Abstract
Understanding the response of drugs and their formulations to thermal stresses is an integral part of the development of stable medicinal products. In the present study, the thermal degradation of two drug samples (cetirizine and simvastatin) was determined by differential scanning calorimetery (DSC) and simultaneous thermogravimetery/differential thermal analysis (TG/DTA) techniques. The results of TG analysis revealed that the main thermal degradation for the cetirizine occurs during two temperature ranges of 165–227 and 247–402 °C. The TG/DTA analysis of simvastatin indicates that this drug melts (at about 143 °C) before it decomposes. The main thermal degradation for the simvastatin occurs during two endothermic behaviors in the temperature ranges of 238–308 and 308–414 °C. The influence of the heating rate (5, 10, 15, and 20 °C min−1) on the DSC behavior of both the drug samples was verified. The results showed that as the heating rate was increased, decomposition temperatures of the compounds were increased. Also, the kinetic parameters such as activation energy and frequency factor for the compounds were obtained from the DSC data by non-isothermal methods proposed by ASTM E696 and Ozawa. Based on the values of activation energy obtained by ASTM E696 method, the values of activation energy for cetirizine and simvastatin were 120.8 and 170.9 kJ mol−1, respectively. Finally, the values of ΔS #, ΔH #, and ΔG # of their decomposition reaction were calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
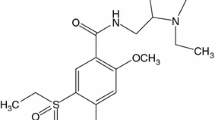
Cetirizine hydrochloride, the active component of ZYRTEC tablets and syrup, is an orally active and selective H1-receptor antagonist [1, 2]. The chemical name is (±)2-(2-{4-[(4-chlorophenyl)(phenyl)-methyl]piperazino}ethoxy)acetic acid dihydrochloride. Cetirizine hydrochloride is a racemic compound with an empirical formula of C21H25ClN2O3∙2HCl. Cetirizine hydrochloride is a white, crystalline powder and is water soluble. The molecular mass is 461.82, and the chemical structure is shown in Scheme 1. Cetirizine belongs to the group of medicines known as antihistamines. It can be used to prevent and treat allergic conditions such as hay fever and some allergic skin reactions.
Also, simvastatin (simvastatinum) or [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate (C25H38O5) belongs to anticholesteremic agents. It is a derivative of lovastatin and potent competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase (hydroxymethylglutaryl CoA reductases), which is the rate-limiting enzyme in cholesterol biosynthesis [3, 4]. It may also interfere with steroid hormone production. Due to the induction of hepatic LDL receptors, it increases breakdown of LDL cholesterol. Scheme 1 shows chemical structures of these drugs.
Thermal analysis methods are well-established techniques for studying the thermal properties of materials in reasonable ways [5–9]. In today’s pharmaceutical industry, discussion on a strategy for characterization of physical and chemical properties of drugs is necessarily complicated. While a “universal” approach is desirable, different drug compounds require different strategies and techniques (to determine thermal stability, particle size control, separation and extraction from matrix). Researchers now have access to many analytical techniques that allow them to study many aspects of drug properties, but a balance between adequacy and depth is essential [10–12].
Thermal analytical techniques can provide important information regarding storage and stability of pharmaceuticals [13, 14]. The most widely used thermal analysis techniques are differential thermal analysis (DTA), differential scanning calorimetry (DSC), and thermogravimetry/derivative thermogravimetry (TG/DTG) [15, 16]. These techniques are widely used in the pharmaceutical sciences for the characterization of solid drugs and excipients. The application of thermoanalytical methods may provide new information about the temperature and energy associated with events, such as melting, oxidation and reduction reactions, glass transition, boiling, sublimation, decomposition, crystallization, or gel to liquid crystal transition [17–19].
Solid-state kinetic studies have increasing importance in thermal analysis, in which the main purposes are to calculate the parameters of the Arrhenius equation and to determine the mechanism(s) of pyrolysis reaction. These data can provide valuable information about time and condition of storage [20–24]. The knowledge of such parameters for pure drugs and for drug–excipient mixtures is also meaningful to elucidate miscibility/incompatibility and its effects on thermal stability.
In this work, thermal behavior of cetirizine and simvastatin drugs were investigated by means of DSC and simultaneous thermogravimetry/differential thermal analysis (TG/DTA). The results allowed us to acquire information concerning these compounds in the solid-state, including their thermal stability and thermal decomposition. Also, this study seeks for determination of kinetic parameters of non-isothermal decomposition of the compounds. Although many investigations have been carried out on synthesis, characterization, purity, and compatibility of pure and formulated cetirizine and simvastatin samples as drugs [1–4], but those with solid samples have rarely been performed [23]. However, literature review illustrated that TG and DSC techniques were used to evaluate thermal stability, compatibility, and morphological properties of simvastatin [23]. Furthermore, to the best our knowledge, there is no report on the thermal behavior and degradation kinetic of these drugs.
