Abstract
The tomato processing wastes are rich in lycopene pigment, which can be consumed as a natural food colorant and bioactive ingredient. The present study investigates the appropriateness of ultrasound pretreatment and enzymatic treatment with cellulase and pectinase enzymes to extract lycopene pigment from tomato processing wastes using response surface methodology (RSM). To achieve this goal, a central composite design consisting of four independent variables including cellulase concentration (0–10%), pectinase concentration (0–5%), ultrasonic time (0–60 min) and pH (3.5–5.5) was used to examine the influences of these process variables on the lycopene extraction. The second order model of lycopene extraction obtained from RSM analysis had the p value less than 0.001 and represented a coefficient of determination (R2) of 93.82%. Thus, a satisfactorily agreement between experimental and predicted values for lycopene content obtained by the second order model was observed. In addition, the Fourier Transform Infrared spectroscopy results did not show any destruction of functional groups of the investigated extracts containing lycopene pigment. Scanning electronic microscopy images from the remaining tomato processing wastes after treatment with more lycopene content extraction demonstrated more porous structure. These results implied that ultrasound-assisted enzymatic extraction was an efficient extracting method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lycopene (C40H56), a red carotenoid pigment, has a free radical quenching ability twice as high as that of β-carotene and 10 times higher than that of α-tocopherol [1]. Therefore, lycopene consumption has been correlated with a lower risk of cancer [2] as well as inflammatory, cardiovascular and coronary heart diseases [3]. Owing to its health benefits, strong color, non-toxicity and great fat and oil solubility, lycopene is used as a natural and functional colorant in the food industry [3] instead of synthetic colorants to enhance sensory quality [1], nutritional advantages and storage stability of foods [3]. It is noteworthy to mention that the solubility of lycopene in vegetable oils is approximately 0.2 g/L at room temperature [4].
Recently, there has been a growing interest and demand for natural lycopene pigment owing to its biodegradability and compatibility with the environment [5]. Thus, lycopene supplements currently arouse interest in food, pharmaceutical, nutraceutical, cosmetic [3] and feed industries [6]. To respond to the growing need for natural lycopene, there has been an increasing interest in the development of lycopene from natural sources [7], especially tomatoes as its main natural source [6]. In addition, other raw materials such as tomato processing waste are considered a rich and interesting source of lycopene [8]. Tomato industrial waste, a by-product during production of tomato juice, paste, puree [9], sauce, and ketchup [10], represents up to 40% of the initial fresh tomato amounts processed [10]. The use of tomato industrial waste as a source of lycopene pigment suggests great potential managing the waste [11] to save money in waste disposal [12].
The conventional solvent techniques of lycopene extraction from plant materials often require a large quantity of solvents [13]. Consumption of a large quantity of solvents is dangerous to humans and the environment [14] due to considerable waste amount of poisonous and toxic solvents [15], being highly dangerous to the operator’s health [16]. In more details, for instance, hexane is highly inflammable and there is still the danger of severe accidents [17]. Methanol and acetonitrile are toxic, environmentally unfriendly and pose more dangers to the operator [16]. In addition, the conventional extraction techniques are time-consuming with low yields [14]. Thus, to reduce the price of lycopene, using appropriate technologies such as ultrasound and enzymatic extraction methods [18] for its recovery seems to be necessary [19].
Ultrasound is now recognized as an economical, simple and effective method in the food industry for extraction purposes [9]. Ultrasound leads to cavitation, being generally described as the creation, growth, and collapse of bubbles in a liquid [14]. Cavitation can result in locally high pressures and temperatures [20] as well as producing disruption of cell walls, turbulence and liquid circulation currents, which can result in better penetration of solvent into the sample matrix and increase of the release of contents [21]. The interest in applying ultrasound to extract natural product has improved due to its advantages of a significant decrease in extraction time, economizing energy consumption [22], low water usage (no reflux is needed) [23], less solvent consumption, lack of toxic chemical involvement and low temperature [24].
Application of hydrolytic enzymes like cellulase and pectinase enzymes, which can degrade the cell wall constituents [25], is an efficient and mild method to facilitate the recovery of lycopene [8]. Theses enzymes have been often employed because cellulose and pectin are the dominant components of the plant cell wall [25]. In addition, these enzymes are effective even at ambient temperatures and at short extraction times to prevent lycopene degradation [19]. Enzymatic process can be used in combination with other methods for lycopene extraction. For instance, it was used as pretreatment for laboratory scale supercritical CO2 extraction of lycopene from tomatoes [26].
