Abstract
Extraction of phenolic compounds was carried out from the waste generated during processing of fruit of Borassus flabellifer, which could not only alleviate the issue of disposing the waste but also provide a useful resource. Using conventional extraction method, phenolic compounds have been extracted from the shell of the fruit using different solvents, like methanol, ethanol, acetone and water, in order to select the most suitable solvent. One factor at a time study was conducted to observe the effect of various parameters, like solid loading, volume of solvent, temperature, concentration of solvent and extraction time and for maximizing the extraction efficiency. The maximum extraction of phenolic compounds (110.3 mg GAE/g and 25.6 mg TAE/g) was achieved while using 5 g solid loading, 200 mL methanol with 75% (v/v) concentration, 65 °C temperature and 8 h extraction time. The process was then improved by incorporating sonication which has resulted in the drastic reduction in extraction time (from 8 h to 8 min) with comparable yield (92.08 mg GAE/g and 25.35 mg TAE/g). A reduction in extraction time has further extended the advantages of reduced power consumption, minimized carbon emission and minimal utility requirement. Hence, ultrasound-assisted extraction can further be explored and has the potential to replace the conventional technique.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Consumption of fruits and vegetables by humans has been observed from ancient times. Scientific studies have shown the positive effect of increased consumption of such fruits and vegetables toward preventing disease-causing conditions because of the presence of phytochemicals [1,2,3]. Polyphenols, vitamins, minerals, proteins, flavonoids and phenolic acids are the examples of phytochemicals that provide numerous immunity benefits due to antioxidant, antifungal, antibacterial activities and so on. Chemicals like polyphenols act as antioxidant and help in scavenging active oxygen species [4]. Reactive oxygen species (ROS) and various oxygen-centered free radicals keep on developing in human body which resulted in the death of cell and damage to the tissue. Cancer, cardiovascular problems and so on are implicated due to such oxygen species and radicals [5]. Oxidation is a process which is naturally occurring in the human body; hence, there is a need to balance the functioning of body by introducing antioxidants to maintain good health. Butylated hydroxyl anisole (BHA) and butylated hydroxy toluene (BHT) are the majorly used synthetic antioxidants which are effective but with some side effects [6]. Hence, there is a growing interest in searching antioxidants from natural resources. Further, promotion of the national health care programs by Government of India encourages the use of traditional herbal medicine since such drugs are easily available, show less or no side effects and have earned trust [7]. It has, therefore, become important to classify and estimate the required components in fruits and vegetables so as to obtain the benefits for health. One such natural source of phenolic contents is Borassus flabellifer Linn, commonly known as palmyra palm or doub palm. It is cultivated around the tropical regions of India. Some countries like Thailand, Malaysia, Indonesia, Myanmar and Sri Lanka also cultivate palmyra palm on a large scale due to favorable climatic conditions [8].
B. flabellifer is known to be used for multiple purposes, viz., anti-laprotic, diuretic, analgesic, antipyretic, antiarthritic antiphlogistic and anti-inflammatory actions. The different parts of the tree have been used in curing various diseases, like fruit is useful in curing hyperdipsia, dyspepsia, skin diseases and fever [9,10,11,12,13]. The extract of the roots of this tree contained various phenolic compounds, that is, tannins, alkaloids, glycosides, terpenoids, flavonoids and saponins [14]. The phytochemical analysis of fruit of B. flabellifer has shown the presence of carbohydrates, amino acids, saponins, tannins, flavonoids and phenolic compounds in the extract [15]. All the parts of the tree like roots, leaves, fruits and seeds possess antioxidant activity due to the presence of phenolic compounds [14,15,16,17,18]. Various articles have reported on the useful activities and phytochemical analysis of various parts of B. flabellifer [10,11,12,13,14,15,16,17,18]. The fruit contains majorly two parts: a hard cover known as shell and gelatinous eatable endosperm, often called a tender fruit. However, there is no work reported on the shell of the fruit and it is the major waste generated after obtaining the fruits.
The objective of the present work was to extract the phenolic compounds from the shell of the fruits of B. flabellifer. The effect of various parameters like type of solvent (methanol, ethanol, acetone and water), solid loading (5–20 g), volume of solvent (50–200 mL), concentration of solvent (0–100%), temperature (25–65 °C) and extraction time (2–10 h) on extracting the phenolic compounds has been studied using solvent extraction technique. Based on parametric study, a combination of optimum conditions has been obtained. The process was then further improved by incorporating sonication in the system since ultrasound-assisted extraction has the ability to achieve a faster rate of extraction with comparable yield.
