Abstract
Although not being the ideal drug due to its low solubility and high toxicity, the benznidazole is the drug currently chosen for Chagas disease treatment. The deep knowledge about the characteristics of the drug in addition to the knowledge of more effective vectorization techniques of drugs in pharmaceutical forms allows a faster and cheaper development of a new therapeutic alternative in comparison to the introduction of a new molecule in the treatment. The aim of this study is the characterization of inclusion complexes Benznidazole and cyclodextrins in solid state. The interactions between Benznidazole (BNZ) and β-cyclodextrins (β-CD) modified: randomly methylated β-CD (RMβCD) and sulfobutylether β-CD (SBβCD) were studied by differential scanning calorimetry (DSC), fourier transform-infrared spectroscopy, RAMAN, and scanning electron microscopy. The preparation of solid-state binary systems by different techniques, namely, kneading, evaporated, and freeze-drying. The results suggest the formation of inclusion complexes of the drug with both CDs types in solid state by the techniques which were used, based on physicochemical data of interaction compared to the drug or the CDs/drug physical mixture. Thus, the preparation technique played an important role in the BNZ and modified CDs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chagas disease is a global problem and estimated to infect 9.8 million people worldwide. Scientists from all over the world have been working on the development of new therapeutic alternatives to treat the disease, since the discovery of new biochemistry targets to the development of new drugs, with potential action against Trypanosoma cruzi . Several chemotherapeutic agents have already been identified, and continue being tested almost a century after the discovery of the disease in 1909, by Carlos Chagas [1].
The morbidity and mortality rates associated with Chagas Disease in Latin America show a greater magnitude than the rates related to malaria, schistosomiasis, and leishmaniasis, which constitutes one of the biggest public health problems of developing nations [1, 2].
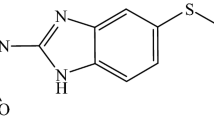
Although not being the ideal drug due to its low solubility and high toxicity, the benznidazole (BNZ), namely, N-benzyl-2-(2-nitroimidazol-1-yl) acetamide (Fig. 1) is the drug currently chosen for Chagas disease treatment [3]. The deep knowledge about the characteristics of the drug in addition to the knowledge of more effective vectorization techniques of drugs in pharmaceutical forms allows a faster and cheaper development of a new therapeutic alternative in comparison to the introduction of a new molecule in the treatment. Few technological boarding had been carried out until the present time aiming the better vectorization of BNZ and, none of them are related to a solid pharmaceutical form [2, 4] had delineated BNZ liposomes, with the aim of developing a pharmaceutical form of parenteral use. Silva et al. [5] obtained the ruthenium complex, trans-[Ru(Bz)(NH3)4SO2](CF3SO3)2 changing the solubility of the compound and increasing the drug effectiveness.
Various techniques such as solid dispersion [6], sol-emulsion [7], inclusion complexation [8], and conjugation with water-soluble polymer or cyclodextrin [9] have been developed to enhance the solubility of various drugs. The potential use of natural cyclodextrins (CDs) and their synthetic derivatives has been extensively studied to improve certain drug properties such as solubility, stability, and/or bioavailability. The property of enhancing solubility may be explained by the formation of water-soluble ‘‘inclusion complexes’’ in which the hollow, truncated, cone-like CD structure encapsulates the hydrophobic drug molecules in the apolar interior. In contrast, the outer, hydrophilic region of the CD enables the solubilization through interaction with water molecules [10].
Nevertheless, natural CDs have limited water solubility. In order to obtain a significant increase in water solubility, some chemical derivatives have been synthesized by derivatization of the free hydroxyl groups of the CDs with alkyl and sulfobutyl groups. The result is hydroxyalkyl, methyl, and sulfobutyl derivatives with higher water solubility [11].
The purpose of this study is the characterization of inclusion complexes BNZ and CDs to develop a promising oral drug delivery system. The possible inclusion complex formation was assessed through different physicochemical techniques, including infrared spectrophotometry (FTIR), RAMAN, differential scanning calorimetry (DSC) [12, 13], and scanning electron microscopy (SEM). In addition, the intramolecular interaction was supported by molecular modeling.
Materials and methods
Materials
Benznidazole, N-benzyl-2-(2-nitroimidazol-1-yl) acetamide was supplied by Roche, Purity estimated by DSC and high-performance liquid chromatography (HPLC) is 99.9%. RMβCD (degree of molar substitution of 0.57) was donated by Roquette (Barcelona, Spain); SBβCD (degree of molar substitution of 1.0) by CyDex (Lenexa, KS). All other materials were of analytical grade. The solutions were prepared using distilled water, filtered through 0.22-μm Millipore filters (Millipore Corp, Billerica, MA).