Experimental
Cetirizine and simvastatin, pharmaceutical grade min. 99.5 % (Abureihan Pharma, Iran) were used without further purification. Thermogravimetery and differential thermal analysis were carried out using a Stanton Redcroft, STA-780 series with an aluminum crucible, applying heating rate of 10 °C min−1 in a temperature range of 50–600 °C, under nitrogen atmosphere with the flow rate of 50 mL min−1. The sample mass used was about 3.0 mg.
The DSC graphs were obtained by Du Pont differential scanning calorimeter model DSC 910S, in temperature range of 50–500 °C using an aluminum crucible, at different heating rates (5, 10, 15 and 20 °C min−1), under nitrogen atmosphere with the flow rate of 50 mL min−1. The instrument was calibrated at each heating rate considered using a dedicated 1 mg indium standard in an aluminum pan. Plotting for exothermic reactions was as downward deflection of the curve peak from the baseline.
Results and discussion
The thermoanalytical graphs of cetirizine are presented in Fig. 1a. The TG/DTA graphs obtained in nitrogen atmosphere showed that cetirizine decomposes during several steps which are endothermic and exothermic. Thermal events for this drug were started at about 165 °C and, as shown in DTA curve, continued with two consecutive endothermic peaks with maximum of 193 and 211 °C. These events match with the decomposition process and it is obvious from the TGA data that some mass loss occurs at the end of this process. During these steps, a relatively mild and continuous mass loss (Δm 1 = 5 %) over 165 °C was observed until 227 °C. However, at higher temperatures, cetirizine presents a main mass loss step between 247 and 402 °C, which is Δm 2 = 92.8 % in this step. The DTA curve showed two thermal events during this temperature range: the first is a broad endothermic behavior (T peak DTA = 312 °C) and another is an exothermic peak (T peak DTA = 382 °C). The total mass loss for this drug is about 97.8 % during 165–402 °C temperature range.
The simultaneous TG/DTA curves obtained in nitrogen atmosphere for the simvastatin are shown in Fig. 1b. In alignment with previous results [23] and according to the TG/DTA data, a sharp endothermic peak was observed at about 143 °C, without any change in the mass of sample, corresponding to the melting point of simvastatin. Up to the melting point, the simvastatin is thermally stable until 230 °C. However, at higher temperatures, simvastatin presents two main mass loss steps between 238 and 414 °C, which is Δm = 99.6 % during this temperature range. In the first step, TG/DTA graphs exhibit a sharp mass loss in 238–308 °C. The DTA curve showed a broad endothermic behavior in this temperature range (T peak DTA = 278.2 °C). The second mass loss is in the range 308–414 °C, which is a relatively mild mass loss step. DTA curve in this temperature range showed another broad endothermic behavior (T peak DTA = 365.4 °C). Table 1 summarizes the experimental results of TG/DTA obtained for each compound.
Effect of heating rate
Table 2 and Fig. 2 show DSC curves for the decomposition of cetirizine and simvastatin at several heating rates. It was found that by increasing the heating rate, the melting peaks and the decomposition temperature of the drugs were shifted to higher temperatures.
Kinetic methods
The ASTM method E698 [25] occupies intermediate position between the model-fitting and model-free methods. It uses a model-free estimate for the activation energy which is evaluated from Kissinger plot of \( {\text{ln }}\left( {{{\upbeta}}\,T_{\text{m}}^{ - 2} } \right) \) against 1/T m [26], where T m is the temperature corresponding to the maximum of dα/dT. In this study, ASTM method was used to determine the Arrhenius parameters for the thermal decomposition of cetirizine and simvastatin. In order to calculate the pre-exponential factor (Z), it was assumed that the decomposition followed first-order kinetics. The DSC curves obtained at various heating rates for the drugs are shown in Fig. 2. Also, Table 2 summarizes the experimental results as well as the maximum peak temperatures (T m) for each compound and heating rates (β) used to perform the calculations in the ASTM E698 method.
The plot of the \( {\text{ln }}({{\upbeta}}\,T_{\text{m}}^{ - 2} ) \) against 1/T m was straight lines (Fig. 3) for cetirizine (r = 0.9992) and simvastatin (r = 0.9989), which indicated that the mechanism of thermal decomposition of these compounds over this temperature range did not vary. The slope of the lines was equal to −E a /R. Therefore, the activation energy (E a) was obtained from the slope of the graph while the log of the pre-exponential factor, log(Z/S −1) was calculated from the expression given in ASTM E698:
Table 3 contains the calculated values of activation energy and frequency factors for both compounds.