Experiments
Materials
The shells of the fruit of B. flabellifer were obtained from the local cultivator in Surat, India. The shells were dried under sunlight for about 4–5 days and then chopped into small pieces. Those pieces were ground in the laboratory grinder and screened to have size of the powdered material around 500 µm. The size reduction would help to improve the mass transfer rate during extraction. Methanol (Rankem Laboratory), ethanol (Advent Chembio Pvt. Ltd.), acetone (Loba Chemie Laboratory Reagents), sodium carbonate (Finar Chem), Folin-Ciocalteu phenol reagent (Sisco Research Laboratories), gallic acid (Finar Chem), tannic acid (S. D. Fine Chem Ltd.) were used for extraction as well as for analysis purposes along with distilled water.
Method
Extraction of Phenolic Compounds. The powdered material having an average size of 500 µm was subjected to extraction using conventional extraction technique. In this process, a fixed amount of powdered material was subjected to extraction along with the solvent. The mixture was kept in a round-bottom flask and a condenser was connected to it to prevent the loss of volatile solvents. The phenomenon of transfer of solute from solid material to solvent is termed as leaching. The leaching operation focuses on extracting valuable compounds, known as solute, from the solid feed using a suitable solvent. In case of natural product extraction, the solvent from the bulk volumes moves toward liquid film surrounding the solid material after which it diffuses through the film toward the solid surface. Once the solvent reaches the surface, it permeates through the cell wall toward the inner part of the cell, wherein the solute resides. The solvent upon reaching the cell dissolves the solute and when it becomes saturated moves outward through cell well and then back diffusion. Finally, it reaches back to the bulk solution. The phenomenon continues till the whole solution becomes saturated and the driving force becomes zero.
Based on the solubility of the phenolic compounds in the solvent and its saturation, temperature, and extraction time, recovery of phenolic compounds changes. Therefore, the effect of various parameters, like type of solvent, solid loading, volume of solvent, concentration of solvent, temperature and extraction time on extracting the phenolic compounds has been studied while power was kept constant. Agitation was kept in the range of 350–400 rpm to ensure uniformity in temperature and mass transfer. The procedure along with the processing variables is presented in Fig. 15.1. Upon completion of the extraction, the solution was filtered to remove solid material and the filtrate was then stored for determining the total phenolic compounds (TPC). In the present work, TPC was measured in terms of gallic acid and tannic acid. Each experiment was conducted twice to ensure repeatability.
Ultrasound-Assisted Extraction. The sonicator bath from Aqua Scientific Instruments (Surat, India) was used for performing extraction in the presence of sonication. Since sonication has the ability to improve the process performance primarily by reducing the extraction time, the aim of this study was to estimate the time required for extracting the phenolic compounds using sonication process. All the parameters except time were kept constant and the extraction was carried out for time period in the range of 2–10 min.
Determination of Total Phenolic Compounds. The filtered extract was analyzed for TPC in terms of gallic acid (as mg of gallic acid equivalent weight (mg GAE)/g of feed material). The sample after dilution if required was assessed using UV-visible spectrophotometer (DR600, HACH). The method for determining TPC using UV-visible spectrophotometer was developed based on Folin-Ciocalteu method [19]. The standard solution of gallic acid was used to prepare the calibration curve (Fig. 15.2).
In addition, TPC was determined in terms of tannic acid (mg of tannic acid equivalent weight (TAE)/g of feed material). The method used UV-visible spectrophotometer (DR600, HACH) for determining the concentration of TAE [20]. Using standard solution of tannic acid, the calibration curve was prepared having R2 = 0.992.
Results and Discussion
Parametric Study
Effect of Nature of Solvent. It is always preferable to select a solvent which has a higher affinity toward the targeted compound, hence different solvents have been used to extract phenolic compounds from the shell and other parameters were kept constant at 5 g of solid loading, 50 mL pure solvent, 25 °C temperature and 6 h of extraction time (Fig. 15.3). As seen from the figure, methanol has provided the highest recovery of phenolic compounds (29.46 mg GAE/g) compared to other solvents while acetone has exhibited the lowest recovery. The solubility of phenolic compounds was more in methanol due to its polarity, and also the methanol was able to penetrate through the solid material easily compared to other solvents. Hence, a higher extraction of phenolic compounds was observed in case of methanol and was used for further studies. Similar results were obtained while extracting rosmarinic acid using methanol [21, 22].