Here, the lycopene extraction from tomato processing waste was investigated by ultrasound-enzymatic method to improve the lycopene content of the extracts. To achieve this goal, the influence of four variables (cellulase concentration, pectinase concentration, ultrasonic time and pH) on the extraction yield of lycopene from the tomato processing waste was investigated using the response surface methodology (RSM). The study also contains understanding the influence of some selected ultrasound-enzymatic extraction treatments on the functional groups of the extracts using Fourier Transform Infrared (FTIR) spectra. In addition, scanning electronic microscopy (SEM) analysis was performed to investigate the dependency of the extraction yield of lycopene on sample microstructures.
Materials and methods
Chemicals and reagents
Acetone, methanol, n-hexane, potassium bromide (KBr), and disodium phosphate (Na2HPO4) were purchased from Merck Company, Darmstadt, Germany. Butylated hydroxytoluene (BHT) and citric acid were purchased from Sigma Chemical Company, St. Louis, MO, U.S.A. Cellulase enzyme (activity of 167 µkatal/g) was purchased from CN LAB Nutrition, Asian Group, Shaanxi P. R. China. Moreover, pectinase enzyme (activity of 500 µkatal/g) was purchased from XI’AN Rongsheng Biotechnology CO., LTD., Shaanxi Province, P. R. China. It is worth mentioning that enzyme activity in the International System (SI) of units is the katal, being defined as the amount of enzyme that converts one mole of substance per second [27].
Sample preparation
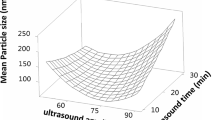
Fresh red tomatoes were purchased from a local supermarket (Shahinshahr, Isfahan, Iran). After removing damaged parts, washing with tap water and chopping into smaller pieces, they were blended using a mechanical blender (Model Depose type 241, Moulinex, Paris, France). The puree was passed through a sieve to obtain the tomato processing waste composed of skin and seeds. Moisture content of fresh tomato processing waste was determined by oven method at 105 °C to obtain constant weight, and was found to be 89.5 ± 1.0 g water/g product. The tomato processing waste was air dried in an oven (Model inc-55, Alfa, Isfahan, Iran) with air circulation at 45 °C for 96 h until obtaining constant weight and moisture content of 6.4 ± 0.1 g water/g product. After that, dried tomato processing waste was powdered in a laboratory mill (Model Depose type 241, Moulinex, Paris, France). Particle size distribution of tomato processing waste powder was determined by static light scattering (Horiba, LA-930, Kyoto, Japan). The results of Fig. 1 show that the mean particle size diameter is 150.7 ± 118.3 (µm). The final sample powder was immediately filled into polypropylene pouches wrapped with an aluminum foil and stored in a refrigerator (Model G26, Samsung Electronics Co., Suwon, Kyunggi Province, South Korea) at − 15 °C prior to later use.
Extraction procedure
1.14 ± 0.003 g of dried tomato processing waste powder was extracted in a glass laboratory tube using 40 mL of a solvent mixture of hexane, acetone and methanol (2:1:1) containing 0.02 g butylated hydroxytoluene (BHT) for each treatment [18]. The suspension was extracted in an ultrasonic bath (Model 300, Pulse, Milan, Italy) at a fixed temperature of 40 °C and at a frequency of 250 Hz. As Table 1 shows, ultrasonic extraction time varied from 0 to 60 min. Table 2 shows the exact ultrasonic pretreatment time for all treatments. After ultrasonic pretreatment, the mixture was transferred into a conical flask. Then, the exact amounts of pectinase and cellulase enzymes (according to Table 2) were dissolved in a 7.5 mL buffer solution of 0.2 mol/L citric acid and 0.2 mol/L disodium phosphate (Na2HPO4) with desired pH (according to Table 2). According to Table 2, the mixture of solvent, sample and enzymes was finally adjusted to the desired pH from 3.5 to 5.5 with the mentioned buffer solution. The obtained slurry was incubated in a shaking incubation (Fan Azma Gostar, Tehran, Iran) at the temperature of 57 °C and regular shaking of 120 rpm for 17 min. It should be mentioned that the incubation temperature of 57 °C and incubation time of 17 min were selected based on the results of some pretests for the most lycopene content extractions. After incubation, the pectinase and cellulase enzymes were inactivated by immersing the conical flask in boiling water for 3 min [28]. After that, the mixture was filtered by filter paper. Next, the sample was left at room temperature in the dark for 5 min to allow the separation of phases. Finally, the upper layer containing lycopene pigment was collected and stored at 4 °C in the dark and the following tests were conducted on the extracts.
