Abstract
In this work, TG/DTG and DSC techniques were used to the determination of thermal behavior of prednicarbate alone and associated with glyceryl stearate excipient (1:1 physical mixture). TG/DTG curves obtained for the binary mixture showed a reduction of approximately 37 °C to the thermal stability of drug (\( T_{{{\text{d}}m/{\text{d}}t = 0\,{\text{DTG}}}}^{\text{Max}} \)). The disappearance of stretching band at 1280 cm−1 (νas C–O, carbonate group) and the presence of streching band with less intensity at 1750 cm−1 (νs C–O, ester group) in IR spectrum obtained to the binary mixture submitted at 220 °C, when compared with IR spectrum of drug submitted to the same temperature, confirmed the chemical interaction between these substances due to heating. Kinetics parameters of decomposition reaction of prednicarbate were obtained using isothermal (Arrhenius equation) and non-isothermal (Ozawa) methods. The reduction of approximately 45% of activation energy value (E a) to the first step of thermal decomposition reaction of drug in the 1:1 (mass/mass) physical mixture was observed by both kinetics methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical kinetic concepts were originally based on empirical studies of homogeneous reactions in the gas phase. Solid-state kinetic concepts did not develop separately. However, applying these concepts was justified in the solid-state because of similarities to some homogeneous reactions [1]. The main objective of kinetic studies of chemical reactions is to determine kinetic parameters that can be used to predict the stability of some chemical species. Thermal analysis has been widely used for studies kinetics of solid-state reactions in order to determine kinetic parameters such as activation energy (E a) and reaction order [2–9].
When the reaction involves mass loss (Δm) as a function of heating, the kinetic study may be performed by thermogravimetry (TG). The kinetic study of solid-state reactions also can be performed by differential scanning calorimetry (DSC), based on heat evolved or consumed. The kinetic of thermally stimulated reaction is usually studied under conditions of isothermal or non-isothermal heating. Conventional method of isothermal study by TG uses the Arrhenius equation to determine these kinetic parameters. Ozawa’s method, originated by linear approximation based on integral calculus from Arrhenius equation, is widely used in non-isothermal study, allowing also determine the kinetic parameters of the reaction [2, 3, 5, 8, 10].
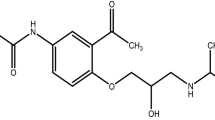
It were presented in this work, the results obtained in compatibility study between prednicarbate and glyceryl stearate excipient, and the results obtained in kinetic study of the first step of thermal decomposition reaction of drug alone and associated with glyceryl stearate (1:1 physical mixture). Prednicarbate is a potent corticosteroid, double-ester derivative of prednisolone (Fig. 1). This drug has a low potential in causing skin atrophy, being frequently used in the treatment of atopic dermatitis [11–16]. Glyceryl stearate excipient is an emulsifier frequently used in semi-solid pharmaceutical form. The kinetic parameters determination of drug decomposition reaction was performed by isohtermal and non-isothermal methods using, respectively, Arrhenius equation and Ozawa’s method.
Materials and methods
Materials
The prednicarbate, a drug used to accomplish the kinetic studies by isothermal and non-isothermal methods, was obtained from the Hawon Biochemical Science Co., Ltd, lot number HW05PDC002. This compound (Fig. 1) has a molecular mass and formula of 488.58 g mol−1 and C27H36O8, respectively. The glyceryl stearate was obtained from Oxiteno Industry, lot number 050917M12676.
Methods
Preparation of the binary mixture
The prednicarbate and glyceryl stearate excipient were ground separately in a mortar for 15 min until obtaining finely divided powder. The 1:1 (mass/mass) homogeneous physical mixture between them was obtained by the mixture of equal quantities of these substances (powders).
Thermal analysis
The DSC curves of prednicarbate, glyceryl stearate and binary mixture between them were recorded using Shimadzu model DSC-50 under dynamic N2 atmosphere (50 mL min−1) and at a heating rate (β) of 10 °C min−1 in the 25–550 °C temperature range, using aluminum crucibles partially closed and weighed approximately 2 mg of each samples. DSC cell was calibrated with In0 (ΔH fus = 28.57 J g−1; m.p. = 156.6 °C) and Zn0 (m.p. = 419.6 °C) standards. The TG/DTG curves of these same samples were obtained with a thermobalance Shimadzu model TGA-51 under dynamic air atmosphere (50 mL min−1) and at β = 10 °C min−1 between 25–900 °C, using platinum crucibles with approximately 25 mg of sample loads.
