Abstract
Several treatments for the removal of toxic heavy metals like uranium from wastewater have been developed, but none of them are sufficiently effective. Biosorption is the most feasible, eco-friendly, cost-effective, reusable technique for uranium removal. The relationship between different influencing factors in biosorption, mechanisms, competency of different biosorbents utilized, and significance of biosorption over other conventional methods have been described elaborately. The use and potentiality of genetically engineered biosorbents, modified nanoparticles for efficient biosorption are also highlighted. Among given biosorbents, Chlorella salina, Laminaria japonica, Candida utilis, chemically modified Spirulina platensis, genetically modified Deinococcus radiodurans, and Brassica juncea showed maximum biosorption capacity considering the reaction parameters. Thus, different biosorbents shows different uranium uptake potentiality due to different pH level and ionic charges present in them. Recent trends of biosorption of uranium and their present and future impact in the field of innovative and advance technology are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent days, the releases of environmental pollutants have increased remarkably due to intensive industrial and urban growth [1, 2]. One of the major contaminations in such pollutants is coming from metals, in particularly from uranium which is the most deleterious metal for the entire environment. Radioactive mining and milling waste gets flushed from the tailings to the rivers and other water sources and it causes a deadly radiological impact in water sites [3]. Due to excessive use of nuclear power plants and nuclear fuel cycle, unprocessed uranium-containing waste liquid directly enters into the natural environment and this uranium migrates to the water bodies and soils due to its serial dissolution and sedimentation and causes damage to DNA, reproductive system of living beings [2, 3]. A certain percentage of U(IV) and U(VI) may causes severe damage to the living system in the environment. Maximum Contaminant Level (MCL) of uranium in potable drinking water has been established as 15 µg/L by World Health Organization (WHO) [4] and beyond this limit, it’s treated as carcinogen for human beings [4]. Therefore, consumption of such contaminated with persistent and non-degradable metal pollutants can cause severe disease in human body [5]. The presence of these heavy metals like lead, mercury, uranium, cadmium etc. in drinking water are the reason of many life-threatening diseases such as cancer, neurological disorders etc. to human. Uranium possesses both radiotoxic as well as chemo toxic property thus consumption of uranium contaminated water leads directly into bloodstream of human beings and results in detrimental effect [6]. Besides, it can even damage the liver and kidneys of humans [7]. Therefore, a special attention is highly required due to its toxicity and potential to bioaccumulation via food chain.
Various chemical and biological methods are available for the removal of heavy metals from wastewater as well as from surface water [8]. Some of the traditionally used processes for the removal of heavy metals especially uranium from any kind of waste water include ion-floatation, ion–exchange, reverse osmosis, ultra-filtration, electrodialysis, electrodialysis reversal, membrane filtration, chemical precipitation, electrocoagulation-flocculation etc. [9] as tabulated in Table 1. From the extensive literature survey, it is clear that the above mentioned conventional methods have several disadvantages, such as high cost, non-reusability, lower efficiency, and are unable to operate at low concentration [25, 26]. Besides, it is also difficult to remove the sludge coming out while using these conventional methods [26]. Therefore, recent research is more concerned for the development of biosorption process which is an inexpensive, highly effective, eco-friendly technique. Biosorption is a segregation process influenced by the concentration gradient in which desired molecules, and ions are diffused into a specialised biosorbent at the solid–liquid interface until the equilibrium is attained [27]. The versatile and recyclable nature of biosorbents have made this technique as the most significant and innovative idea for complete uranium biosorption. Uranium is a positively charged radionuclide which confers strong binding affinity with negatively charged biosorbents. There are several stages involved in uranium adhesion and remediation from the wasteeffluent, namely, ion-exchange, complexation, and physical or surface adsorption. Each mechanisms depends on some influencing factors. For example, effects of pH condition on U(VI) species is a crucial parameter for biochemical interactions between biosorbents and uranium ions in the biosorption process. If pH of the solution is less than 4 then UO22+ divalent cation is a dominant species of hexavalent uranium regardless of the air [28]. Thus, any negatively charged biosorbents could strongly bind U(VI) through ionic interaction (electrostatic interaction) [28]. Whereas, In basic & neutral pH condition, UO2(OH)+, (UO2)3(OH)5+, (UO2)4(OH)7+, UO2CO3, and UO2(OH)2 are the dominant species of uranium radionuclide respectively, thereby any kind of positively charged biosorbents can easily sorbed U(VI) ions through other biochemical interactions [28]. At high pH condition (> pH 8), negatively charged UO2(CO3)34−, and UO2(OH)3− are dominant species. From this concept, we can say that gradual