Quantification of lycopene content
The absorbance value of the extracts at 503 nm (λ) in a 1 cm path length quartz cuvette was performed to determine lycopene content using a UV visible spectrophotometer (Rayleigh-UV 9200, Beijing, China). The lycopene content (mg/kg or µg/g) was calculated using the following formula [25]:
where, Absorbance503 is absorbance of the extract at 503 nm and 31.2 is a constant [29].
Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy has unique advantages such as simple sample preparation, rapid analysis and satisfactory precision and sensitivity. These features make it an outstanding method to quantify specific compounds within food [30]. To perform this test, a potassium bromide (KBr) pellet was prepared. Next, 1 to 2 drops of extract were poured on KBr pellet using capillary tube. The spectra of the extracts were obtained with the spectral region of 450–4000 cm− 1 to gain more information about their characteristic functional groups and to examine the possibility of their damage due to extraction processes [31, 32]. The spectra of the three selected extracts obtained from the first treatment of Table 2 (cellulase concentration of 5 g/100 g DM, pectinase concentration of 0 g/100 g DM, sonication time of 30 min and pH of 4.5) having the highest lycopene content extraction, the second treatment of Table 2 (cellulase concentration of 5 g/100 g DM, pectinase concentration of 2.5 g/100 g DM, sonication time of 30 min and pH of 3.5) having the least lycopene content extraction, and the optimal treatment (cellulase concentration of 2.5 g/100 g DM, pectinase concentration of 0.8 g/100 g DM, sonication time of 60 min and pH of 5.3), being explained in the optimization section, were obtained at room temperature using FTIR spectroscopy (Model Spectrum 65, Norwalk, Connecticut, PerkinElmer, U.S.A).
Scanning electron microscopy (SEM)
To evaluate any changes in the microstructure of tomato waste powders as a result of the examined extraction treatments, the photos of remaining residues obtained after the extraction treatment having the highest lycopene content extraction (the first treatment of Table 2) and the extraction treatment having the least lycopene content extraction (the second treatment of Table 2) were taken by the Scanning Electron Microscope (Model EM3200, KYKY, Beijing, China) at an accelerating voltage of 26 kV. The mentioned treatments were compared with each other and with control sample (tomato processing waste powder without application of any type of extraction processes on it). To take the SEM images, the surface of a low amount of sample was plated with a conductive material (a fine layer of gold) using a gold deposition device (Model SBC12, KYKY, Beijing, China) to ensure sufficient electron refraction.
Experimental design and statistical analysis
Response surface methodology or RSM is the collection of statistical methods that are effective in analyzing, modeling and optimizing dependent responses [33, 34]. In this study, a central composite design (CCD) was adopted using Minitab software version 16.1.1.0 to evaluate how the lycopene content of the extracts was influenced by independent variables, including cellulase concentration (g/100 g DM, X1), pectinase concentration (g/100 g DM, X2), ultrasonic time (min, X3) and pH (X4). Table 1 presents the coded and uncoded levels of the mentioned independent variables used in the RSM design. The experimental design needed thirty-one experimental settings with seven replicated center points (as Table 2 shows) to allow the estimation of the pure error. The following second-order polynomial regression equation was used to express the dependent variable (Y) as a function of the independent variables [35]:
where Y represents the predicted response of the lycopene content; β0 is the model intercept, βi, βii, and βij are linear, quadratic, and interaction coefficients, respectively; and Xi and Xj are uncoded independent variables (cellulase concentration, pectinase concentration, ultrasonic time and pH). The significance of the coefficients of the quadratic polynomial model was determined with the aid of the t-test with a confidence level of 95.0% (p ≤ 0.05). The coefficients of the quadratic polynomial model will be more significant if the absolute t value becomes larger and the p-value becomes smaller [36, 37].
