S.M. Zaidul, M.M. Rahman, K.M. Sharif, A. Mohamed, F. Sahena, M.H.A. Jahurul, K. Ghafoor, N.A.N. Norulaini, A.K.M. Omar, J. Food Eng. 117, 426–436 (2013)
A.J. Purohit, P.R. Gogate, Sep. Sci. Technol. 50, 1507–1517 (2015)
N. Sarkar, S.K. Ghosh, S. Bannerjee, K. Aikat, Renew. Energy 37, 19–27 (2012)
S.A. Zauro, M.T. Abdullahi, A. Aliyu, A. Muhammad, I. Abubakar, Y.M. Sani, Appl. Chem. 96, 41479–41483 (2016)
N. Ullah, A. Khan, F.A. Khan, M. Khurram, M. Hussan, M. Amin, S.M.U. Khayam, J. Hussain, Middle-East J. Sci. Res. 9, 496–502 (2011)
L.S. Lee, N. Lee, Y.H.E.H. Kim, C.H. Lee, S.P. Hong, Y.W. Jeon, Y.H.E.H. Kim, Molecules 18, 13530–13545 (2013)
A.P. Schwab, J. Su, S. Wetzel, S. Pekarek, M.K. Banks, Environ. Sci. Technol. 33, 1940–1945 (1999)
J.F.G. Martín, Applications of Ultrasound in the Beverage Industry. (Nova Science Publishers, New York, 2016)
F. Chemat, M.K. Khan, Ultrason. Sonochem. 18, 813–835 (2011)
S. Dey, V.K. Rathod, Ultrason. Sonochem. 20, 271–276 (2013)
T. Roukas, F. Mantzouridou, Appl. Biochem. Biotechnol. 90, 37–46 (2001)
S. Hecker, Extraction of β-carotene from orange peel and carrot waste for cotton dyeing, Thesis for the Degree of Master in Science with a major in Textile Engineering, The Swedish School of Textiles, (2014)
S. Rebecca., A.N. Boye, S. Chandran, Afr. J. Biotechnol. 9, 1450–1454 (2010)
S. Haji Heidari, S. Taghian Dinani, Eur. J. Lipid Sci. Technol. 120, 1700252–1700264 (2018)
S. Taghian Dinani, N. Hamdami, M. Shahedi, M. Havet, D. Queveau, Food Bioprod. Process. 95, 83–95 (2015)
Y. Sun, G. Ma, X. Ye, Y. Kakuda, R. Meng, Ultrason. Sonochem. 17, 654–661 (2010)
F. Khalili, S. Taghian Dinani, J. Food Meas. Charact. (2017) https://doi.org/10.1007/s11694-017-9712-1
Y. Sun, D. Liu, J. Chen, X. Ye, D. Yu, Ultrason. Sonochem. 18, 243–249 (2011)
M.A. Hejazi, R.H. Wijffels, Biomol. Eng. 20, 171–175 (2003)
W.-J. Yen, L.-W. Chang, P.-D. Duh, LWT: Food Sci. Technol. 38, 193–200 (2005)
E. Ginting, J. Teknol. dan Ind. Pangan 24, 81–88 (2013)
S. Okuno, M. Yoshinaga, M. Nakatani, K. Ishiguro, M. Yoshimoto, T. Morishita, T. Uehara, M. Kawano, Food Sci. Technol. Res. 8, 154–157 (2002)
B. Saberi, S. Chockchaisawasdee, J.B. Golding, C.J. Scarlett, C.E. Stathopoulos, Int. J. Biol. Macromol. 104, 345–359 (2017)
M.M. Kamil, G.F. Mohamed, M.S. Shaheen, J. Am. Sci. 27, 253–260 (2011)
T. Suganya, S. Renganathan, Bioresour. Technol. 107, 319–326 (2012)
P.J. Munde, A.B. Muley, M.R. Ladole, A.V. Pawar, M.I. Talib, V.R. Parate, 3 Biotech 7, 206 (2017)
T. Shahzad, I. Ahmad, S. Choudhry, M.K. Saeed, M.N. Khan, Int. J. Pharm. Pharm. Sci. 6, 223–228 (2014)
J.J. Chavan, D.M. Ghadage, P.R. Kshirsagar, S.S. Kudale, J. Liq. Chromatogr. Relat. Technol. 38, 963–969 (2015)
R. Konwarh, S. Pramanik, D. Kalita, C.L. Mahanta, N. Karak, Ultrason. Sonochem. 19, 292–299 (2012)
K. Sinha, P.D. Saha, S. Datta, Dye. Pigment. 94, 212–216 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirheli, M., Taghian Dinani, S. Extraction of β-carotene pigment from carrot processing waste using ultrasonic-shaking incubation method. Food Measure 12, 1818–1828 (2018). https://doi.org/10.1007/s11694-018-9796-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9796-2