Analytical methods
The UV spectrophotometric method was used according to a previously reported method Soares-Sobrinho et al. [14] for quantitative determination of BNZ, with the use of an UV–visible spectrophotometer Agilent 8453 (Agilent Corp, Santa Clara, CA) with photodiode array detector at 324 nm. Calibration curve in water was made with the use of standard solutions in the range of 4–40 μg mL−1. No effect of CDs addition on the UV spectrum of BNZ solution was verified for any of the CDs studied.
Molecular modeling
The geometry optimization of the BNZ-RMβCD and BNZ-SBβCD inclusion complexes was performed in vacuum and in the presence of water molecules using MM2 force field as an implement in HiperChem (release 6.03 for windows). The presence of water was simulated by placing the solute molecules into a 20 × 20 × 20 Å box with approximately 200 water molecules and a minimum distance of 2.0 Å between BNZ and water molecules. The most stable complex conformation was considered through the lowest interaction energy [15, 16].
Preparation of solid-state complexes
Preparation of physical mixture
A physical mixture (PM) of BNZ and the cyclodextrins RMβCD or SBβCD was obtained by mixing in a Turbula WAB T2C mixer (Willy A. Bachofen Corp, Basel, Switzerland) in 1:1 M ratio for 25 min.
Preparation of kneaded systems
The kneaded system (KN) was prepared from PM in a mortar by the slow addition of ethanol/water (1:1 v/v), in an amount of 20% wt/wt, and mixed until the homogeneous system was obtained [17]. Samples were dried for 24 h in a 40 °C oven and powdered.
Freeze-dried systems preparation
The PM was dissolved in ethanol:water (1:1 v/v). The solution was frozen by immersion in liquid nitrogen and freeze-dried (FD) in a Labconco apparatus (Labconco Corp, Kansas City, MO).
Evaporated
The PM was dissolved in ethanol:water (1:1 v/v). The solution was evaporated (EV) under vacuum at 50 °C in a rotary evaporator (Büchi Rotavapor R-200)
Solid-state complexes characterization
Differential scanning calorimetry
Samples weighing 3–4 mg were placed in aluminum open pans and heated from 30 to 250 °C at a rate of 10 °C min−1 using a temperature modulated DSC Q100 calorimeter (TA Instruments, New Castle, DE). Nitrogen was used as purge gas at a flux rate of 50 mL min−1. Temperature calibration and heat flow were performed with standard indium samples.
Fourier transform-infrared spectroscopy
Infrared spectra were obtained using a Bruker IFS-66 V spectrometer (Bruker Daltonics Inc, Bremen, Germany). Samples were ground, thoroughly mixed with potassium bromide, and compressed by a hydraulic press. Thirty-two scans were obtained at a 4 cm−1 resolution.
Raman spectroscopy
The Raman spectra of the samples (Sp and complex) were collected employing a 180° Raman spectrometer. The Raman spectrometer was equipped with a ZnSe beam splitter and a TE cooled InGaAs detector. Rayleigh filtering was afforded by a set of two Holographic technology filters. The laser power on the sample was 800 MW. The spectra were collected with 2 cm−1 resolution by coadding the results of about 2000 scans. Resolution: 4 cm−1/Pot laser: 90–100/Scans: 120.
Scanning electron microscopy
Surface morphologies of samples were examined using a LEO-435VP SEM (Leica Microsystems, Cambridge, UK). Particles were fixed on a brass stub using double-sided tape and vacuum-coated gold.
Results and discussion
Molecular modeling
From BNZ, chemical structure analysis (Fig. 1) is possible to distinguish two regions which could be included themselves inside the CD cavity—the molecule imidazolic portion and its benzenic group. All simulations considered both possibilities. Molecular modeling was carried out on vacuum, and the presence of water as solvent was also taken into consideration. The energies calculated for the stoichiometry systems 1:1 and 1:2 (drug: cyclodextrin) are described in Tables 1 and 2. The inclusion stoichiometry 2:1 cannot be determined since the mathematical model of optimization expelled one of the drug molecules from the cavity due to its eventual association high instability, which was probably caused by steric impediment.
In all cases, the more stable conformations were reached in the presence of solvent. The 1:1 stoichiometry was the one which presented the higher stability. In the complexes of 1:2 stoichiometry (drug:CD) the volume of the substituents of the modified CDs (RMβCD and SBβCD) disabled the formation of complexes with such stoichiometry, which also occurred due to a matter of steric, presenting very high enthalpy values. In the case of SBβCD, electrostatic repulsion caused by the substituent groups contributes to highlight the enthalpic differences when compared to 1:1 stoichiometry (Tables 1 and 2) [18]. The inclusion complexes more energetically favorable were obtained with the RMβCD.