Results and discussion
Model fitting
Table 2 shows the complete design matrix and corresponding experimental data obtained for the lycopene content response. Table 3 represents the estimated coefficients and t test results. In this table, the p value was used to investigate the significance of each coefficient. Equation 3 shows the second-order polynomial model of the lycopene content of the extracts (Y) as a function of the mentioned independent variables (in terms of significant uncoded levels):
where X1 is the cellulase concentration, X2 is pectinase concentration, X3 is ultrasonic time, and X4 is pH. From Table 3 and Eq. (3), it can be observed that the parameters with the highest influence on the lycopene content were the linear (p ≤ 0.001) and quadratic (p ≤ 0.001) terms of pectinase concentration, the linear (p ≤ 0.001) and quadratic (p ≤ 0.01) terms of pH, followed by the linear terms of ultrasonic time (p ≤ 0.001) and cellulase concentration (p ≤ 0.01). Moreover, the interaction terms of cellulase concentration × ultrasonic time (p ≤ 0.01), cellulase concentration × pH (p ≤ 0. 05), pectinase concentration × ultrasonic time (p ≤ 0.05), and ultrasonic time × pH (p ≤ 0.001) had high significant influence on the lycopene content of the extracts. The other terms did not have any significant influence on the lycopene content (p > 0.05) and were not shown in Eq. (3). Table 4 shows that the model p-value for the lycopene content response was less than 0.001 and statistically significant. It also shows that the R2 and R2 (adj) of this model were 0.93 and 0.88, respectively, specifying that the precision and accuracy of this polynomial model are satisfactory. Moreover, the ‘lack of fit’ of the model (presented in Table 4) was not significant (p > 0.05). Totally, the model p-value ≤ 0.001, an insignificant lack of fit (p > 0.05) and the high R2 and R2 (adj) confirm that the quadratic regression model (Eq. 3) for lycopene content could satisfactorily predict the lycopene content response [38].
Effect of process variables on the lycopene content
As Table 2 shows, the lycopene content obtained by ultrasonic-enzymatic extraction method ranged from 19.7 to 94.3 mg/kg DM. To envisage the effect of the four independent variables on the lycopene content, the contour plots were drawn using the fitted quadratic polynomial equation (Eq. 3) obtained from regression analysis. Figure 2a illustrates the effects of cellulase concentration and ultrasonic time on the lycopene content of the extracts at the fixed pectinase concentration of 2.5 g/100 g DM and pH of 4.5. As the two-dimensional diagram of Fig. 2a shows, the lycopene content more than 70 mg/kg DM was obtained at the concentration of 0 to 3 g/100 g DM cellulase enzyme and at ultrasonic pretreatment time of 0 to 18 min. Figure 2b illustrates the effects of pectinase concentration and ultrasonic time on lycopene content of the extracts at the fixed cellulase concentration of 5 g/100 g DM and pH of 4.5. Figure 2b shows that the highest amount of lycopene content is obtained at pectinase concentration from 0 to 0.6 g/100 g DM and ultrasonic pretreatment time from 0 to 20 min. This suggests that by applying the cellulase and pectinase enzymes at appropriate concentrations, the high amount of lycopene content can be obtained. It was reported by Choudhari and Ananthanarayan [6] that pectinase enzyme at the concentration of 0.5% w/w was highly effective for a high amount of lycopene extraction. Thus, this study showed that by enormous increase of pectinase enzyme concentration, there will not be a significant proportional increase in extraction efficiency. This study is consistent with our study. It is interesting to note that very high enzyme concentration resulted in very fast total hydrolysis and probably inhibition of end-product [25]. Choudhari and Ananthanarayan [6] and Strati et al. [28] reported that both cellulase and pectinase enzymes enhanced the recovery of lycopene from various tomato materials such as tomato waste. In addition, Zuorro et al. [19] indicated that the lycopene recovery from tomato peel processing waste could be significantly improved using enzymes with cellulolytic and pectinolytic activities. These studies are in good accordance with the present study.