Effect of Solid Loading. Solid loading assists in determining the capacity of the process and it is always desirable to operate the process at its maximum capacity. In order to observe the effect of solid loading, it was varied from 5 to 20 g while volume of the solvent, solvent concentration, temperature and extraction time were maintained at 100 mL, 100% methanol, 25 °C and 6 h, respectively. The influence was observed by determining the phenolic contents in terms of gallic acid and tannic acid (Fig. 15.4). A recovery of phenolic compounds has experienced a decreasing trend with a higher solid loading due to decrease in solvent to solid loading ratio. A higher amount of solid material in a fixed volume of solvent increases the solution viscosity which may impart resistance in mass transfer leading to reduced recovery of the solute [23]. TPC in terms of gallic acid equivalent was higher compared to TPC in terms of tannic acid equivalent. However, both have shown similar behavior upon increasing the solid amount. The highest recovery was observed at 5 g (52.33 mg GAE/g and 14.04 mg TAE/g), and therefore, it was used for rest of the experiments. Similar observations have been made while isolating geraniol from the leaves of palmarosa [24].
Effect of Solvent Volume. In order to observe the influence of solvent volume on extraction of phenolic compounds, it was varied in the range of 50–200 mL while maintaining other parameters constant, that is, solid loading: 5 g, solvent concentration: 100% methanol, temperature: 25 °C and extraction time: 6 h. A higher solvent volume provides a higher solubilization capacity as well as improved driving force for mass transfer. It is based on the principle of mass transfer between solid and liquid medium, which provides increased concentration gradient [25]. As seen from Fig. 15.5, the highest extraction (67.29 mg GAE/g and 17.3 mg TAE/g) was observed for 200 mL.
Effect of Temperature. Diffusivity of solute toward solvent from solid and solubility of solute in solvent increase when temperature is increased [25, 26]. Hence, leaching is always preferred at an elevated temperature. Also, a higher temperature reduces the viscosity and surface tension of solvent which further enhances the rate of extraction [27]. However, one needs to ensure that there is no degradation of the solute in case of heat-sensitive compounds [25, 28]. In the present study, the temperature was varied from 25 to 65 °C and the other parameters were kept constant at 5 g of solid loading, 200 mL pure methanol and 6 h of extraction time (Fig. 15.6). At 65 °C, boiling of the solvent was observed; hence, the highest recovery might be expected. The phenolic compounds have exhibited a higher extraction upon increasing the temperature and the highest extraction was observed at 65 °C (94.42 mg GAE/g and 24.06 mg TAE/g).
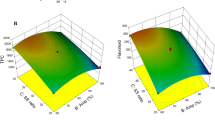
Effect of Solvent Concentration. A change in the concentration of the solvent may lead to a change in the recovery of phenolic compounds due to the presence of two solvents (methanol and water) at the same time. The concentration of methanol was varied from 0 to 100% (v/v) while keeping other parameters constant, viz., solid loading: 5 g, solvent volume: 200 mL, temperature: 65 °C and extraction time: 6 h (Fig. 15.7). There was an increase in the TPC as the purity of solvent was changed from 0 (pure water) to 100 (pure methanol) % (v/v) which might be due to the higher affinity of the phenolic compounds toward methanol. However, at 75% (v/v) concentration the extraction efficiency was similar to that with the pure methanol, that is, 93.53 mg GAE/g and 23.67 mg TAE/g against 94.42 mg GAE/g and 24.06 mg TAE/g, respectively. Since the solubility of phenolic compounds was improved because of methanol, desorption of solute has become easier due to the presence of water [29], and such extraction efficiency could be expected. Hence, 75% (v/v) methanol was used for next experiments.
Effect of Extraction Time. A process should be provided with sufficient time so that the completion of the extraction can be achieved. A lesser time may lead to incomplete extraction, while a higher time may result in degradation of the solute due to its exposure to the elevated temperature for a longer period. In the present work, the extraction time was varied in the range of 2–10 h, while keeping other parameters constant (5 g of solid loading, 200 mL methanol with 75% (v/v) concentration and 65 °C temperature). The highest recovery of phenolic compounds was achieved at 8 h that is, 110.3 mg GAE/g and 25.6 mg TAE/g, as shown in Fig. 15.8. As the time of extraction was further increased, a decrease in the extraction efficiency was observed. Since the compounds were exposed to elevated temperature for a longer time as well as they were prone to oxidation, a degradation of the compounds has been observed [30].