Also, activation energy (E a) for these compounds was calculated by Ozawa method [27, 28]. In this method, activation energy could be determined from plots of the logarithm of the heating rate versus the inverse of the temperature at the maximum reaction rate in constant heating rate experiments. The activation energy can be determined by Ozawa method without a precise knowledge of the reaction mechanism, using the following equation:
The plot of logarithm of heating rates versus reciprocal of the absolute peak temperature for cetirizine and simvastatin was straight lines with r = 0.9993 and 0.9990 (Fig. 3), respectively. On the other hand, frequency factor (Z) was found for both compounds from the equation 1 [29].
All resulted data are summarized in Table 3. Comparing the results of the application of the two methods, we observe that values calculated for both compounds by Ozawa method are slightly higher than those of ASTM method. However, both methods reveal the same trend of activation energies for the whole conversion range studies.
After the kinetic parameters E a and Z were obtained, the thermodynamic parameters of activation can be calculated from the following equations [30]:
where, ΔG #, ΔH #, and ΔS # are free energy, enthalpy and entropy of the activation, and receptivity. υ is the υ = K B T/h (where K B and h are Boltzmann and Plank constant, respectively). Table 3 gives the computed thermodynamic parameters for both compounds studied.
Reaction rate constant determination
Assuming a first-order decomposition, the rate constant (K) for decomposition reaction could be calculated by the following equation [19]:
which was for the temperature of 25 °C; and the equation was solved for K using activation energies (E a) and frequency factors (Z) obtained above. Table 3 listed the Log K for each compound. By considering reaction rate constant calculated for drug samples, cetirizine reaction rate constant was compared with simvastatin reaction rate constant. It was found that the reaction rate constant of cetirizine is considerably (106) higher than that calculated for the simvastatin.
Comparison thermal behavior of drug samples
In this study, the thermal behavior of two drug samples was studied in identical conditions. These drugs showed different thermal behavior and stability ranges. For cetirizine, as seen in Fig. 1 and Table 1, initial decomposition occurred at about 165 °C, and the first step of decomposition process for this drug occurred during an endothermic phenomenon with two maximum temperatures of 193 and 211 °C with 5 % mass loss. The results show that simvastatin melts at 143 °C and its decomposition starts above 240 °C and take places during two endothermic steps. A comparison of decomposition temperature of these samples is shown in Table 1.
The values of the kinetic parameters that were obtained by the ASTM and Ozawa methods for these samples confirm the higher thermal stability of simvastatin in comparison with cetirizine. The values of kinetic parameters (activation energy and frequency factor) of simvastatin are about 1.5 times higher than the values for cetirizine. On the other hand, from the data presented in Table 3, it was found that the ratio of decomposition rate constant for cetirizine to simvastatin is about 106 and hence, rate of decomposition for cetirizine is considerably higher than simvastatin. These results show that cetirizine in comparison with simvastatin is a heat sensitive drug, has a shorter shelf-life, and needs more care during storage period.
Conclusions
The thermal stability of two drug samples was determined by DSC and TG/DTA. Also, the influence of the different heating rates on the DSC behavior of the cetirizine and simvastatin was verified. Activation energies and frequency factors for the decomposition were calculated by different methods. According to the TG/DTA data, it was verified that the thermal decomposition of cetirizine started during 165–215 °C with some successive phenomenon. However, simvastatin was decomposed at about 240 °C, after its melting at temperature of 143.4 °C. On the other hand, both the drug samples were decomposed completely until 450 °C.
The values of the kinetic parameters that were obtained by the ASTM and Ozawa methods for cetirizine and simvastatin showed good correlation, but the values obtained by the Ozawa method were slightly higher compared to those obtained by the ASTM method. On the other hand, the values of ΔS #, ΔG #, and ΔH # of the decomposition reaction of drugs were computed. Our finding showed that the values of the ΔG #, activation enthalpies (ΔH #), and activation entropies (ΔS #) for the decomposition of simvastatin are considerably higher than cetirizine.
References
Chen X, Ji ZL, Chen YZ. TTD: therapeutic target database. Nucleic Acids Res. 2002;30:412–5.
Tillement JP, Testa B, Bree F. Compared pharmacological characteristics in humans of racemic cetirizine and levocetirizine, two histamine H1-receptor antagonists. Biochem Pharmacol. 2003;66:1123–6.
Huang CY, Wu TC, Lin WT, Leu HB, Lin CP, Lin SJ, et al. Effects of simvastatin withdrawal on serum matrix metalloproteinases in hypercholesterolaemic patients. Eur J Clin Invest. 2006;36:76–84.
Lazzerini PE, Capecchi PL, Nerucci F, Fioravanti A, Chellini F, Piccini M, et al. Simvastatin reduces MMP-3 level in interleukin 1β stimulated human chondrocyte culture. Ann Rheum Dis. 2004;63:867–9.