Figure 2c depicts the contour plot of the lycopene content as a function of the two independent variables of cellulase concentration and pH. It can be observed in this figure that the pH of 5–5.5 at all cellulase concentration has led to a higher lycopene yield (Fig. 2c). This suggests that the most cell wall destruction occurs by the cellulase enzyme at pH from 5 to 5.5. Figure 2d shows the effect of pH and ultrasonic time on the lycopene content of the extracts. To obtain the highest extraction yield of lycopene, pH should be increased from 5.3 to 5.5, at ultrasonic pretreatment of 45–60 min (Fig. 2d). It was reported that the application of ultrasound in food processing led to increase of the extraction yield [21], since it can effectively increase mass and heat transfer [9]. In fact, the extraction process can be enhanced with the disruption of cell walls and the release of cellular materials attributed to cavitation phenomena [39]. In summary, the RSM results show that the maximum lycopene content was predicted to be 100.0 mg/kg DM by combination of the cellulase concentration of 3 g/100 g DM, pectinase concentration of 1 g/100 g DM, ultrasonic time of 60 min and pH of 5.3.
Overall optimization and comparison
To optimize the lycopene extraction process from tomato processing waste, the parameters of cellulase concentration (0–10 g/100 g DM), pectinase concentration (0–5 g/100 g DM), ultrasonic time (0–60 min) and pH (3.5–5.5) in ultrasound-enzymatic extraction method were examined for maximum lycopene content extraction, color parameters of L, a and b and antioxidant activity of the extracts (the data of color parameters of L, a and b and antioxidant activity are not shown in this paper). The numerical optimization estimates a point with maximized desirability function. The desired aim was selected by modifying the weight or importance that can modify the features of a goal. The importance of a dependent variable can be changed sequentially according to its increasing importance in relation to the other dependent variables for optimization. It can range from 1 (least importance) to 5 (most important) [40]. An importance of 1 was selected for antioxidant activity and an importance of 2 was selected for the other responses. One optimal extraction condition of cellulase concentration of 2.5 g/100 g DM, pectinase concentration of 0.8 g/100 g DM, sonication time of 60 min and pH of 5.3 was obtained. Results show that the experimental value of lycopene content at optimal treatment (93.4 ± 0.8 mg/kg DM) is in satisfactory agreement with the predicted value of lycopene content (100 mg/kg DM). Therefore, the mentioned RSM model (Eq. 3) for lycopene content is suitable in the prediction. The effectiveness of the optimal ultrasound-enzymatic extraction of lycopene was compared with the conventional stirring incubation method at the similar extraction time (17 min + 60 min = 77 min) of optimal extraction treatment without ultrasound pretreatment, cellulase and pectinase enzymes. This treatment was performed at the incubation temperature of 57 °C and regular shaking of 120 rpm for 77 min. Moreover, it had 1.14 g of dried tomato processing waste and 40 mL of a solvent mixture of hexane, acetone and methanol (2:1:1) containing 0.02 g butylated hydroxytoluene (BHT) at pH of 5.3. Results confirms that optimal ultrasound-enzymatic extraction treatment resulted in more lycopene content (93.4 ± 0.8 mg/kg DM) than that of stirring incubation treatment (26.1 ± 1.0 mg/kg DM). Furthermore, the optimal extraction treatment was compared to a treatment with similar conditions (cellulase concentration of 2.5 g/100 g DM, pectinase concentration of 0.8 g/100 g DM, sonication time of 60 min, pH of 5.3 and incubation time for 17 min at the temperature of 57 °C), but using distilled water solvent. Results showed that the optimal ultrasound-enzymatic extraction technique suing a solvent mixture of hexane, acetone and methanol led to more lycopene content than that of similar treatment using distilled water solvent (13.2 ± 0.1 mg/kg DM).
Scanning electron microscopy (SEM) images
To investigate and authenticate the influence of ultrasonic-enzymatic extraction treatments on the solid matrix of tomato processing waste powders, SEM analysis at a magnification factor of × 1000 was performed on (a) untreated tomato processing waste powder, (b) the dried residues after the second treatment of Table 2 having the lowest lycopene content extraction, and (c) the dried residues after the first treatment of Table 2 having the highest lycopene content extraction. Figure 3a–c present the images. It is obvious that tomato waste after the second treatment of Table 2 (cellulase concentration of 5 g/100 g DM, pectinase concentration of 2.5 g/100 g DM, ultrasonic time of 30 min and pH of 3.5) with the lowest lycopene content extraction was relatively smooth and intact (Fig. 3b), and it was similar to that of untreated tomato processing waste powder. However, it showed a greater amount of particulates on the surface in comparison to the untreated tomato waste powder due to extraction processes. In the case of Fig. 3c in which the tomato waste underwent the first treatment of Table 2 (cellulase concentration of 5 g/100 g DM, pectinase concentration of 0 g/100 g DM, ultrasonic time of 30 min and pH of 4.5), particle deposition is even more pronounced than that of Fig. 3b. Another noteworthy issue is that the surface of the tomato waste in Fig. 3c presented cracks and disruption. Thus, more appropriate digestion occurred in this treatment. Totally, these morphological changes represent that an ultrasonic-enzymatic extraction method under suitable conditions effectively liberated the bound lycopene by increasing surface area and improving solvent distribution. Therefore, the first treatment of Table 2 disrupted tissues, decreased the particle size, increased solvent distribution and enhanced the efficiency of mass transfer rate resulted in higher extraction yield of lycopene and a different SEM image (Fig. 3c).