increment of pH value of wasteeffluent favours strong binding affinity between positively charged biosorbents and negatively charged uranium ions. In sum, considering pH value of solution could help researchers in selecting efficient biosorbents for the uranium biosorption. Another important fact is that when initial pH of solution is relatively low, uranyl cation remains extremely mobile and are not easy to capture [28]. Many hydrions in the solution ensure that the functional groups (binding sites), namely, hydroxyl, carboxyl, amino groups, thiols, and phosphate group present on the biosorbent surface are deprotonated and negatively charged, which accelerate the biosorption of positively charged uranyl ions because of specific biochemical interactions [27, 28]. In contrast, if we use positively charged biosorbent for uranyl cation removal, then the same hydrions present in the solution ensure that the binding sites on the biosorbent surface are substantially protonated which retards the biosorption process because of electrostatic (ionic) repulsion [28]. Carbohydrates, proteins, teichuronic acid, peptidoglycan, teichoic acid, lipids and other biomolecules are dispersed on the cell wall surface (mosaic form) of biosorbents and provide distinct functional groups responsible for uranium ion binding [29]. The biosorbent dosage at a particular initial uranium ion concentration in solution determines the biosorption capacity and the removal capacity of the biosorbent towards the uranium removal. The biosorbent dosage is directly proportional to the higher percentage remediation of the uranium ions. Increase in biosorbent dosage implies increase in the number of binding sites on the surface of biosorbent. Another influencing factor is adsorption isotherm that helps us to understand biosorbent’s maximum biosorption capacity as well as biosorption process. Langmuir, Freundlich, Dubinin-Raduskevich, and Temkin isotherm are commonly used to understand this process. Thermodynamic parameters value reveal that whether the biosorption process is endothermic or exothermic and the spontaneity of the process. At a specific point of time, the biosorbents become saturated with the uranium ions and then results in desorption process. At the equilibrium time, the biosorption and desorption rate become equal. Once the biosorption process reached the equilibrium state, there will not be any further interaction of the uranium and biosorbent, thus known as contact time. Additionaly, increase in temperature induces enlargement of biosorbent pore size, increase of biosorbent functional groups, reduction in the diffusion layer thickness around biosorbent, increment of uranium ions mobility, and enrichment of biosorbent surface activity that eventually aids in efficient biosorption of uranium ions within less time of contact [29]. All these parametes are discussed in detail in this review.Microorganism or biological materials have been used for the effective removal of toxic heavy metal ions especially uranium from the waste effluent in bisorption process [26, 30, 31]. Non-living modified or unmodified algal biomass, bacterial biomass and fungal biomass are used as biosorbent due to their higher biosorption capacity [32], low-cost, eco-friendly and reusability properties. Moreover, On the basis of 16S rRNA gene PCR amplification, high-throughput sequencing, and phospholipid-fatty acid evaluation, it has been reported that three phyla, namely Proteobacteria, Firmicutes, and Actinomycetes are commonly found in U(VI) contaminated sites [33]. They can survive in oligotrophic ecosystems and exhibit radionuclide resistance, thereby play an imperative role in immobilization and biosorption of uranium [33]. Among bacteria, Shewanella sp. are used as a model organism for U(VI) remediation research [33]. Vegetative cells as well as spores of Clostridium acetobutylicum, Geobacter, and Desulfovibrio remediate uranium ion species using H2 as the electron donor and form U(VI) precipitate [33], Among algal biosorbents, Spirulina platensis biomass has is an effective algal biosorbent for the biosorption of uranium ions, other radioactive ions and heavy metal ions [34]. The dry algal biomass of Spirulina platensis has been modified by using amidoximethatis composed of basic amino group and acidicoxime group [35]. These modifications have been done to improve and enhance the biosorption capacity of algal biomass. Such modifications result in more number of functional groups in the binding sites of algal biomass cell surface and adsorbed more number of uranium ions wastewater. Quercetin (3,3’,4’,5,7-Pentahydroxy-flavone) is a natural flavonoid compound, acting as an antioxidant, anti-cancer and anti-inflammatory agent [36, 37]. It is mainly found in many plants, fruits and vegetables like broccoli, onions, apples, green tea [38] etc. Quercetin acts as a chelator for metal ion [39] as it contains a functional group such as hydroxyl group (-OH) in its structure.
The present review deals with the removal of uranium ions from the waste effluent by using different microorganisms peel waste, chemically modified microorganisms, nanoparticles and genetically engineered microorganisms. The relationship between several reaction parameters such as pH, biosorbent dose, retention time, reaction temperature, functional groups etc. and the removal efficiency in biosorption process have been intensively investigated. Besides, mechanism, adsorption kinetics and thermodynamics studies, advantages and disadvantages of biosorption process are also highlighted.
