Abstract
In this paper, the thermal behaviours of two organophosphorous compounds, N,N-dimethyl-N′,N′-diphenylphosphorodihydrazidic (NDD) and diphenyl amidophosphate (DPA), were studied by thermogravimetery (TG), differential thermal analysis (DTA) and differential scanning calorimetery (DSC) techniques under non-isothermal conditions. The results showed that NDD melts about 185 °C before it decomposes. NDD decomposition occurs in two continuous steps, in the 190–410 °C temperature range. First thermal degradation stage for NDD results a broad exothermic peak in the DTA curve that is continued with a small exothermic peak at the end of decomposition process. On the other hand, applying TG-DTA techniques indicates that DPA melts about 150 °C before it decomposes. This compound decomposes in the temperature range of 230 to 330 °C in two steps. These steps are endothermic and exothermic, respectively. Activation energy and pre-exponential factor for the first step of decomposition of each compound were found by means of Kissinger method and were verified by Ozawa–Flynn–Wall method. Activation energy obtained by Kissinger method for the first stage of NDD and DPA decompositions are 138 and 170 KJ mol−1, respectively. Finally, the thermodynamic parameters (ΔG #, ΔH # and ΔS #) for first step decomposition of investigated organophosphorous were determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorous (OP) compounds are commonly used as pesticides and herbicides in agricultural industry and as chemical warfare agents in military practice. OPs can inhibit enzyme acetylcholinesterase (AChE) and cause permanent nervous system damage. The use of OPs as insecticides in the agricultural and urban settings is still high and is expected to remain so, at least in the near future. While other classes of insecticides are gaining market share (e.g. pyrethroids) and new classes have been developed (e.g. neonicotinoids), the efficacy of OPs, their relatively low cost and their lack of bioaccumulation in the ecosystems would support this prediction [1, 2].
Mixing, formulation and storage of organophosphorous compounds at appropriate sites implies a series of safety problems which are generally well known [3, 4]. There is in fact an agreement among the experts about the fact that the risk is mainly associated with accidental combustion of flammable organic compounds that are used in the formulation and are normally stored in the same industrial area as the pesticide. The identification and quantification thermal hazard of organophosphorus compounds as well as their degradation products and metabolites in environmental and biological samples is an analytical problem of increasing importance [5–7]. Thermal analysis techniques are particularly useful for solving these problems owing to their potential for trace analysis of compounds in complex matrices [8–11].
Studies on thermal behaviours of organophosphorous are an essential part of research and development in this area [12–14]. This is in view of safety as well as performance parameters. Kinetic parameters and mechanistic aspects are highly useful for making predictions of performance parameters, e.g. ‘shelf life’ [15–18].
N,N-dimethyl-N′,N′-diphenylphosphorodihydrazidic (NDD) and diphenyl amidophosphate (DPA), with the structures shown in Fig. 1, are two relatively new synthesized organophosphorus compounds; and hence, acquiring information concerning these compounds in the solid-state, including their thermal stability and thermal decomposition, is necessary. This research was initiated to improve the knowledge about thermal stability of the organophosphorous compounds by means of differential scanning calorimetery (DSC) and simultaneous thermogravimetery-differential thermal analysis (TG-DTA). Also, this study seeks for determination of kinetic parameters of non-isothermal decomposition of the compounds. To the best of our knowledge, there is no report on the thermal behaviour of these organophosphorous compounds.
Experimental
N,N-dimethyl-N′,N′-diphenylphosphorodihydrazidic (NDD) and diphenyl amidophosphate (DPA) compounds were synthesized as proposed by [19]. Thermogravimetery (TG) and differential thermal analysis (DTA) were carried out using a Stanton Redcroft, STA-625 series with alumina crucibles, with a heating rate of 10 °C min−1 in a temperature range of 50 to 700 °C, under nitrogen atmosphere with a flow rate of 50 mL min−1. The sample mass was about 5 mg.
DSC experiments were conducted using the DuPont Instrument Model DSC 910S differential scanning calorimeter. Aluminum crucibles containing the samples were purged with nitrogen at a flow rate of 50 mL min−1. The DSC experiments were carried out in scanning mode at heating rates of 5, 10, 15 and 20 °C min−1 in the temperature range of 25 to 700 °C. The instrument was calibrated at each heating rate considered using a dedicated 1 mg indium standard in an aluminum pan. Plotting for exothermic reactions was as downward deflection of the curve peak from the baseline.
