Abstract
Quercetin is a flavonoid very well studied and has already entered clinical trials emerging as prospective anticancer drug candidate. In addition, quercetin has being reported to its free-radical scavenging activity and suggests potential uses for the prevention and treatment of pathologies as atherosclerosis, chronic inflammation, and others. However, quercetin is sparingly soluble in water, which may be responsible for its limited absorption upon oral administration. The solid dispersion of quercetin with polyvinylpyrrolidone Kollidon® 25 (PVP K25) suggests an interesting way to increase quercetin solubility, antioxidant activity, and consequently bioavailability. Then, the purpose of this study was to prepare solid dispersions of quercetin with PVP K25 and evaluate their thermal characterization, antioxidant activity and quercetin improvement solubility. For this purpose, quercetin-PVP K25 solutions were dried and quercetin-PVP K25 solids were obtained. The formation of quercetin-PVP K25 solid dispersion was evaluated by solubility studies, powder X-ray diffraction (XRD), fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC), thermogravimetry (TG), and antioxidant activity. It was observed that PVP K25 was able to provide quercetin clear aqueous solutions and that quercetin solubility was increased in a PVP K25 concentration dependent manner, improving solubility even 436-fold the pure quercetin. The results obtained with XRD, FT-IR, DSC, and TG demonstrated possible quercetin-PVP K25 solid dispersion formation. Besides, the antioxidant activity of the quercetin-PVP K25 solid dispersions dissolved in aqueous solution and pure quercetin dissolved in methanol showed IC50 value of 0.61 ± 0.03 and 1.00 ± 0.02 μg/mL, respectively, demonstrating that the solid dispersions presented a significant increase in antioxidant activity (P < 0.05). Putting results together, it was possible to conclude there was the formation of quercetin-PVP K25 solid dispersion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
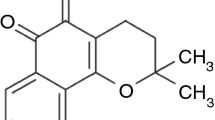
In recent years, there has been an intensive researcher’s interest in evaluating and standardizing natural/herbal extracts and compounds obtained from natural sources and the antioxidant activity is one of the several ways to evaluate and standardize them [1]. Quercetin (Fig. 1) and other flavonoids are related to their free-radical scavenging activity and suggest their antioxidant activity being focused on their potential uses for the prevention and treatment of related oxidative pathologies such as atherosclerosis, cancer, and chronic inflammation [4–9]. Quercetin is very well studied and has already entered clinical trials emerging as prospective anticancer drug candidate [10–12]. It belongs to a large group of naturally occurring flavonoid compounds found in plants, foods, and beverages. Flavonoids represent a sub-group of intensely colored polyphenolic phytochemicals. They contribute to plant color, providing a spectrum of colors from red to blue in flowers, fruit and leaves. Due to some interesting health-benefiting properties, flavonoids are widely examined in terms of chemistry as well as biological activity. The antioxidant, antitumor, and antibacterial activity of flavonoids is the focus of the attention of many researchers in pharmaceutical and medicine chemistry [4, 9–14].
However, quercetin is sparingly soluble in water, which may be responsible for its limited absorption upon oral administration [2–4, 6, 9]. The pharmacokinetics of quercetin demonstrated that after oral administration no measurable plasma concentrations could be detected and no quercetin was found in urine, neither unchanged nor in a metabolized form. These results exclude absorption of more than 1% of unchanged drug [15]. Therefore, it is interesting to develop novel quercetin soluble formulations in order to improve its bioavailability.
To improve solubility of little soluble molecules and to control their enhancing, there are several techniques like the obtainment of nanoparticles, liposomes, microspheres, microemulsions, cyclodextrin, and polyvinylpyrrolidone complexation. However, the complexation with cyclodextrin is associated with a risk of nephrotoxicity and employing liposome might incur stability problems during storage [16]. Therefore, it is clear that a safe, stable, and efficient delivery system is necessary to assure that the increasing of quercetin solubility be warranted.
The polyvinylpyrrolidone Kollidon® 25 (PVP K25) is a polymer used to improve the solubility and bioavailability of little soluble molecules [17–21]. The soluble grades of Kollidon® are obtained by free-radical polymerization of vinylpyrrolidone in water or isopropanol according to the cGMP regulations, yielding the chain structure of polyvinylpyrrolidone, showed in Fig. 2.