Hazardous effects of uranium
Anthropogenic activities are responsible for influencing the environment by producing huge amount of toxic liquids containing heavy metals, metalloids, radio nuclides and other various organic pollutants [40]. Uranium (U) is one such naturally occurring radioactive substance, found in various form, out of which the most common is in the form of uranyl ion (UO22+) in acidic condition present in solution [41, 42]. When the toxicity level of uranium that mainly found in the form of either uranyl ion or U(VI) exceeds beyond the threshold limit, then it becomes a perilous element for the environment, water, human health, animals etc. [43]. It is responsible for causing cancer, brain damage, DNA damage, infertility problems, high blood pressure and renal failure to human beings [44] as depicted in Fig. 1. Due to its high radioactivity along with high toxicities, these long-live radionuclides are extremely hazardous element. Abshire et al. [45], showed that uranium can be present in water effluents in the form of its isotopes such as, U-238 (99.27%), U-235 (0.72%) and U-234 (0.01%). Several studies have reported that uranium contamination in aquatic invertebrates may cause malformations, growth inhibition and survival time reduction [46, 47].
Effects of uranium on human health
Mitochondrion of human cells is the main target for Depleted uranium (DU) which simply leads to apoptosis. DU affects bronchial cells of the human lungs and leads to cancer. Moreover, uranyl acetate damages the human chromosomes. Several reports suggested that the individuals who were residing near the uranium-mining wastes had higher chances of having cells with chromosomal aberrations. One example is rogue cells which are common in uranium miners. This implies that uranium shows genotoxic effects on humans. Birth defects are also found due to uranium exposure. Uranium also has toxic effects on human kidneys [7, 48]. High uranium intakes cause acute renal failure and even death; damage proximal tubules. Accumulation of uranium in bone causes severe bone cancer, thyroid cancer etc. Another most common disease is lung cancer which is being caused by the accumulation of uranium in human body [49]. Abnormal gene expression, infertility, gulf war syndrome, neurological disorders and many others occur due to uranium contamination in human body as shown in Fig. 1.
Effects of uranium on plants
Uranium is a natural radionuclide exists in its isotopic forms 238U; 235U; 234U and these isotopes are used in medical treatment, agricultural purposes, mining and other industries. It is reported that higher concentration of uranium inhibits seed germination. Uranium also effects leaf area, root length, plant height etc. It also induces oxidative damage due to increase in Reactive Oxygen Species (ROS) in plants which lead in blockage of photosynthesis [50,51,52,53] as shown in Fig. 1.
Effects of uranium on animals
Uranium being naturally-occurring radioactive element, has hazardous effects on animals, especially aquatic species such as; fishes and terrestrial animals. These accumulate in the food chain and then cause deformities and reproductive issues for them as explained in Fig. 1. Waterborne uranium causes thyroid disruption in Zebra-fish [54]. Another report suggests that exposure of aquatic animals to uranium inhibits ATPase activity and ATP production respectively [55,56,57]. Exposure of lower concentration of uranyl ions to Calamoceras marsupus inhibited its signal transduction and cell growth regulation enzyme Na+/K+ ATPase activity [46, 58]. One reason behind uranium entering into animal organs is due to the formation of uranium-oxyhemoglobin complex [46, 59, 60]. Exposure of uranium also leads to DNA damage of animals by binding of uranyl ions to nucleotides through phosphoric groups present in DNA strands [61]. Several mineral supplements containing dicalcium phosphate are given to the cattle in order to enhance their reproductive capacity and milk production since, these supplements contain high amount of natural uranium and daily consumption of these supplements damages cattle’s intestine and kidneys [62, 63]. On the other hand consumption of uranium contaminated water and soil by animals cause kidney damage [48].