Apparently, there are no significant differences between the assessed conformations according to the vacuum simulations, which suggest the coexistence of all conformations in the inclusion complexes with BNZ. However, in the simulation of BNZ–RMβCD complexes in aqueous medium it is possible to notice a little enthalpy difference for the conformation whose the aromatic portion of the molecule is inserted in the narrow part of CD, being apparently more stable than the other ones (Table 1).
Characterization of solid-state complexes
Differential scanning calorimetry
The thermal analysis of the processed samples noticed displacement at lower temperatures and endothermic peak enlargement corresponding to the fusion of the drug, more pronounced in mixtures with RMβCD (Fig. 2). The same effect is also found with a lower intensity in the PM and may be explained by the interaction provided by the heating process inherent to the DSC test.
One may still observe an exothermic rise in the PM of BNZ–RMβCD after the fusion of the drug. The rise may be related to the formation of inclusion complexes from the fusion (Fig. 2). This phenomenon is less noticeable in the KN, FD, and EV processed since these already present a fraction of the mixture which was previously complexed.
Another point that should be also commented is the thermal profile presented by KN from the BNZ–RMβCD binary mixture which shows a marked loss of resolution in the fusion peak of BNZ. The hypothesis of thermal superposition between possible different crystalline phases is not discarded.
Fourier transform-infrared and Raman spectroscopy
Results from FTIR and Raman for BNZ and RMβCD mixtures in Fig. 3 show minor changes in the region which corresponds to stretching vibrations of the BNZ benzene group (3100–2900 cm−1), mainly for the EV and FD samples. One also observes changes in the bands which correspond to the drug in the regions of 1300–1000 cm−1, related to the amide C–N bonds. These results point to a higher interaction in the central-aromatic portion of the drug, which indicates a possible inclusion of BNZ in the CD through its benzene group. The spectroscopic data are in accordance with the results found on the molecular modeling, which suggest that more favorable values of enthalpy for the inclusion complex obtained by the aromatic part of the BNZ included by the narrow part of the CD (Table 2).
FTIR and Raman spectra (Fig. 4) show no appreciable interactions for the BNZ and SBβCD mixtures. Only slight displacements or reduction of relative intensity in the position of some bands corresponding to the drug may be assessed in the regions of 3300–3200 cm−1 and 1300–1200 cm−1. These regions virtually coincide with those found for BNZ and RMβCD mixtures; however, changes are much less visible in this case.
Scanning electron microscopy
Morphological changes can be used as evidence to verify interactions between the molecules and complexes formation [19, 20]. The selected photomicrographs of BNZ, RMβCD, and SBβCD, besides the processed binary systems are represented in Fig. 5. Regular crystals characterize BNZ [21], while amorphous particles originally compose RMβCD and SBβCD [8].
(I) Scanning electron microphotographs of benznidazole (a), randomly methylated-β-CD (b), physical mixture (c), kneaded (d), lyophilized (e), and evaporated (f) systems. (II) Scanning electron microphotographs of benznidazole (g), sulfobutylether β-CD (h), physical mixture (i), kneaded (j), lyophilized (l), and evaporated (m) systems
In general, all the processed samples change in the morphological aspect of particles. Amorphous particles characteristics of the non-manipulated CDs were not observed. The KN form aggregates (granules) that are somewhat uniform sized. The FD of both CDs and the EV of RMβCD show a reduction in the size of particles that take the form of needles which are completely different from the starting materials. The changing of shape and size reduction of particles seems less intense for the EV of SBβCD.
Conclusions
In conclusion, the results suggest possible formation of inclusion complexes by EV, freeze-drying, and kneading techniques with compared to the drug or the CD/drug PM. The results obtained by SEM, DSC, FTIR/RAMAN, and Molecular Modeling show that a BNZ:CDs inclusion complex could be prepared at a 1:1 M ratio for all methods. Taking these results into account, we believe that the complexation effectively will enhance the solubility of BNZ, which may consequently increase its bioavailability and might improve its pharmaceutical potential.
References
Coura JR, Castro SL. A critical review on chagas disease chemotherapy. Mem Inst Oswaldo Cruz. 2002;97:03–24.
Lamas MC, Villagi L, Nocito I, Bassani G, Leonardi D, Pascutti F, Serra E, Salomón CJ. Development of parenteral formulations and evaluations of the biological activity of the trypanocide drug benznidazole. Int J Pharm. 2006;307:239–43.