Isothermal thermogravimetric studies were performed using the drug alone and 1:1 physical mixture between prednicarbate and glyceryl stearate. The experiments were carried out using the same equipment and conditions of the dynamic TG, however, the samples were heated to 10 °C lower than T i (where T i is the isothermal temperature) at a β of 20 °C min−1 and that at 2 °C min−1 to reach T i. The initial value of T i was determined in the DTG curve for both the samples, corresponding to the maximum temperature that dm/dt had become different of zero. From this temperature (\( T_{{{\text{d}}m/{\text{d}}t = 0\,{\text{DTG}}}}^{\text{Max}} \), Table 1), other values were determined with temperature increases of 5 to 5 °C, allowing the construction of the Arrhenius plot. Thus, the drug samples in isothermal study were kept at 195, 200, 205, 210, and 215 °C, to 10% of mass loss. For the binary mixture, other T i values were used (Table 1): 150, 155, 160, 165, and 170 °C, also to 10% of mass loss. The Arrhenius plot (ln t, min vs. 1/T, K−1) was constructed with the experimental data obtained for both samples, based on 5% of mass loss [2, 5, 8, 10, 17].
Ozawa dynamic method was applied to the samples of drug alone and associated with glyceryl stearate (1:1 physical mixture), using TG kinetic analysis program installed in Shimadzu data acquisition system. The experiments were carried out using the same conditions and equipment of the dynamic TG, however, five different heating rates (β) were used: 2.5, 5.0, 10.0, 15.0, and 20.0 °C min−1 [5, 8, 17].
IR spectroscopy
IR spectra of prednicarbate, glyceryl stearate and 1:1 (mass/mass) physical mixture between them were recorded in equipment model MB102 (Bomem), at room temperature in the 4000–400 cm−1 range in KBr pellets.
Results and discussion
Compatibility study between prednicarbate and glyceryl stearate by TG/DTG, DSC and IR spectroscopy
TG/DTG curves of the prednicarbate sample (Fig. 2) showed no mass loss up to 200 °C. Above this temperature, four mass loss steps were observed. The DSC curve (Fig. 2) showed an endothermic event in the 175–200 °C temperature range; however, the TG/DTG curves showed no mass loss in this temperature interval. The sharp endothermic peak corresponded to melting process of prednicarbate (T onset = 182.7 °C; T peak = 186.2 °C; ∆H fus = 75.6 J g−1). The second thermal event observed in the DSC curve was endothermic and began immediately after the melting process and it corresponds to the first step of thermal decomposition of the drug. This first mass loss event occurred in the 207–291 °C (T onset TG = 243 °C; \( T_{{{\text{d}}m/{\text{d}}t = 0\,{\text{DTG}}}}^{\text{Max}} \) = 195 °C; T peak DTG = 264 °C) temperature range and it represents 20.7% (Δm 1) of mass loss [18]. The third event observed in the DSC curve is an exothermic one (T peak = 332.5 °C) and it corresponds to the second step of thermal decomposition observed in TG/DTG curves (Δm 2 = 28.4%, T peak DTG = 339 °C). The third and fourth mass loss steps (Δm 3 = 7.6% and Δm 4 = 42.6%, respectively) correspond to the secondary thermal decomposition.
Regarding to glyceryl stearate sample, its TG/DTG curves (Fig. 3) showed no mass loss up to 100 °C. Above this temperature, two mass loss steps were observed, corresponding to thermal decomposition events of this substance (T peak DTG = 253.4 °C and Δm = 17.2%, T peak DTG = 403.4 °C and Δm = 79.1%, respectively). The DSC curve obtained for this same sample (Fig. 3) showed two endothermic events in the 25–75 °C temperature range; however, the TG/DTG curves showed no mass loss in this temperature interval. An exothermal event was observed in DSC curve after 400 °C (T peak = 437.4 °C), temperature interval that TG/DTG curves showed mass loss, being assigned to this event a new thermal decomposition step.