Biosorption
Biosorption is a reversible physicochemical process that permits certain biosorbents to passively adhere toxic ions or other heavy metals onto their cellular surface. In this process, biomasses of microorganisms or plants are used for the adsorption of toxic heavy metal and uranium ions from the wastewater. It can also be defined as uptake of toxic pollutants from wastewater by using non-living biosorbents. It can remove inorganic, organic, soluble, insoluble toxic pollutants from the waste effluent. Biosorption is known as metabolic-independent bioremediation process due to its independency on cellular metabolism during the removal of toxic contaminants from waste effluents. Numerous researches are still going on for the development of novel techniques involving complete and efficient bioremediation of toxic heavy metals including radioactive metals. Heavy metals such as, cadmium (Cd), zinc (Zn), lead (Pb), chromium (Cr), copper (Cu), nickel (Ni), manganese (Mn), mercury (Hg), arsenic (As) and radioactive metals, like uranium (U), radium (Ra), polonium (Po), thorium (Th), plutonium (Pu), strontium (Sr), etc. are toxic and hazardous for the environment. Their accumulation in the environment inhibits photosynthesis, reduction of chlorophyll production, improper seed germination, and functioning of enzymes. These toxic pollutants are also responsible for skin infections, chronic asthma, nervous system deregulation, kidney dysfunction, various types of cancers (bone cancer, liver cancer, lung cancer and breast cancer), diarrhea, Wilson disease, gulf war syndrome, neurological disorders etc. Even today, complete remediation of these toxic pollutants especially uranium by using chemical techniques showed incomplete removal along with several additional drawbacks as discussed in previous section. Therefore, bioremediation process is one of the best possible alternatives to resolve these issues. Several biosorbents such as, macro-algae; micro-algae; bacteria; genetically engineered bacteria, fungi; plants; transgenic plants; biopolymers; nanoparticles such as graphene-oxide; iron-oxide; human black hair; fruit peels such as, pomelo peels; pomegranate peels; chitosan; mesoporous silica; other waste products of fermentation industries etc. are used for the efficient biosorption of uranium and other toxic heavy metals from wastewater.
In this process, uptake of uranium and other heavy metal ions involve either active or passive transportation. There are mainly two phases involved in this process, (a) Solid phase or biosorbent (e.g., bacteria, algae, fungi, plants etc.) and (b) Liquid phase or sorbate (metal ions present in wastewater) [64]. This technique is reported as the most effective in detoxifying all types of toxic metals including uranium in lower concentration. Every cell surface of biosorbents contains biosorption sites such as, cell wall with different functional groups (e.g. carboxyl; amine group; hydroxyl; sulfhydryl; phosphoryl etc.), contributing in biosorption of metal ions from wastewater. Optimum pH; temperature; contact time of biosorbent and metal ions (uranium); initial and final uranium concentration; binding sites etc. play a vital role in enhancing the biosorption capacity of biosorbents. Recently, researchers have reported that isotherm models and thermodyanic studies are also important factors for facilitating efficient biosorption and removal of uranium and other heavy metal ions [65, 66]. Biosorption capacity of the biosorbent can be calculated using the following formula [64, 67],
Here, qe = capacity of biosorption.
Co and Ce = initial and equilibrium concentration of metal ion solution (mg/L) respectively.
V = volume of metal ion solution (L).
W = amount of dose of biosorbent (g).
Biosorption process involves physico-chemical sorption, electrostatic interaction, ion-exchange, complexation, chelation and lastly precipitation. In bioaccumulation, living- biomass is only eligible for the removal of toxic pollutants, but, in biosorption process non-living biosorbents are more efficient than living-biosorbents. Recently, membranes such as, silicon rubber membrane tubes can be used as biosorbent for successful uranium removal. These membrane biosorbents are also applicable for the removal of gas stream from the waste gas stream. In spite of the high efficiency of this process, few researches have been reported till date. Therefore, more studies on membrane based biosorption of uranium from wastewater are required.
Significance of biosorption
There are many methods for the remediation of all kinds of heavy metals from the wastewater. Especially bioaccumulation and biosorption process are used synonymously, but on the basis of removal mechanism of pollutants, both the process are different from each other. In, bioaccumulations, remediation of pollutants are done using living biosorbents as explained in Fig. 2. On the other hand, based on several published research articles, dead-biomass is only involved in biosorption process as they have more capability for complete and efficient remediation of pollutants [68, 69]. Therefore, biosoption is a significant approach for the bioremediation of heavy metals especially uranium. It is a reversible process where used biosorbents can be reused and regenerated further for next biosorption cycle. The rate of remediation is also faster than bioaccumulation and other conventional methods reported. Till now, the reasons of better biosorption capacity of non-living biosorbents comparative to the living-biosorbents are not well understood. Researchers are trying to realize the exact mechanism behind this phenomenon. In exploration of this, several novel proteins, enzymes, genes have been discovered which are responsible for this process. Moreover, in this study, several genetically engineered biosorbents have been discussed. Studies on combined use of dead-microbial biomass and plant wastes as biosorbents in bioremediation processes are in progress.