Results and discussion
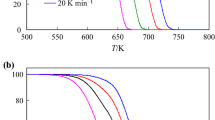
The TG and DTA curves at a heating rate of 10 °C min−1 in nitrogen atmosphere for both compounds are given in Fig. 2. As shown in Fig. 2a for NDD, no thermal event was observed until 180 °C. Above this temperature, melting occurs at 185 °C during a sharp endothermic phenomenon. No change in the mass of compound was observed until 190 °C. Above this temperature, two exothermic peaks occurred with a major fall in the sample’s mass until about 410 °C. During these two steps, the TG curve indicates a continuous mass loss that continues up to 410 °C. The results indicated a mass loss in TG curve about 58% for these two steps.
Figure 2b shows the TG-DTA curves of DPA. As shown in this figure, melting occurs at 150 °C during a sharp endothermic phenomenon and as seen in the TG curve of DPA, up to 230 °C, no change in the mass of the compound was observed. Such behaviour suggests that DPA is kinetically stable at its melting point. After this, as seen in the DTA curve in Fig. 2b, there are long intervals of temperature between DPA undergoing fusion at 150 °C and it first decomposing endothermically at (262.5 °C). Decomposition started above 230 °C and the TG curve showed strong mass loss after this temperature. The decomposition took place in two steps. These steps are consecutive endothermic and exothermic decomposition processes. A total mass loss observed in the TG curve was about 82%.
The general information about thermal behaviour of NDD and DPA compounds in terms of mass loss, peak temperature and percentage of mass loss corresponding to each compound is presented in Table 1.
Effect of heating rate
Figure 3 shows the DSC curves for NDD and DPA at several heating rates. The data presented in this figure shows that as the heating rate was increased, the melting peak of the compounds shifted to higher temperatures. These shifts in melting points are shown in Table 2 and Fig. 4. On the other hand, as shown in Table 2 and Fig. 4, there was an increase in the temperature of the decomposition peaks of both compounds as the heating rate increased. Also, Fig. 5 and Table 3 show variation of the enthalpy for melting and decomposition obtained by area under DSC peaks at various heating rates.
Thermal decomposition kinetics
In view of general complexity in the processes involved in solid-state reactions, [20–23], two thermal analysis kinetic methods were jointly employed in this study to process the DSC data to calculate the Arrhenius parameters, such as the activation energy (E) and the pre-exponential factor (A) for both the compounds studied. These methods are as follows: Kissinger equation [20]:
where φ is heating rate, T m is the maximum peak temperature and R is the gas constant.Ozawa–Flynn–Wall equation [21]:
where g(α) is the integral function of conversion. This equation is valid in the range 20 < X = E/RT < 60 (Doyle’s approximation); in our study, the x factor for both the compounds was in this range.
These multiple scanning methods are model free isoconventional methods and allow the activation energy to be independently obtained [24]. The E values calculated using each method was used to check the constancy and validity of activation energy obtained by another method.
On the other hand, the Arrhenius pre-exponential factor (A) was found for both the compounds from the following relation [25, 26]: \( A = \varphi E\exp (E/RT_{\text{m}} )]/RT_{\text{m}}^{2} \).
The values of activation energy and pre-exponential factor of decomposition calculated by the Kissinger method and those calculated according to the Ozawa–Flynn–Wall method have been compared (Table 4). As could be found in this table, the activation energies and pre-exponential factors calculated by Kissinger method for both the compounds were comparable with those obtained by Ozawa–Flynn–Wall method.
After obtaining the kinetic parameters (E and A), the thermodynamic parameters of activation can be calculated from the following equations [27, 28]:
where ΔG #, ΔH # and ΔS # are free energy, enthalpy and entropy of the activation, respectively. υ is υ = K B T/h (where K B and h are Boltzmann and Plank constant, respectively). Table 4 gives the computed thermodynamic and kinetic parameters for both the studied compounds. Based on the kinetic data, activation energy of NDD is approximately 32 kJ mol−1 lower than DPA, and hence, the relative stability of these organophosphorous compounds was found to obey in this order: DPA > NDD. Also, same trend could be seen in the values of thermodynamic parameters (ΔH #, ΔG #) calculated for both organophosphorous compounds. These results could be used in environmental science for prediction of lifetime. Also, these data can provide valuable information about time and condition of storage.