Scanning electron microscopy images of tomato waste powders a untreated, b the remained solids after the second treatment of Table 2 (cellulase concentration of 5 g/100 g DM, pectinase concentration of 2.5 g/100 g DM, sonication time of 30 min and pH of 3.5) having the lowest lycopene content extraction, and c the remained solids after the first treatment of Table 2 (cellulase concentration of 5 g/100 g DM, pectinase concentration of 0 g/100 g DM, sonication time of 30 min and pH of 4.5) having the highest lycopene content extraction
Fourier transform infrared (FTIR) spectroscopy analysis
In this study, FTIR spectroscopic test was used to investigate the characteristic functional groups and the possible changes of the functional groups during the treatment having the highest lycopene content extraction (cellulase concentration of 5 g/100 g DM, pectinase concentration of 0 g/100 g DM, sonication time of 30 min, and pH of 4.5), the treatment having the least lycopene content extraction (cellulase concentration of 5 g/100 g DM, pectinase concentration of 2.5 g/100 g DM, sonication time of 30 min, and pH of 3.5), and the optimal extraction treatment having maximum lycopene content, L, a and b color parameters and antioxidant activity (cellulase concentration of 2.5 g/100 g DM, pectinase concentration of 0.8 g/100 g DM, sonication time of 60 min, and pH of 5.3). Figure 4 presents the FTIR spectra of these treatments. Although the mentioned FTIR spectra displayed numerous peaks, only sharp ones were considered for further investigations. In the FTIR spectra of Fig. 4, the bandwidth centered at 655.43 cm− 1 can be attributed to CH=CH stretching vibration [41]; the peak of 1014.58 cm− 1 represents a trans-R–HC=CH–R [42]; the peak of 1229.58 cm− 1 confirms a C–O stretching [43]; the peak of 1370 cm− 1 represents the –CH3 bond [44]; the strong peak at 1641.59 cm− 1 can be attributed to the C=C aromatic stretching [43, 45]; the peak at 1737.63 cm− 1 represents the carbonyl stretching (C=O bond) [46]; the peak at 2075.70 is indicative of C≡C symmetry stretching vibration [41]; the peaks of 2970.76 and 2843.62 cm− 1 indicate asymmetric and symmetric stretching of the functional C–H bond in CH2 groups [42, 45, 46] and the broad band at 3431.82 cm− 1 represents the hydroxyl group (O–H stretch) [45, 47]. By comparing the FTIR spectra, it can be concluded that application of ultrasound from 30 to 60 min, pectinase enzyme from 0 to 2.5 g/100 g DM, cellulase enzyme from 2.5 to 5 g/100 g DM as well as pH from 3.5 to 5.3 for lycopene pigment extraction from tomato processing waste powder did not destroy the functional groups of the extracts, since the position and type of functional groups did not change. These results are in line with the results obtained by Konwarh et al. [48] reporting no changes in the position and type of the functional groups of the FTIR spectra of the extracted biomolecules with and without application of sonication and enzyme.