Advantages of biosorption over other methods
There are several advantages of biosorption process over other traditional methods,
-
Eco-friendly and low cost
-
No energy required
-
Less time consumption (rapid process)
-
Regeneration and reuse of biosorbents
-
Metals or solutes can be recovered from the biosorbent surface by using different desorbing agents
-
Not a complex process
-
All the biosorbents have an excellent biosorption capacity
-
Excellent biosorbent-sorbate interaction
-
Applicable for all kinds of waste effluents
-
Applicable for all kinds of toxic heavy metals especially uranium
-
Agricultural wastes are also applicable for bisorption of uranium
-
Metabolic-independent process
-
Aseptic conditions not required
-
Like living-biomass, non-living biomass also have same biosorption capacity
-
In-situ biosorption process is applicable at the contaminated site
-
No secondary pollutions
-
Applicable for dyes, antibiotics and other pollutants removal
-
Capable of removing soluble or insoluble – organic or inorganic toxic pollutants
-
Biosorption is a broad-spectrum process
-
No additional nutrient requirements
Thus, based on the above mentioned advantages, one can easily say that biosorption process is a significant approach in the field of environmental microbiology and environmental biotechnology.
Mechanism of biosorption
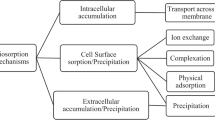
Several studies demonstrated different mechanisms involved in biosorption of uranium or other heavy metals based on the type of biosorbents used. We have already discussed different classes of biosorption based on location in previous section. Metabolism-independent biosorption mechanism consists of ion-exchange, physical adsorption, complexation and sometimes precipitation as shown in Fig. 3 [26]. In spite of many mechanisms involved in biosorption process, very few have been discovered properly according to the published literature from 1996 to 2021. It’s due to the complexity in structures of biosorbents such as microbial biomass, human black hair or other fermentation wastes. Several researches are still going on to understand its mechanism properly in order to put more emphasis on genetically designed biosorbents further.
Ion-exchange
Ion-exchange mechanism involves exchange of metal ions with the counter ions of the biosorbents [70]. Here, functional groups of some biosorbents are involved [71] for the uranium biosorption, especially microorganisms such as, microalgae, bacteria, fungi etc. In case of microalgae, their cell wall participates in this mechanism. It is composed of proteins, lipids and polysaccharides, extracellular polysaccharides and contains functional groups like carboxyl, hydroxyl, phosphate, amino and aromatic compounds [69, 72]. Sometimes, sulfhydryl functional groups are also present which contribute a negative charge on their cell surface and thereby uranium ions binds on their cell surface and involved in metal exchange via this mechanism [73]. These ligand arrangements remain proper on the algal cell surface due to the porous structure of the cell wall [74, 75]. But, in case of microalgae biosorbent, two mechanisms are involved called passive and active biosorption [76].
In case of bacteria, such as, Pseudomonas halodenitrificans cell wall, calcium ions and lipopolysaccharides (LPS) are present due to which, cobalt and uranium ions were easily biosorbed through this ion-exchange mechanism [77]. Bacterial cell wall consists of various kinds of functional groups such as carboxyls, phosphomono-esters, phosphodi-esters, amines, hydroxyls, etc. [77]. Uranium and zinc sorption by using Pseudomonas putida biofilm showed carboxyl like complexes where ion-exchange mechanism showed an efficient biosorption of uranium as well as zinc respectively [78, 79].
Complexation
Complexation or co-ordination step can be defined as combination of cationic sorbates and anionic biosorbents. Due to electrostatic interactions between the uranium ions, chelating agents and biosorbents, complex formation occurs [80]. Furthermore, during the interaction between uranium and biosorbents on biological surface, uranium ions co-ordinates in formation of complex species [81]. The mechanism of all uranium biosorption process from the wastewater has been illustrated in Fig. 3.
During the biosorption of uranium ions, Pseudomonas putida cell wall contains carboxyl and phosphoryl groups which contributed in the formation of uranium-phosphoryl complexes as well as uranium-carboxyl complexes [82]. On the cell wall of Bacillus sp., an inner sphere complex forms between U(VI) and phosphate groups at pH 4.5–5.0 [83, 84]. Cell wall of Escherichia coli contains amido and hydroxyl groups which formed complexes with uranium ions and showed efficient biosorption capacity for this toxic metal ion [85]. Thus, bacterial cell wall compositions and structure plays an important role in biosorption process. Therefore, complexation or co-ordination mechanism occurs due to bio-surfactants, proteins, lipids, nucleic acids and polysaccharides present on some cell surface of biosorbents [86].