Reaction rate constant determination
Assuming first-order decomposition, the rate constant (k) for decomposition reaction could be calculated by the following equation [29]:
which is for the temperature of 25 °C and using activation energies (E a) and frequency factors (Z) obtained in the above; the equation was solved for k. Table 4 listed the log k for each compound. By considering the reaction rate constant calculated for the samples, NDD reaction rate constant was compared with DPA reaction rate constant. It was found that the reaction rate constant of NDD is higher than that calculated for the DPA.
Comparison thermal behaviour of organophosphorous compounds
In this study, the thermal behaviour of two organophosphorous compounds was studied under identical conditions. For NDD, as seen in Fig. 2 and Table 1, melting phenomena occurred about 35 °C higher than the DPA sample. Also, for the NDD sample, the decomposition happened about 19 °C lower than DPA. On the other hand, the first decomposition peak for DPA is endothermic, whereas this peak for NDD is a broad exothermic peak. A comparison of the decomposition temperature of these samples is shown in Table 1.
The values of the kinetic parameters that were obtained by the Kissinger and Ozawa- Flynn- Wall methods for these samples confirm the higher thermal stability of DPA compared to NDD. The values of kinetic parameters (activation energy and frequency factor) of DPA are higher than those for NDD. On the other hand, from the data presented in Table 4, it was found that the ratio of decomposition rate constant for NDD to DPA is about 850, and hence, the rate of decomposition for NDD is considerably higher than that for DPA. These results show that NDD, in comparison with DPA, is a heat sensitive organophosphorous compound and needs more care during the storage period.
Conclusions
The thermal behaviour of two Organophosphorus (OP) componds, N,N-dimethyl-N′,N′-diphenylphosphorodihydrazidic (NDD) and diphenyl amidophosphate (DPA), was studied using TG-DTA and DSC. The results showed that NDD and DPA melted at about 185 and 150 °C, respectively, before they decompose. On the other hand, both the compounds showed that their mass losses are consecutive and there are no plateaus in the decomposition temperature interval in the TG curve.
The values of the kinetic parameters that were obtained by the Kissinger and Ozawa–Flynn–Wall methods for NDD and DPA showed good correlation, but the values of activation energy and frequency factor obtained by Kissinger method were slightly lower than those obtained by Ozawa–Flynn–Wall method. On the other hand, the values of ΔS #, ΔG # and ΔH # of the first stage of decomposition reaction of NDD and DPA were computed. Our finding showed that the values of ΔG # and ΔH # for the decomposition of NDD are lower than those for DPA.
References
Jamal E, Shashidhar N, Adrea M, William S. Prediction of organophosphorus acetylcholinesterase inhibition using three-dimensional quantitative structure-activity relationship (3D-QSAR) methods. Toxicol Sci. 2001;63:223.
Kamil K, Jirl B, Jirl C, Jirl K. Synthesis of a new reactivator of tabun-inhibited etylcholinesterase. Bioorg Med Chem Lett. 2003;13:3545.
Ghadimi S, Mousavi S, Javani Z. Structure-activity study of phosphoramido acid esters as acetylcholinesterasf inhibitors. J Enzym Inhib Med Chem. 2008;23:213.
Hansch C, Deutsch E. The use of substitution constants in the study of structure–activity relationships in cholinesterase inhibitors. Biochim Biophys Acta. 1966;126:117.
Hosa N, Radic Z, Tsigeling I, Berman H, Quinn D, Taylor P. Aspartate74 as a primary determinant in acetylcholinesterase governing specificity to cationic organophosphates. Biochem J. 1996;35:995.
Singh AK. Quantitative structure-activity relationships for phosphoramidothioate toxicity in housefly. Comp Biochem Phys A. 1999;123:241–55.