The complexation of quercetin with PVP suggests an interesting way of increasing quercetin solubility, antioxidant activity, and consequently bioavailability. Then, the purpose of this study was to prepare solid dispersions of quercetin with PVP K25 and evaluate their thermal characterization, antioxidant activity, and quercetin improvement solubility. For this purpose, quercetin-PVP K25 solutions were dried and quercetin-PVP K25 solids were obtained.
Materials and methods
Preparation of solid dispersion
The quercetin sample (lot number MM 2-6003) was courtesy of Merck® and PVP K25 (Polyvinylpyrrolidone Kollidon 25)—molecular mass between 28,000 and 34,000 g/mol—(lot number 28764336W) was courtesy of Basf®. All other chemicals and solvents were analytical grade and recent distilled water.
For the preparation of quercetin-PVP K25 aqueous solution, constant and excess amount of quercetin (5 mg/g) were added to increasing concentrations of PVP K25 solutions, ranging from 1.3 to 47.4% m/m, in dibasic/monobasic sodium phosphate buffer (0,1 M, pH = 7,0). The preparations were maintained at 200 rpm using a magnetic stirrer, at a controlled temperature of 323 K during 24 h. Soon after, samples were filtered through a 0.45 μm cellulose filter to obtain the aqueous solution. The dried samples were obtained by carefully evaporating the water at 323 K. Physical mixtures were prepared in the same proportions. The quercetin-PVP K25 solid dispersions were analyzed by X-ray diffraction (XRD), Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), and thermogravimetry (TG).
X-Ray diffraction
X-rays diffraction patterns were obtained using a Kristalloflex Simens Diffractometer with a Ni filter and CuKα radiation, step pass of 0.02° and a step time of 3 s, from 4 to 70° (2θ angle).
Fourier transform infrared spectroscopy
The FT-IR spectra for all dried samples were obtained on a PerkinElmer Spectrum 1000 FT-IR Spectrophotometer. Pellets were prepared from mixtures of the samples and KBr (1:100 in weight mass). Scans were done at a resolution of 4 cm−1.
Thermogravimetry
The TG curves were obtained from SDT equipment from TA Instruments. The conditions used in the experiments were: nitrogen atmosphere at a flow rate of 50 mL min−1, heating rate of 283 K min−1 from 283 to 873 K and alumina pans.
Differential scanning calorimetry
The DSC curves were obtained from ambient temperature to 673 K from DSC equipment, model Q600 from TA Instruments using a sealed aluminum pan under a nitrogen atmosphere (50 mL min−1) and a heating rate of 283 K min−1. The instrument was calibrated (temperature and enthalpy) using the indium standard.
Solubility studies
The solubility studies were to quantify the quercetin content that was able to be solubilized by different concentrations of PVP K25 and compare to the pure quercetin content in aqueous solution. The quantitative analyses for determination of quercetin content were obtained according to the methodology developed by [22, 23]. For that, UV-spectrophotometry was used (Shimadzu UV min-1240) at the wavelength of 425 nm. The several samples were diluted with methanol to the linear range previously determined. Then, they were reacted with aluminum chloride and, after 30 min reaction, samples were analyzed. The quercetin content was calculated against an analytical curve of the quercetin standard prepared under the same conditions.
Antioxidant activity
The antioxidant activity was evaluated by the DPPH methodology [24]. For that, a series of concentration of pure quercetin was dissolved in methanol. The quercetin-PVP K25 solid dispersion was dissolved in distilled water to obtain 50 μg/mL of quercetin content. For the reaction solution was added 1 mL of acetate buffer 0.1 M pH = 5.5, 1 mL of ethanol, 1 mL of DPPH solution 125 M, and 50 μL of the sample. The reaction solutions were incubated and protected of light at room temperature for 30 min. The absorbance was measured in UV–vis spectrophotometer at 517 nm. The percentage of inhibition of DPPH for each sample was calculated according to the following equation:
The concentration of 50% of inhibition of DPPH (IC50) was determined and used to compare the antioxidant activity. All the determinations were performed in triplicate. The t-Student test was used to calculate statistical significance (P = 0.05).