Physical adsorption
Physical adsorption is a phenomenon of adhesion of uranium ions on the surface of biosorbent [87]. In Fig. 3, it has been described that physical adsorption occurs due to various types of interactions between sorbate and biosorbent such as van-der waal’s force, electrostatic interactions, covalent bonds, redox reactions and few other interactions [88]. Surface area of biosorbents is the most important parameter for the physical adsorption mechanisms. In some studies, it is reported that pH of solution also plays an important role in efficient adsorption. Several studies suggested that, human black hair [89], modified wheat bran [90] and some fruit peels such as, pomelo peels [91], pomegranate peels [92] biosorbed U(VI) ions through physical adsorption mechanism. In this process, a layer of sorbate forms on the surface of the biosorbents [93]. This layer may be of monolayer, bilayer or multilayer. In case of modified wheat bran, langmuir isotherm confirmed monolayer adsorption for the uranium (VI) removal [90]. In this mechanism, uranium contains positively charged ions while biosorbents for uranium uptake are negatively charged. Therefore, uranium ions are attracted by the negative charge containing biosorbent on the cell surface. From this, it is clear that adsorption depends on the pH of the process solution. Another report regarding bacteria as biosorbents suggests that extracellular polymeric substance (EPS) which is produced by bacteria also involve in bioremediation of uranium ions by physical adsorption mechanism.
Biosorbents and its role in uranium biosorption
Biosorbents are required for the bioremediation of uranium ions, which simply bind on the cell surface of various kinds of biosorbents. It could be any biological materials like bacteria, fungi, algae, agricultural wastes, polysaccharide etc. and capable of sorption of heavy metal ions including uranium from aqueous solution. In this context, these biosorbents are categorized into solid phase as liquid phase signifies wastewater where toxic radioactive materials such as, uranium, thorium, strontium then toxic heavy metals such as, cadmium, zinc, lead are present. So, genetically engineered microorganisms, bacteria, fungi, algae, are named as living biosorbents, whereas plant wastes, fruit wastes, agricultural wastes, exo-polysaccharide materials and other biopolymers such as, alginate, chitosan and peat moss are non-living biosorbents. An ideal biosorbents should be easily available, cheaper, reusable, pollution free with more number of functional groups on the surface, have the ability to be genetically or chemically modified for enhanced biosorption capacity and available on large scale for its application.
Most of the biosorbents with hydroxyl, carboxyl, amino, sulfhydryl, phosphate, ester group on the cell surfaces are charged biosorbents. The cell surfaces possess an overall negative charge and act as a strong binding site for cationic uranium ions. During the interaction (due to van-der-waals force or electrostatic interaction) between positively charged uranium ions and proteins or genes or binding group present on the cell surface of biosorbents, a complex species is formed due to pH effect. In this way uranium ions are being adsorbed onto the cell surface of the biosorbents and removed from the wastewater in less time.
Algae as biosorbents
Algal cells are eukaryotic cells containing membrane-bound organelles, mainly nucleus, chloroplast and mitochondria. There are several types of algae based on their habitat e.g., aquatic algae which are predominantly found in fresh water, ponds, pools, lakes, rivers, tanks, marine water etc. Few other algae are Cladophora sp., Volvox sp., Microcystis sp., Chara sp., planktonic algae (found in free-floating water surface), benthic algae (attached to the bottom of the shallow pools), endozoic algae (grows inside the body of aquatic animals or vertebrates), epizoic algae (grows on the surface of other aquatic animals), epiphytic algae (known to grow on the surface of aquatic plants) [94]. Halophytic algae (found in extremely high salinity type of area) such as, Chlamydomonas ehrenbergii, Oscillatoria sp., Ulothrix sp. Thermophytic algae (found in hot springs that is in extreme temperature above 85◦C); some of them are Synechoccus sp., Synechocystis sp., Phormidium sp., Scytonema sp. Most importantly, Cyanophyceae group of algae belongs to this thermophytic algae group. Cryophytic algae grows on the surface of mountains, snow and in polar regions such as,Chlamydomonas nivalis, Chlamydomonas yellowstonensis, Nostoc sp. Parasite algaelive as parasite and semi-parasite on the algal surface. Symbiotic algae grow in association with plants such as, Anabaena, Azollae–Azolla sp.Terrestrial algae are found in soils, moist rocks, moist wood logs etc.such as, Anabaena sp., Euglena sp., Nostoc sp., Vaucheria sp [95]. Most of the algal cells contain cell wall which consists of Fibrillar cellulose, mannans, xylans and amorphous alginic acid, fucoida, galactans. Algae are of two types which includes microalgae and macroalgae [96, 97].