Basak S, Magnuson V, Niemi G, Regal R, Veith G. Topological indices: their nature, mutual relatedness, and applications. Math Model. 1986;8:300.
Hosseini SG, Pourmortazavi SM, Hajimirsadeghi SS. Thermal decomposition of pyrotechnic mixtures containing sucrose with either potassium chlorate or potassium perchlorate. Combust Flame. 2005;141:322.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Fathollahi M, Hosseini SG. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel. 2008;87:244.
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combust Sci Tech. 2008;180:2093.
Pourmortazavi SM, Hajimirsadeghi SS, Hosseini SG. Characterization of the aluminum/potassium chlorate mixtures by simultaneous thermogravimetry—differential thermal analysis. J Therm Anal Calorim. 2006;84:557.
Hajimirsadeghi SS, Teimouri MB, Rahimi-Nasrabadi M, Dehghanpour S. Non-isothermal kinetic study of the thermal decomposition of N-{bis[benzyl(methyl)amino]phosphoryl}-2,2-dichloroacetamide and N-{bis[dibenzylamino]phosphoryl}-2,2-dichloroacetamide. J Therm Anal Cal. 2009. doi: 10.1007/s10973-009-0293-z.
Senneca O, Scherillo F, Nunziata A. Thermal degradation of pesticides under oxidative conditions. J Anal Appl Pyrolysis. 2007;80:61.
Andreozzi R, Ialongo G, Marotta R, Sanchirico R. Thermal decomposition of ethyl parathion. J Loss Prevent Proc. 1999;12:315.
Fathollahi M, Pourmortazavi SM, Hosseini SG. Particle size effects on thermal decomposition of energetic material. J Energ Mater. 2008;26:52.
Bohn MA. Kinetic modeling of the concentrations of the stabilizer DPA and some of its consecutive products as function of time and temperature. J Therm Anal Calorim. 2001;65:103.
Kohsari I, Pourmortazavi SM, Hajimirsadeghi SS. Non-isothermal kinetic study of the thermal decomposition of diaminoglyoxime and diaminofurazan. J Therm Anal Calorim. 2007;89:543.
Eslami A, Hosseini SG, Pourmortazavi SM. Thermoanalytical investigation on some boron-fuelled binary pyrotechnic systems. Fuel. 2008;87:3339.
Edmundson RS. Dictionary of organophosphorus compounds, Published by CRC Press, 1988, ISBN 0412257904, 9780412257902.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881.
Hatakeyama T, Quinn FX. Thermal analysis: fundamentals and applications to polymer science. New York: Wiley; 1994.
Rong L, Binke N, Yuan W, Zhengquan Y, Rongzu H. Estimation of the critical temprature of thermal explosion for the highly nitrated nitrocellulose using non-isothermal DSC. J Therm Anal Calorim. 1999;58:369.
Pineda EAG, Ferrarezi ADM, Ferrarezi JG, Hechenleitner AAW. Thermal decomposition of enalapril maleate studied by dynamic isoconversional method. J Therm Anal Calorim. 2005;79:259.
ASTM E 698, Test methods for Arrhenius kinetic constants for thermally unstable materials.
Pourmortazavi SM, Kohsari I, Teimouri MB, Hajimirsadeghi SS. Thermal behaviour kinetic study of the dihydroglyoxime and dichloroglyoxime. Mater Lett. 2007;61:4670.
Criado JM, Perez-Maqueda LA, Sanchez-Jimenez PE. Dependence of the preexponential factor on temperature. J Therm Anal Calorim. 2005;82:671.
Pourmortazavi SM, Hosseini SG, Rahimi-Nasrabadi M, Hajimirsadeghi SS, Momenian H. Effect of nitrate content on thermal decomposition of nitrocellulose. J Hazard Mater. 2009;162:1141.
Krabbendam-LaHaye ELM, de Klerk WPC, Krämer RE. The kinetic behaviour and thermal stability of commercially available explosives. J Therm Anal Calorim. 2005;80:495.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sovizi, M.R., Anbaz, K. Kinetic investigation on thermal decomposition of organophosphorous compounds. J Therm Anal Calorim 99, 593–598 (2010). https://doi.org/10.1007/s10973-009-0502-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0502-9