Results and discussion
X-Ray diffraction
Diffractograms patterns presented in Fig. 3 refers to the quercetin, PVP K25, quercetin-PVP K25 solid dispersion, and physical mixture. Quercetin, Fig. 3a, shows crystalline nature, which is confirmed by sharp peaks on 14, 26.5, 27.3 and 44.1 in 2θ angle [25, 26]. PVP K25 is described in literature as predominantly amorphous polymer, as observed in Fig. 3b. In the diffractograms Fig. 3c–e and f quercetin peaks are absent, suggesting quercetin is well dispersed in PVP K25 in the solid dispersions, in physical mixtures. X-Ray diffraction shows that quercetin crystals were converted into microcrystalline or amorphous forms [27].
Fourier transform infrared spectroscopy
The infrared spectra of quercetin, PVP K25, solid dispersions and physical mixtures are shown in Fig. 4. The bands of quercetin were visible on their spectra and also in physical mixture and solid dispersions on the spectra were maintained, becoming more or less intense as the concentration of the polymer varies. Pure quercetin, Fig. 4a, shows specific bands in 1664 and 3560 cm−1, referring to carbonylic stretching (C=O) groups and O–H stretching, respectively [26]. PVP K25 has specific bands in 1400 up to 1680 cm−1, relative to amide groups stretching [25], and which represents high intermolecular binds, lead of the large absorption peak evident at 1668 cm−1 in Fig. 4b [28], which corresponds to a mixing band of C=O and C–N stretching vibrations [25]. It can be viewed in spectra of quercetin-PVP K25 physical mixture 50:1 and 108:1, Fig. 4c and d, a similar behavior as pure PVP K25, mainly due to the large amounts of PVP K25 in this sample. Quercetin-PVP K25 solid dispersion 50:1, Fig. 4e, shows less amplitude peak at 1660 cm−1 and changes in this absorption interval, but no other important consideration can be assigned. Meanwhile, quercetin-PVP K25 solid dispersion 108:1 spectra, Fig. 4f, shows abroad shift peak on 1660 cm−1, due to the fact that PVP K25 is more concentrated in the sample. The changes of the, Fig. 4f, spectra provide us the hypothesis of solid dispersion formation, to verify the other analysis.
All the samples of quercetin-PVP K25 mixture showed less expressive peaks on OH stretching, suggesting the formation of hydrogen bonding between carbonyl groups presents in PVP K25, more specifically pyrrolidone ring and phenols aromatic group presents on quercetin [26].
Thermal behavior
The TG and DSC curves are shown in Figs. 5 and 6, respectively, for the samples quercetin, PVP K25, quercetin-PVP K25 solid dispersion, and physical mixture.
Quercetin shows a mass loss (around 5%), Fig. 5a that occurs from ambient temperature up to 423 K. This event is attributed to surface water evaporation. The thermal decomposition of quercetin starts at about 523 K, and a great event involving mass loss (55%) was observed in the temperature range 523–873 K. DSC curve for quercetin, (Fig. 6) curve a, shows a sharp endothermic peak around 601 K, due to its melting, and an exothermic peak near 617 K, which refers to its decomposition [29].
According to the TG curve, Fig. 5b, PVP K25 has two events involving mass loss, the first one occurs 298–423 K due to water loss, and the second event beginning at 663 K assigned to the polymer decomposition. These events are confirmed in DSC curve, Fig. 6b, by an endothermic peak near 373 K, and an exothermic peak near 613 K [28, 30, 31].
The TG curves for all quercetin-PVP K25 show a similar profile as compared to pure PVP K25. TG and DSC curves of quercetin-PVP K25 suggest that quercetin is well dispersed in PVP K25. This hypothesis is confirmed as DSC curves, as shown in Fig. 5c–e, which revealed an absence of fusion peaks characteristic of the pure quercetin [28, 31]. According to TG and DSC curves of quercetin-PVP K25 systems, from Figs. 5c–e and 6c–e, the obtained materials present a thermal stable profile, which is closed to the thermal decompositions of pure PVP K25, showing that the presence of quercetin do not interfere the thermal profile of the PVP K25. These data strongly suggest a good interface between PVP K25 and quercetin.
Solubility studies
The quercetin-PVP K25 aqueous solutions obtained were clear and bright yellow. The visual aspect showed that the PVP K25 made quercetin more soluble being an evidence of solid dispersion formation. As showed in Fig. 7, the increase of quercetin solubility happened as a PVP K25 concentration dependent manner. In a 47% m/m PVP K25 concentration, the quercetin solubility improvement was 436-fold the pure quercetin (Fig. 7).