The DSC curve obtained to 1:1 (mass/mass) physical mixture between prednicarbate and glyceryl stearate did not show the drug melting event, being assigned to this observation a previous dissolution of drug in the melt of excipient (Fig. 4). The changes observed in thermoanalytical profile of the prednicarbate in DSC curve demonstrated a sort of interaction due to heating. The TG/DTG curves of this binary mixture (Fig. 5) showed that the beginning of the first thermal decomposition step of drug was shifted towards a lower temperature (\( T_{{{\text{d}}m/{\text{d}}t = 0\,DTG}}^{\text{Max}} \)) [19]. The value observed to this temperature was 37 °C lower than the value obtained to the corresponding event in TG/DTG curves from drug alone (Table 1) [6, 20].
The IR spectra of prednicarbate and 1:1 (mass/mass) physical mixture samples submitted at 220 °C (Fig. 6) demonstrated a sort of interaction between these substances due to heating, according to the results obtained by thermal analysis. The IR spectrum of prednicarbate sample, before heating (Fig. 6), showed absorption band assigned to C=O group (νC=O) at 1752 cm−1 that is characteristic of the ester (C22) and carbonate (C25) groups. Furthermore, absorption bands at 1282 and 1083 cm−1 assigned to asymmetric stretching of C–O (νas C–O) in carbonate group and symmetric stretching of C–O (ys C–O) in ester group can be also observed. The presence of these representative bands in the IR spectrum of prednicarbate treated at 220 °C confirms its thermal stability up to this temperature. The disappearance of stretching band at 1280 cm−1 together with the presence of stretching band with lower intensity at 1750 cm−1 in the IR spectrum of 1:1 (mass/mass) physical mixture between drug and glyceryl stearate treated at 220 °C (Fig. 6) showed a supposed interaction in the drug–excipient mixture, indicating some changes in the chemical structure of prednicarbate [19].
Determination of the kinetic parameters of thermal decomposition reaction of prednicarbate by TG using the isothermal and non-isothermal methods
Figures 7, 8, 9, and 10 show the TG curves obtained in the isothermal kinetic study of the first step of thermal decomposition reaction of prednicarbate alone and associated with glyceryl stearate, and the Arrhenius plot (ln t, min vs. 1/T, K−1) constructed with these results. Figures 7 and 8 illustrate TG curves obtained under isothermal conditions, showing the correlation between mass loss (Δm, %) and temperature. The larger the temperature used, smaller the time necessary, so that the same mass loss occurs (5%). From the linear regression method, it were constructed Arrhenius plot and their equations were obtained (Figs. 9, 10). The activation energy (E a) could be calculated by the multiplication of slope value (Arrhenius equation) by the molar gas constant (R = 8.314 J mol−1 K−1). Table 2 lists the values of E a and linear correlation coefficient (r) obtained for both samples in the isothermal study.
The superposition of TG curves obtained in non-isothermal study (Ozawa’s method) to different heating rates (β), for the first thermal decomposition step of prednicarbate alone and associated with glyceryl stearate, is shown, respectively, in Figs. 11 and 12. In these figures were presented the respective graphics ln β versus 1/T (K−1), which demonstrated a fairly good correlation at five heating rates. Activation energy (E a) to this thermal decomposition step of drug was also calculated by Ozawa’s method for both the samples. Table 2 lists the values of E a (kJ mol−1), reaction order and reaction frequency factor (A, min−1) obtained by non-isothermal kinetic study.
The percentage reduction of E a value obtained by the Ozawa’s method to the first step of the thermal decomposition reaction of drug associated with glyceryl stearate (81 kJ mol−1) when compared with E a observed to the drug alone (145 kJ mol−1), was in good agreement to the results of isothermal kinetic analysis (from 302 to 160 kJ mol−1), being approximately equal to 45%. These results confirmed the lower thermal stability of drug in the presence of glyceryl stearate, according with results obtained in the compatibility study performed by thermal analysis and IR spectroscopy.
Conclusions
The results obtained in the compatibility study between prednicarbate and glyceryl stearate by thermal analysis and IR spectroscopy presented a modification of the thermal stability of drug in the presence of excipient (\( \Updelta T_{{{\text{d}}m/{\text{d}}t = 0\,{\text{DTG}}}}^{\text{Max}} \) = 37 °C), showing there was a strong chemical interaction between these substances due to heating. The comparative assessment of E a value obtained to the first step of thermal decomposition reaction of prednicrabate alone and associated with the excipient, by isothermal (Arrhenius equation) and non-isothermal (Ozawa’s method) kinetics studies, confirmed the results from compatibility study. The reduction of approximately 45% of E a value obtained by both kinetics methods for this thermal decomposition step of drug when in the presence of excipient, showed a lower thermal stability of drug.