Ultrasound-Assisted Extraction
In conventional extraction (CE), solvent has to penetrate through the cell wall to solubilize the solute and then transfer back to the bulk solution. This step is regarded as the slowest step in the extraction process and leads to extended time of extraction [23, 24, 27]. Ultrasound-assisted extraction (UAE) has the ability improve the mass transfer by damaging the cells. In sonication, the propagation of sonic waves leads to the formation of bubbles, which in turn gets ruptured after attaining the maximum size. This rupture results in release of tremendous amount of energy at a molecular level. The energy has the sufficient intensity to damage the cell wall either by creating cracks, pores, or by breaking the cell walls. Also, a micro-jet phenomenon may assist in erosion of the cell wall or detexturation. These phenomena allow easy access of solute, better interaction between solute and solvent, and finally accelerate the dissolution of the solutes which are either residing in the cell or able to come out from the cell [27, 31]. Thus, the rate-limiting step observed in the convention extraction can be eliminated using sonication and the extraction time can be reduced which may further help in improving the production capacity [31].
In the present study, extraction of phenolic compounds was carried out in sonication bath at different time intervals (Fig. 15.9) with the combination of parameters which have provided a better extraction in conventional method except temperature, that is, solid loading: 5 g; solvent volume: 200 mL, concentration of solvent: 75% (v/v) methanol and temperature: 30 °C. As seen from the figure, the extraction time was greatly reduced from 8 h to 8 min when sonication was employed in the process and TPC was obtained as 92.08 mg GAE/g and 25.35 mg TAE/g.
Apart from the benefit of reduction in extraction time, other advantages are also obtained upon employing UAE like reduced power consumption, shrunken carbon footprint and almost negligible utility requirement in terms of cooling water. The comparison of both the techniques is shown in Fig. 15.10, where conventional method has been considered as a reference (100%). Further improvement in the sonication process can be achieved by varying the sonication parameters, like frequency, amplitude and pulse ratio.
Conclusion
Borassus flabellifer is the natural source of phenolic compounds that can be used as an antioxidant for different medicinal purposes. The waste material, that is, shell left after using the fruit of this plant was evaluated for its possible utilization as the source of phenolic compounds. Among various solvents like methanol, ethanol, acetone and water, extraction of phenolic compounds was efficiently carried out using methanol. Various parameters like solid loading, volume of solvent, temperature, concentration of solvent and extraction time have been assessed to understand their impact on the recovery of phenolic compounds. The phenolic contents have been estimated in terms of gallic acid equivalent and tannic acid equivalent. From the one factor at a time study, a combination of optimum parameters was obtained as solid loading: 5 g, solvent volume: 200 mL, temperature: 65 °C, concentration of solvent: 75.5 (v/v) methanol and extraction time: 8 h. Under the set of optimized conditions, TPC was obtained as 110.3 mg GAE/g and 25.6 mg TAE/g. In order to overcome the limitation of the conventional technique, ultrasound-assisted extraction was employed which has reduced the extraction time from 8 h to 8 min, thereby providing sustainable alternative to the conventional technique. A detailed study on the process parameters of ultrasound-assisted extraction will further add value to the process.