These results were consistent with the literature [3, 25]. The former presented that PVP K30 was able to improve PVP/aglycone flavonoids solubility and the latter found that 20% m/v PVP K30 solution was able to improve 200-fold quercetin solubility at 293 K compared to the pure quercetin. Besides, the quercetin increasing solubility was a PVP K30 concentration dependent manner probably explained by a decrease of the interfacial tension between quercetin and the dissolving solution.
Antioxidant activity
The scavenging activity of the DPPH radical is a very simple method, which is currently used in laboratory, due to its rapidity and low costs. DPPH is a stable free radical that potentially reacts with the compounds able to donate H+, generating a deep violet solution in organic solvents. Its progressive discoloration, when in the presence of quercetin, indicated that it is acting as an antioxidant. Furthermore, since the mechanism of DPPH reduction is known, the amount of both reagents remaining may be determined. The rate of the DPPH scavenging reaction was measured by monitoring the decrease in absorbance at 517 nm, which corresponds to a second order decay [6].
The inhibition concentration of 50% of DPPH (IC50) obtained in the present study to pure quercetin dissolved in methanol was 1,0 ± 0,02 μg/mL (Fig. 8) and IC50 of the quercetin-PVP K25 solid dispersion dissolved in aqueous solution was 0,61 ± 0,03 μg/mL, showing that the solid dispersion had a significantly higher antioxidant activity than the pure quercetin (P < 0.05). It was interesting when the results of the present study were compared to the obtained by [16], where quercetin-loaded nanoparticle was prepared by a nanoprecipitation technique with Eudragit® E (EE) and polyvinyl alcohol (PVA) as carriers. In that study, the antioxidant effects of quercetin in DMSO, quercetin in water and finally quercetin nanoparticles were studied by DPPH scavenging method and the results (IC50) were 4.24 ± 0.48, 3746.99 ± 611.68, and 7.04 ± 0.85 μg/mL, respectively, showing that the quercetin-PVP K25 solid dispersion proposed in this work presented excellent DPPH radical scavenge activity and higher than the quercetin nanoparticles, suggesting that a simpler technique used here furnished a better antioxidant result.
Conclusions
The thermal characterization in addition to the solubility studies got to the conclusion that it is possible to obtain quercetin-PVP K25 solid dispersions. Besides, the visual aspect showed crystalline solutions being an evidence of solid dispersion formation. The antioxidant activity was higher to the quercetin-PVP K25 solid dispersion than the pure quercetin and the quercetin-loaded nanoparticles obtained by [16]. Probably, these antioxidant activity results were possible because of the quercetin solubility increasing due to the solid dispersion formation.
References
Marquelle-Oliveira F, Fonseca YM, Georgetti SR, Vicentini FTMC, Bronzati V, Fonseca MJV. Evaluation of the antioxidant activity as an additional parameter to attain the functional quality of natural extracts. Latin Am J Pharm. 2008;27:325–32.
Singh K, Marangoni DG. Microcalorimetric determination of effect of the antioxidant (Quercetin) on polymer/surfactant interactions. J Therm Anal Calorim. doi:10.1007/s10973-010-0864-z.
Kanaze FI, Kokkalou E, Niopas I, Georgarakis M, Stergiou A, Bikiaris D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J Therm Anal Calorim. 2006;83:283–90.
Rice-Evans CA, Miller NJ, Paganga G. Structure antioxidant activity relationship of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–56.
López-Revuelta A, Sánchez-Gallego JI, Hernández-Hernández A, Sánchez-Yagüe J, Llanillo M. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact. 2006;161:79–91.
Cook NC, Samman S. Flavonoids, chemistry, metabolism, cardioprotective effects and dietary sources. J Nutr Biochem. 1996;7:66–76.
Bors W, Heller W, Michel C, Saran M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–55.
Lu J, Zheng Y, Luo L, Wu D, Sun D, Feng Y. Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171:251–60.
Budavari S, O’Neil MJ, Smith A, Heckelman PE, Kinneary JF. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. 12th ed ed. Whitehouse Station: Merck & Co; 1996.