FTIR spectra of the extracts obtained by three extraction methods: first treatment of Table 2 having the highest lycopene content extraction (cellulase concentration of 5 g/100 g DM, pectinase concentration of 0 g/100 g DM, sonication time of 30 min and pH of 4.5), second treatment of Table 2 having the lowest lycopene content extraction (cellulase concentration of 5 g/100 g DM, pectinase concentration of 2.5 g/100 g DM, sonication time of 30 min and pH of 3.5) and optimal extraction treatment (cellulase concentration of 2.5 g/100 g DM, pectinase concentration of 0.8 g/100 g DM, sonication time of 60 min and pH of 5.3)
Conclusion
Recently, there has been a growing interest and demand for natural dyes such as lycopene pigment. In the present study, the influence of four independent variables, including cellulase concentration (0–10 g/100 g DM), pectinase concentration (0–5 g/100 g DM), ultrasonic time (0–60 min) and pH (3.5–5.5) was optimized in a combined ultrasonic pretreatment-enzymatic process to extract lycopene pigment from tomato processing waste using response surface methodology. The main conclusions can be summarized as follows:
-
The overall optimization for maximum lycopene content extraction, color parameters of L, a and b and antioxidant activity of the extracts led to an optimal extraction condition of the cellulase concentration of 2.5 g/100 g DM, pectinase concentration of 0.8 g/100 g DM, sonication time of 60 min and pH of 5.3.
-
Scanning electronic microscopy results demonstrated that the treatment resulted in higher lycopene extraction, presented more cracks and disruption in the residues, which could be the probable mechanism for the increase of lycopene extraction yield.
-
Fourier Transform Infrared spectroscopy results did not show any destruction of the functional groups of the investigated extracts containing lycopene pigment.
According to the obtained results, it is suggested that ultrasound-enzymatic extraction method can be considered an appropriate industrial method resulting in higher extraction yield of lycopene from tomato processing waste. It may result in considerable economic and environmental benefits in terms of producing natural valuable colorants from inexpensive processing waste.
Abbreviations
- RSM:
-
Response surface methodology
- FTIR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning electronic microscopy
- BHT:
-
Butylated hydroxytoluene
- KBr:
-
Potassium bromide
- DM:
-
Dry matter
References
A. Saeid, J.B. Eun, M.S.A. Sagor, A. Rahman, M.S. Akter, M. Ahmed, J. Food Sci. 81, C2630–C2638 (2016)
J. Liao, N. Zheng, B. Qu, Food Anal. Methods 9, 2288–2298 (2016)
M.M. Poojary, P. Passamonti, Food Chem. 188, 84–91 (2015)
M.S. Lenucci, A. Caccioppola, M. Durante, L. Serrone, R. Leonardo, G. Piro, G. Dalessandro, J. Sci. Food Agric. 90, 1709–1718 (2010)
K. Sinha, S. Chowdhury, P. Das Saha, S. Datta, Ind. Crops Prod. 41, 165–171 (2013)
S.M. Choudhari, L. Ananthanarayan, Food Chem. 102, 77–81 (2007)
G. Özkan, S.Ersus Bilek, Food Chem. 176, 152–157 (2015)
A. Zuorro, R. Lavecchia, Biotechnol. Biotechnol. Equip. 24, 1854–1857 (2010)
A. Amiri-Rigi, S. Abbasi, M.G. Scanlon, Innov. Food Sci. Emerg. Technol. 35, 160–167 (2016)
A.F. Silva, M.M.R. de Melo, C.M. Silva, J. Supercrit. Fluids 95, 618–627 (2014)
R. Lavecchia, A. Zuorro, Eur. Food Res. Technol. 228, 153–158 (2008)
U. Topal, M. Sasaki, M. Goto, K. Hayakawa, J. Agric. Food Chem. 54, 5604–5610 (2006)