Docampo R. Recent developments in the chemotherapy of Chagas disease. Curr Pharm Des. 2001;7:1157–64.
Morilla MJ, Montanari JA, Prieto MO, Lopez MO, Petray PB, Romero EL. Intravenous liposomalbenznidazole as trypanocidal agent: increasing drug delivery to liver is not enough. Int J Pharm. 2004;278:311–8.
Silva-Nogueira S, Jerley J, Pavanelli WR, Gutierrez FRS, Lima FCA, Silva ABF, Silva SJ, Franco WD. Complexation of the anti-Trypanosoma cruzi drug benznidazole improves solubility and efficacy. J Med Chem. 2008;51:4104–14.
Jung J, Yoo S, Lee S, Kim K, Yoon D, Lee K. Enchanced solubility and dissolution rates of itraconazole by a solid dispersion technique. Int J Pharm. 1999;187:195–201.
Akkar A, Muller RH. Intarvenous itraconazole emulsions produced by solemuls technology. Eur J Pharm Biopharm. 2003;56:29–36.
Figueiras A, Carvalho RA, Ribeiro L, Torres-Labandeira JJ, Veiga FJB. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified β-cyclodextrin. Eur J Pharm Biopharm. 2007;67:531–9.
Rodriguez-Tenreiro C, Alvarez-Lorenzo C, Concheiro A, Torres-Labandeira JJ. Characterization of cyclodextrin–carbopol interactions by DSC and FTIR. J Therm Anal Calorim. 2004;77:403–11.
Piel G, Pirotte B, Delneuville I, Neven P, Llabres G, Delarge J, Delattre L. Study of the influence of both cyclodextrins and l-lysine on the aqueous solubility of nimesulide; isolation and characterization of nimesulide-l-lysine–cyclodextrin complexes. J Pharm Sci. 1997;86:475–80.
Ventura CA, Giannone I, Paolino D, Pistara V, Corsaro A, Puglisi G. Preparation of celecoxib-dimethyl-b-cyclodextrin inclusion complex: characterization and in vitro permeation study. Eur J Med Chem. 2005;40:624–31.
Bruni G, Milanese C, Berbenni V, Sartor F, Villa M, Marini A. Crystalline and amorphous phases of a new drug. J Therm Anal Calorim. 2010;102:297–303.
Pinto MF, Moura EA, Souza FS, Macêdo RO. Thermal compatibility studies of nitroimidazoles and excipients. J Therm Anal Calorim. 2010;102:323–9.
Soares-Sobrinho JL, Silva ALM, Grangeiro-Júnior S, Medeiros FPM, Rolim-Neto PJ. Desenvolvimento e validação do método analítico para o doseamento de benznidazol. Rev Bras Farm. 2006;87:78–80.
Lipkowitz KB. Applications of computational chemistry to the study of cyclodextrins. Chem Rev. 1998;5:1829–73.
Pose-Vilarnovo B, Perdomo-Lopez I, Echezarreta-Lopez M, Schroth-Pardo P, Estrada E, Torres-Labandeira JJ. Improvement of water solubility of sulfamethizole through its complexation with b- and hydroxypropyl-b-cyclodextrin. Characterization of the interaction in solution and in solid state. Eur J Pharm Sci. 2001;13:325–31.
Hedges AR. Industrial applications of cyclodextrins. Chem Rev. 1998;98:2035–44.
Okimoto K, Rajewski RA, Uekama K, Jona JA, Stella VJ. The interaction of charged and uncharged drugs with neutral (HP-beta-CD) and anionically charged (SBE7-beta-CD) beta-cyclodextrins. Pharm Res. 1996;13:256–64.
Fernandes CM, Vieira TM, Veiga FJB. Physicochemical characterization and in vitro dissolution behavior of nicardipine–cyclodextrins inclusion compounds. Eur J Pharm Sci. 2002;15:79–88.
Cirri M, Rangoni C, Maestrelli F, Corti G, Mura P. Development of fast-dissolving tablets of flurbiprofen–cyclodextrin complexes. Drug Dev Ind Pharm. 2005;31:697–707.
Soares-Sobrinho JL, Cunha-Filho MSS, Rolim-Neto PJ, Torres-Labandeira JJ, Dacunha-Marinho B. Benznidazole. Acta Cryst. 2008;E64:o634.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soares-Sobrinho, J.L., de La Roca Soares, M.F., Rolim-Neto, P.J. et al. Physicochemical study of solid-state benznidazole–cyclodextrin complexes. J Therm Anal Calorim 106, 319–325 (2011). https://doi.org/10.1007/s10973-010-1186-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1186-x