References
Dragsted LO (2003) Antioxidant actions of polyphenols in humans. Int J Vitam Nutr Res 73(2):112–119
Hertog MGL, Fesrens EJM, Hollman PCH, Katan MB, Kromhout D (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly study. The Lancet 342(8878):1007–1011
Yang CS, Landau JM, Huang MT, Newmark HL (2001) Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr 21:381–406
Sakihama Y, Cohen MF, Gace SC, Yamasaki H (2002) Plant phenolic antioxidant and prooxidant activities: phenolics induced oxidative damage mediated by metals in plants. Toxicology 177(1):67–80
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Branen AL (1975) Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc 52(2):59–63
www.ayush.gov.in (Access date: 08/05/2020)
Naknean P, Meenune M, Roudaut G (2010) Characterization of palm sap harvested in Songkhla province, Southern Thailand. Int Food Res J 17(4):977–986
Kapoor LD (2000) Handbook of ayurvedic medicinal plants: herbal reference library. CRC Press, Florida
Jamkhande PG, Suryawanshi VA, Kaylankar, TM, Patwekar SL (2016) Biological activities of leaves of ethnomedicinal plant, Borassus flabellifer Linn. (Palmyra palm): an antibacterial, antifungal and antioxidant evaluation. Bull Fac Pharm, Cairo University 54(1):59–66
Alamelumangai M, Dhanalakshmi J, Mathumitha M, Renganayaki RS, Muthukumaran P, Saraswathy N (2014) In vitro studies on phytochemical evaluation and antimicrobial activity of Borassus flabellifer Linn against some human pathogens. Asian Pac J Trop Med 7(Suppl 1):S182–S185
Paschapur MS, Patil MB, Kumar R, Patil SR (2009) Influence of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) on chemically induced acute inflammation and poly arthritis in rats. Int J PharmTech Res 1(3):551–556
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Saravanan C, Priya B, Asir BS, Uma S (2012) Preliminary phytochemical screening of antibacterial activity of Palmyra Palm (Borassus flabeliffer) root extract. Int J Pharm Sci Res 3(11):4489–4491
Pramod HJ, Yadav AV, Raje VN, Mohite M, Wadkar GH (2013) Antioxidant activity of Borassus flabellifer (linn.) fruits. Asian J Pharm Technol 3(1):16–19
Sahni C, Shakil NA, Jha V, Gupta RK (2014) Screening of nutritional, phytochemical, antioxidant and antibacterial activity of the roots of Borassus flabellifer (Asian Palmyra Palm). J Pharmacogn Phytochem 3(4):58–68
Kommu S, Chiluka VL, Gowri Shankar NL, Matsyagiri L, Shankar M, Sandhya S (2011) Antioxidant activity of methanolic extracts of female Borassus flabellifer leaves and roots. Der Pharmacia Sinica 2(3):193–199
Arunachalam K, Saravanan S, Parimelazhagan T (2011) Nutritional analysis and antioxidant activity of Palmyrah (Borassus flabellifer L.) seed embryo for potential use as food source. Food Sci Biotechnol 20(1):143–149
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
de Amorim ELC, de Castro VTNA, de Melo JG, Correa AJC, Sobrinho TJSP (2012) Standard operating procedures (SOP) for the spectrophotometric determination of phenolic compounds contained in plant samples. In: Akyar I (ed) Latest research into quality control. IntechOpen Limited, London, pp 47–66
Albu S, Joyce E, Paniwnyk L, Lorimer JP, Mason TJ (2004) Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason Sonochem 11(3–4):261–265
Paniwnyk L, Cai H, Albu S, Mason TJ, Cole R (2009) The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason Sonochem 16(2):287–292
Desai MA, Parikh JK (2012) Hydrotropic extraction of citral from Cymbopogon flexuosus (Steud.) Wats. Ind Eng Chem Res 51(9):3750–3757
Thakker MR, Parikh JK, Desai MA (2018) Ultrasound assisted hydrotropic extraction: a greener approach for the isolation of geraniol from the leaves of Cymbopogon martini. ACS Sustain Chem Eng 6(3):3215–3224
Pinelo M, Rubilar M, Jerez M, Sineiro J, Nunez MJ (2005) Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem 53(6):2111–2117
Spigno G, de Faveri DM (2007) Antioxidants from grape stalks and marc: influence of extraction procedure on yield, purity and antioxidant power of the extracts. J Food Eng 78(3):793–801
Chemat F, Rombaut N, Sicaire A, Meullemiestre A, Fabiano-Tixier A, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. mechanisms, techniques, combinations, protocols and applications. a review. Ultrason Sonochem 34:540–560
Spigno G, Tramelli L, de Faveri DM (2007) Effect of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 81(1):200–208
Yilmaz Y, Toledo RT (2006) Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J Food Compos Anal 19(1):41–48
Chew KK, Ng SY, Thoo YY, Khoo MZ, Wan Aida WM, Ho CW (2011) Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int Food Res J 18(4):571–578
Solanki KP, Desai MA, Parikh JK (2018) Sono hydrodistillation for isolation of citronella oil: a symbiotic effect of sonication and hydrodistillation towards energy efficiency and environment friendliness. Ultrason Sonochem 49:145–153
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bageshwar, A.Y., Desai, M.A. (2021). Extraction of Phenolic Compounds from the Waste of Borassus flabellifer: A Step Toward Waste Valorization. In: Kumar, S., Rajurkar, K.P. (eds) Advances in Manufacturing Systems. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-33-4466-2_15
Download citation
DOI: https://doi.org/10.1007/978-981-33-4466-2_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-4465-5
Online ISBN: 978-981-33-4466-2
eBook Packages: EngineeringEngineering (R0)