Indap MA, Bhosle SC, Tayade PT, Vavia PR. Evaluation of toxicity and antitumour effects of a hydroxypropyl β-cyclodextrin inclusion complex of quercetin. Indian J Pharm Sci. 2002;64:349–53.
Hirpara KV, Aggarwal P, Mukherjee AJ, Joshi N Burman AC. Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bioavailability. Med Chem. 2009;9:138–61.
Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Ye B, Wang R, Diao P, Zhang W, Wu HB, Zhao X, Wei YQ. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res. 2006;12:3193–9.
Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann NY Acad Sci. 1998;854:435–42.
Jullian C, Moyano L, Yañez C, Azar-Olea C. Complexation of quercetin with three kinds of cyclodextrins: an antioxidant study. Spectrochimica Acta Part A Mol Biomol Spectrosc. 2007;67:230–4.
Gugler R, Leschik M, Dengler HJ. Disposition of quercetin in man after single oral and intravenous doses. Eur J Clin Pharm. 1975;9:223–34.
Wu TH, Yen FL, Lin LT, Tsai TR, Lin CC, Cham TM. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int J Pharm. 2008;346:160–8.
Papageorgiou GZ, Docoslis A, Georgarakis M, Bikiaris D. The effect of the physical state on the drug dissolution rate: miscibility studies of nimodipine with PVP. J Therm Anal Calorim. 2009;95:903–15.
Karavas E, Georgarakis E, Bikiaris D. Adjusting drug release by using miscible polymer blends as effective drug carries. J Therm Anal Calorim. 2006;84:125–33.
Friedrich H, Fussnegger B, Kolter K, Bodmeier R. Dissolution rate improvement of poorly water-soluble drugs obtained by adsorbing solutions of drugs in hydrophilic solvents onto high surface area carriers. Eur J Pharm Biopharm. 2006;62:171–7.
Ammar HO, Ghorab M, El-Nahhas SA, Makram TS. Improvement of the biological performance of oral anticoagulant drugs.1. Warfarin. Pharmazie. 1997;52:627–31.
El-Arini SK, Leuenberger H. Dissolution properties of praziquantel-PVP systems. Pharm Acta Helvetiae. 1998;73:89–94.
Dowd LE. Spectrophotometric determination of quercetin. Anal Chem. 1959;31:1184–7.
Jay M, Gonnet JF, Wollenwebwer E, Voirin B. Sur lánalyse qualitative des aglycones flavoniques dans une optique chimiotaxinomique. Phytochem. 1975;14:1605–12.
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30.
Zhu J, Yang ZG, Chen XM, Sun JB, Awuti G, Zhang X, Zhang Q. Preparation and physicochemical characterization of solid dispersion of quercetin and polyvinylpyrrolidone. J Chin Pham Sci. 2007;16:51–6.
Pralhad T, Rajendrakumar K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J Pharm Biomed Anal. 2004;34:333–9.
Zheng Y, Chow AHL. Production and characterization of a spray-dried hydroxypropyl-beta-cyclodextrin quercetin complex. Drug Dev Ind Pharm. 2009;35:727–34.
Marin MT, Margarit MV, Salcedo GE. Characterization and solubility study of solid dispersions of flunarizine and polyvinylpyrrolidone. II Farmaco. 2002;57:723–7.
Rosenkrantz H, Skogstron P. Characteristic infrared absorption bands of steroids with reduced ring A. I. tetrahydro compounds. J Am Chem Soc. 1955;77:2237–41.
Costa EM, Filho JMB, Nascimento TG, Macêdo RO. Thermal characterization of the quercetin and rutin flavonoids. Thermochimica Acta. 2002;392:79–84.
Razzak MT, Zainuddin E, Dewi SP, Lely H, Taty E, Sukirno. The characterization of dressing component materials and radiation formation of PVA–PVP hydrogel. Radiat Phys Chem. 1999;55:153–65.
Acknowledgements
The authors are grateful to APIS FLORA (Apis Flora Indl. Coml. Ltda.), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPQ-Brasil (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Mello Costa, A.R., Marquiafável, F.S., de Oliveira Lima Leite Vaz, M.M. et al. Quercetin-PVP K25 solid dispersions. J Therm Anal Calorim 104, 273–278 (2011). https://doi.org/10.1007/s10973-010-1083-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1083-3