Eslami A, Hosseini SG, Pourmortazavi SM. Thermoanalytical investigation on some boron-fuelled binary pyrotechnic systems. Fuel. 2008;87:3339–43.
Sovizi MR, Anbaz K. Kinetic investigation on thermal decomposition of organophosphorous compounds, N,N-dimethyl-N′,N′-diphenylphosphorodihydrazidic and diphenyl amidophosphate. J Therm Anal Calorim. 2010;99:593–8.
Hosseini SG, Eslami A. Thermoanalytical investigation of relative reactivity of some nitrate oxidants in tin-fueled pyrotechnic systems. J Therm Anal Calorim. 2010;101:1111–9.
Hosseini SG, Eslami A. Investigation on the reaction of powdered tin as a metallic fuel with some pyrotechnic oxidizers. Propellant Explos Pyrot. 2011;36:175–81.
Eslami A, Hosseini SG. Improving safety performance of lactose-fueled binary pyrotechnic systems of smoke dyes. J Therm Anal Calorim. 2011;104:671–8.
Huimei Y, Lingjun Q, Qinghong Z, Danyu J, Changwei L. Application of TA–MS combined with Pulse TA for characterization of materials. J Therm Anal Calorim. 2011;106:47–52.
Soares-Sobrinho JL, de La Roca Soares MF, Rolim-Neto PJ, Torres-Labandeira JJ. Physicochemical study of solid-state benznidazole–cyclodextrin complexes. J Therm Anal Calorim. 2011;106:319–25.
Yoshida MI, Oliveira MA, Gomes ECL, Mussel WN, Castro WV. Thermal characterization of lovastatin in pharmaceutical formulations. J Therm Anal Calorim. 2011;106:657–64.
Gaisford S, Buanz ABM. Pharmaceutical physical form characterisation with fast (>200 °C min−1) DSC heating rates. J Therm Anal Calorim. 2011;106:221–6.
Salvio Neto H, Nova’k C, Matos JR. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97:367–74.
Maximiano FP, Novack KM, Bahia MT, de Sá-Barreto LL, da Cunha-Filho MSS. Polymorphic screen and drug–excipient compatibility studies of the antichagasic benznidazole. J Therm Anal Calorim. 2011;106:819–24.
de Mello Costa AR, Marquiafável FS, de Oliveira Lima Leite Vaz MM, Rocha BA, Bueno PCP. Quercetin-PVP K25 solid dispersions, preparation, thermal characterization and antioxidant activity. J Therm Anal Calorim. 2011;104:273–8.
Neto HS, do Rosário Matos J. Compatibility and decomposition kinetics studies of prednicarbate alone and associated with glyceryl stearate. J Therm Anal Calorim. 2011;103:393–9.
Brown ME, Glass BD. Decomposition of solids accompanied by melting-bawn kinetics. J Pharm Biomed. 2003;254:255–61.
Rodomonte A, Antoniella E, Bertocchi P, Gaudiano MC, Manna L, Bartolomei M. Different crystal morphologies arising from different preparation methods of a same polymorphic form may result in different properties of the final materials: the case of diclofenac sodium trihydrate. J Pharm Biomed. 2008;48:477–81.
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, Hajimirsadeghi SS, Momenian H. Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;162:1141–4.
Sovizi MR, Hajimirsadeghi SS, Naderizadeh B. Effect of particle size on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;168:1134–9.
Sovizi MR. Thermal behavior of drugs investigation on decomposition kinetic of naproxen and celecoxib. J Therm Anal Calorim. 2010;102:285–9.
de Oliveira MA, Yoshida MI, de Lima EC. Thermal analysis applied to simvastatin characterisation in pharmaceutical formulations. Quim Nova. 2010;33:1653–7.
Eslami A, Hosseini SG, Shariaty SHM. Stabilization of ammonium azide particles through its microencapsulation with some organic coating agents. Powder Technol. 2011;208:137–43.
ASTM E698-05. Standard test method for Arrhenius kinetic constants for thermally unstable materials. doi:10.1520/E0698-05.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B. 1966;4:323–8.
Sunitha M, Reghunadhan Nair CP, Krishnan K, Ninan KN. Kinetics of alder-ene reaction of tris(2-allylphenoxy)triphen oxycyclotriphosphazene and bismaleimides: a DSC study. Thermochim Acta. 2001;374:159–69.
Tompa AS, Boswell RF. Thermal stability of a plastic bonded explosive. Thermochim Acta. 2000;357–358:169–75.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sovizi, M.R., Hosseini, S.G. Studies on the thermal behavior and decomposition kinetic of drugs cetirizine and simvastatin. J Therm Anal Calorim 111, 2143–2148 (2013). https://doi.org/10.1007/s10973-012-2651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2651-5