Thus, the compatibility and kinetics results showed that the association of the prednicarbate with glyceryl stearate to develop a semi-solid pharmaceutical form is not recommended. It was also noted that the kinetic study performed by thermogravimetry can be a fast alternative or a complementary method to assess the stability of drugs, especially in the presence of excipients during the development of pharmaceutical formulation.
References
Galwey AK, Brown ME. Application of the Arrhenius equation to solid state kinetics: can this be justified. Thermochim Acta. 2002;386:91–8.
Cheng Y, Huang Y, Alexander K, Dollimore D. A thermal analysis study of methyl salicylate. Thermochim Acta. 2001;367–8:23–8.
Rodante F, Catalani G, Tomassetti M. Application of TA and kinetic study to compatibility and stability problems in some commercial drugs. J Therm Anal Calorim. 2001;66:155–78.
Burnham L, Dollimore D, Alexander KS. Kinetic study of the drug acetolamide using thermogravimetry. Thermochim Acta. 2002;392:127–33.
Rodante F, Catalani G, Vecchio S. Kinetic analysis of single or multi-step decomposition processes. Limits introduced by statistical analysis. J Therm Anal Calorim. 2002;68:689–713.
Oliveira GGG, Ferraz HG, Matos JR. Thermoanalytical study of Glibenclamide and excipient. J Therm Anal Calorim. 2005;79:267–70.
Skaria CV, Gaisford S, O’Neill MAA, Buckton G, Beezer AE. Stability assessment of pharmaceuticals by isothermal calorimetry: two components systems. Int J Pharm. 2005;292:127–35.
Cides LCS, Araujo AAS, Santos-Filho M, Matos JR. Thermal behaviour, compatibility study and decompositions kinetics of glimepiride under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2006;84:441–5.
Felix FS, Cides da Silva LC, Angnes L, Matos JR. Thermal behavior study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95(3):877–80.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6.
Lange K, Gysler A, Bader M, Kleuser B, Korting HC, Schafer-Korting M. Prednicarbate versus conventional topical glucocorticoids: pharmacodynamic characterization in vitro. Pharm Res. 1997;14:1744–9.
Gysler A, Kleuser B, Sippl W. Skin penetration and metabolism of topical glucocorticoids in reconstructed epidermis and in excised human skin. Pharm Res. 1999;16:1386–91.
Lange K, Kleuser B, Gysler A. Cutaneous inflammation and proliferation in vitro differential effects and mode of action of topical glucocorticoids. Skin Pharmacol Phys. 2000;13:93–103.
Almawi WY, Tamim H. Posttranscriptional mechanisms of glucocorticoid antiproliferative effects: glucocorticoids inhibit IL-6-induced proliferation of B9 hybridoma cells. Cell Transpl. 2001;10:161–4.
Krakowski AC, Eichenfield LF, Dohil MA. Management of atopic dermatitis in the pediatric population. Pediatr. 2008;122(4):812–24.
Leung DY, Eichenfield LF, Boguniewicz M. Atopic dermatitis (atopic eczema). In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s dermatology in general medicine. New York: McGraw-Hill Companies; 2008. p. 146–58.
Ozawa T. Thermal analysis—review and prospect. Thermochim Acta. 2000;355:35–42.
Salvio Neto H, Barros FAP, Carvalho FMS, Matos JR. Thermal analysis of prednicarbate and characterization of thermal decomposition product. J Therm Anal Calorim. 2010;102(1):277–83.
Salvio Neto H, Novák C, Matos JR. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97(1):367–74.
Verma RK, Garg S. Selection of excipients for extended release formulations of glipizide through drug–excipient compatibility testing. J Pharm Biomed Anal. 2005;38:633–44.
Acknowledgements
The authors acknowledge to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Stiefel Laboratories for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salvio Neto, H., Matos, J.R. Compatibility and decomposition kinetics studies of prednicarbate alone and associated with glyceryl stearate. J Therm Anal Calorim 103, 393–399 (2011). https://doi.org/10.1007/s10973-010-1080-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1080-6