Macroalgae as biosorbents
Macroalgae have three groups that are, (Chlorophyta) green algae, (Phaeophyta) brown algae and (Rhodophyta) red algae. These groups are based on their chlorophyll pigmentation. Macro-algae are multicellular autotrophic organisms found in fresh water and saltwater. Macroalgae are also known as seaweed. Macroalgae are commonly famous for its high biosorption capacity for uranium and other radioactive as well as toxic heavy metals from its surrounding environment. Padina pavonia which is a brown marine macroalgae has a higher biosorption capacity for the uranium ions from the wastewater at pH 4 in 120 min at 323 K temperature [98] as explained in
Supplimentary Table 1. Sargassum sp.,brown algae also have higher biosorption capacity for the uranium at pH 5. In case of brown algae, carboxylic acid; sulfonic acid of fucoidan are the most abundant functional group. At low pH, sulfonic acid functional group of brown algae uptake uranium ions, while, carboxylic acid of brown algae binds with uranium at high pH. The brown algae most frequently studied for uranium biosorption, Laminaria japonica, Sargassum sp., Fucus vesiculosus, Gelidiella acerosa, Padina sp. as explained in Fig. 4.
Rhodophyta, more commonly known as red algae are photosynthetic multi cellular eukaryotes mainly found in marine water, less in fresh water and also contain floridean starch as a reserve food outside of their plastids [99]. The presence of red pigment, r-phycoerythrin is responsible for their red colour. They lack flagella and their cell wall consists of cellulose and sulfated phycocolloids. Gracilaria corticata, a photosynthetic red algae (found in both sea coast and fresh water regions) having carboxyl and hydroxyl functional groups on their cell surface showed maximum biosorption capacity (200 mg/g) at pH 4.5 within 3 h of contact time and this process was spontaneous, followed Freundlich isotherm model (R2 = 0.988) which means these given sorbent’s surface was heterogenous or was in linear form and followed pseudo-second order kinetic model [100]. Catenella repens, a red algae having functional groups carboxylates, hydroxyl, sulfate, and phosphate are present on their cell surface biosorbed more than 90% of U(VI) ions within 30 min between 15 and 55 °C at pH 2.5 from any kind of wastewater.
Microalgae as biosorbents
Microalgae belong to the group of photosynthetic organisms and are found in fresh water as well as in marine water. It has several advantages as biosorbents for uranium removal from wastewater like rapid and higher biosorption capacity, less time consuming, eco-friendly; reusable, recyclable, polynomial, low-cost, easy recovery and regeneration capability. Most common microalgae studied for uranium biosorption are, Chlamydomonas reinhardtii, Dunaliella salina, Chlorella sp., Scenedesmus abundans, Spirulina platensis, Spirogyra sp., etc. During the photosynthesis, these microalgal biomass produce peptide bond and this peptide bond binds to uranium ions and thereby forms organometallic complex and thus the toxic effects of uranium get neutralized. Both macroalgae and microalgae differs from each other in their biosorption capacity. In 2014, Lee et al. [101] have reported that Laminaria japonica have maximum biosorption capacity for uranium ions at pH 4.5 at temperature 30 °C within 60 min as shown in Supplementary Table 1.
Bacteria as biosorbents
Bacteria are of two types, gram-positive bacteria and gram-negative bacteria which differ from each other in their cell wall composition and thickness. In gram-positive bacteria, peptidoglycan is thicker and this macromolecule is made up of series of glycan cross-linked by peptide side branches. Gram-positive bacteria have higher bioremediation capacity for cationic uranium than gram-negative bacteria [102, 103]. This is due to the presence of significant electronegative charge density as they have teichoic and teichuronic acids linked by phosphodiester bonds attached to peptidoglycan of their cell wall. In case of bacteria as biosorbents for uranium removal, functional groups such as, carboxyl, phosphate, amino is involved [78, 104, 105]. Several reports suggest that oxygen-containing functional groups were responsible for the uranium ions biosorption [82, 106, 107].
Role of bacterial biofilms as biosorbents
Biofilms are complex consortium of surface-attached microbial cells which are enclosed in the extracellular polymeric substances (EPS). Bacteria; fungi; algae etc. are capable of forming biofilms. Bacterial biofilms play an important role by providing protection from fluctuating pH; dehydration; anti-microbial agents. Especially, bacterial biofilms are suitable and for removal of uranium ions from wastewater as shown in Supplimentary Table 1. The reason of using bacterial biofilms is due to their fast growth and stability in extreme environmental conditions. Enzymatic activity of bacteria plays an important role in the biofilm formation and is involved in remediation of uranium ions. Biofilms of Staphylococcus aureus, Pseudomonas aeruginosa, Deinococcus radiodurans are mainly studied and used for uranium biosorption [108,109,110] and are shown in Fig. 5. Functional group of biofilm biomass confirmed the involvement of phosphate, hydroxyl and carboxyl groups in uranium ions binding [111].