R. Chen, F. Meng, S. Zhang, Z. Liu, Sep. Purif. Technol. 66, 340–346 (2009)
S.R.R. Shirsath, S.H.H. Sonawane, P.R.R. Gogate, Chem. Eng. Process. Process Intensif. 53, 10–23 (2012)
M. Roiaini, H.M. Seyed, S. Jinap, H. Norhayati, Int. Food Res. J. 23, 47–54 (2016)
L. Guo, M.Y. Xie, A.P. Yan, Y.Q. Wan, Y.M. Wu, Anal. Bioanal. Chem. 386, 1881–1887 (2006)
A. Rosenthal, D.L. Pyle, K. Niranjan, Enzym. Microb. Technol. 19, 402–420 (1996)
S. Kumcuoglu, T. Yilmaz, S. Tavman, J. Food Sci. Technol. 51, 4102–4107 (2014)
A. Zuorro, M. Fidaleo, R. Lavecchia, Enzym. Microb. Technol. 49, 567–573 (2011)
Y. Sun, D. Liu, J. Chen, X. Ye, D. Yu, Ultrason. Sonochem. 18, 243–249 (2011)
A.L.-S.S. Eh, S.-G.G. Teoh, Ultrason. Sonochem. 19, 151–159 (2012)
Z. Lianfu, L. Zelong, Ultrason. Sonochem. 15, 731–737 (2008)
R.-E. Ghitescu, I. Volf, C. Carausu, A.-M. Bühlmann, I.A. Gilca, V.I. Popa, Ultrason. Sonochem. 22, 535–541 (2015)
S. Feng, Z. Luo, B. Tao, C. Chen, LWT 60, 970–976 (2015)
R.C. Ranveer, S.N. Patil, A.K. Sahoo, Food Bioprod. Process. 91, 370–375 (2013)
M.S. Lenucci, M. De Caroli, P.P. Marrese, A. Iurlaro, L. Rescio, V. Böhm, G. Dalessandro, G. Piro, Food Chem. 170, 193–202 (2015)
L. Manzocco, S. Plazzotta, S. Spilimbergo, M.C. Nicoli, LWT 85, 363–371 (2017)
I.F. Strati, E. Gogou, V. Oreopoulou, Food Bioprod. Process. 94, 668–674 (2015)
S. Joseph, M.K. Chatli, A.K. Biswas, J. Sahoo, J. Food Sci. Technol. 51, 3208–3216 (2012)
X. Lu, B.A. Rasco, Crit. Rev. Food Sci. Nutr. 52, 853–875 (2012)
M. Mirheli, S. Taghian Dinani, J. Food Meas. Charact. (2018). https://doi.org/10.1007/s11694-018-9796-2
F. Khalili, S. Taghian Dinani, J. Food Meas. Charact. 12, 974–981 (2018)
S. Taghian Dinani, N. Hamdami, M. Shahedi, J. Keramat, J. Food Process. Preserv. 38, 1269–1278 (2014)
A. Patil, A.K. Singh, J. Food Process Eng. 40, e12340 (2017)
Y.Z. Ghasemi, S. Taghian Dinani, J. Food Process Eng. (2018). https://doi.org/10.1111/jfpe.12696
H. Mirhosseini, C.P. Tan, N.S.A. Hamid, S. Yusof, B.H. Chern, Food Hydrocoll. 23, 271–280 (2009)
Z.J. Wei, A.M. Liao, H.X. Zhang, J. Liu, S.T. Jiang, Bioresour. Technol. 100, 4214–4219 (2009)
F. Chen, Q. Zhang, H. Gu, L. Yang, J. Chromatogr. A 1471, 68–79 (2016)
Y. Zou, C. Xie, G. Fan, Z. Gu, Y. Han, Innov. Food Sci. Emerg. Technol. 11, 611–615 (2010)
J.P. Maran, S. Manikandan, B. Priya, P. Gurumoorthi, J. Food Sci. Technol. 52, 92–104 (2015)
K. Sinha, P. Das Saha, S. Datta, Dye. Pigment. 94, 212–216 (2012)
J. López-Cervantes, D.I. Sánchez-Machado, K.P. Valenzuela-Sánchez, J.A. Núñez-Gastélum, A.A. Escárcega-Galaz, R. Rodríguez-Ramírez, Int. J. Food Sci. Nutr. 65, 187–193 (2014)
J.J. Chavan, D.M. Ghadage, P.R. Kshirsagar, S.S. Kudale, J. Liq. Chromatogr. Relat. Technol. 38, 963–969 (2015)
P. Gupta, M.P. Bansal, A. Koul, Phyther. Res. 27, 448–456 (2012)
M.M. Kamil, G.F. Mohamed, M.S. Shaheen, J. Am. Sci. 27, 253–260 (2011)
P.R. Bhutada, A.J. Jadhav, D.V. Pinjari, P.R. Nemade, R.D. Jain, Ind. Crops Prod. 82, 74–80 (2016)
T. Suganya, S. Renganathan, Bioresour. Technol. 107, 319–326 (2012)
R. Konwarh, S. Pramanik, D. Kalita, C.L. Mahanta, N. Karak, Ultrason. Sonochem. 19, 292–299 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimpour, S., Taghian Dinani, S. Lycopene extraction from tomato processing waste using ultrasound and cell-wall degrading enzymes. Food Measure 12, 2394–2403 (2018). https://doi.org/10.1007/s11694-018-9856-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9856-7