Fungi as biosorbents
Recent studies reported that, fungi have rich cell wall content which signifies of having huge amount of functional groups favoring the biosorption process [112]. The fungal cell wall is consists of chitin; cellulose; β-glucan; α-glucan; chitosans; glycoproteins; polyuranides; lipids and other inorganic salts. Fungi can be easily grown and cultivated in a low-cost. Aspergillus fumigatus removed U(VI) rapidly within 1 h at pH 5.0 at the temperature of 5–50 °C [113]. Mangrove endophytic fungus Fusarium sp. #ZZF51 from the South China Sea removed uranium rapidly within 60 min at pH 4.0 [114] as explained in Supplimentary Table 1. Trichoderma harzianum removed U(VI) ions from milling waste water rapidly within 1 h at the temperature of 303 K at pH 6.0 [115]. Several studies reported that hydroxyl; amino and carboxyl groups are the most common functional group present on the cell surface of fungi responsible for uranium biosorption [116, 117]. Trichoderma harzianum, chemically modified Lentinus concinnus and Aspergillus fumigatus have maximum uranium biosorption capacity as shown in Fig. 6.
Nanoparticles as biosorbents
One of the key factors for the biosorption of uranium by using nano sized materials is the high surface to volume ratio of nanoparticles (NPs) [118]. They are selective and have high capacity for removing uranium from waste effluents. Till now, microbial cells immobilized on magnetic NPs have been used a biosorbent for uranium biosorption and considered them as an innovative technique for efficient treatment of wastewater [104]. Even though nanoparticles based biosorption of uranium is most efficient process, but very limited research has been carried out related to graphene-based nanoparticles. As mentioned above, due to the high surface area of the NPs, maximum number of uranium ion uptake is possible within a very less time [119, 120]. Amine-functionalized magnetite-silica NPs showed efficient biosorption of U(VI) ions within 30 min at the temperature of 293–313 k [121]. Chitosan-coated magnetic silica NPs showed efficient biosorption capacity at pH 4.0 [122] as tabulated in Supplimentary Table 1.
Rice and coffee husks as biosorbents
Rice husks with –OH, –O–CH3, Si–H, C–C functional groups are most stable biosorbents used for uranium, thorium and other heavy metal removal from wastewater [123, 124]. Some of the research articles reported that chemical modifications of these rice husks can biosorb huge amount of uranium ions from wastewater. Rice husks are feasible, easily available and one of the potential candidates to be used as biosorbent to reduce efficient amount of environmental pollutions in a cheap cost.
Coffee husks are comprised of dry outer skin, pulp and parchment, having –COOH, C = O and –OH functional groups and have good capacity of uranium biosorption. Very limited research has been reported regarding rice and coffee husks for uranium ions biosorption. The biosorption capacity of these two huskes has been documented in Supplimentary Table 1. They have higher and good biosorption capacity for uranium ions removal within 2 h at pH 4–7.5 [125].
Wheat bran, eggshell membrane, sugar beet pulp and pomelo peels as biosorbents
Wheat bran, a by-product of conventional wheat milling industries is composed of phenolics and flavonoids [126, 127]. It has many nutritional as well as other important properties as biosorbent. Several researchers have shown the successful biosorption of uranium using wheat bran, which is easily biodegradable, economically viable, commercially available [90, 128]. The use of wheat bran over microorganisms in biosorption is due to the unexpected microbial growth during higher uranium ion concentrations in solution.
Recent studies also reported that the magnetically modified wheat brans exhibit enhanced biosorption activity. Wang et al. have used nano and micro level magnetic iron oxide nanoparticles for the modification of wheat brans which showed excellent biosorption capacity at pH = 7.0 at 30◦C within 45 min [90]. Therefore, magnetic wheat bran is the most promising biosorbent for bioremediation of uranium from any kind of waste effluents. The eggshell membranes are composed of protein fibers such as Type I, V, X collagen, osteopontin and sialoprotein [129,130,131]. The structure of eggshell membrane (ESM) has amino, amido and carboxyl functional groups and these ESM can easily biosorbed uranium ions from all kinds of radioactive wastewater [132]. In most of the published research article, carboxyl-rich agents are used for enhancing the biosorption capacity for uranium biosorption. Thus, ESM-COOH showed an excellent biosorption of selective U(VI) ions from radioactive wastewater and can be reused for further biosorption process [129]. The adsorption capacity was around 84% of the initial value after six cycles as reported in Supplimentary Table 1. Beside, both sugar beet pulp and pomelo peels are novel biosorbents that have been used for biosorption of U(VI) ions from wastewater [91, 133]. All these reports suggest that, biological wastes are usable for the bioremediation of large concentration of uranium ions from wastewaters as explained in Figs. 7 and 8.
Advantages of biosorption over conventional methods, bioaccumulation, bioprecipitation and bioreduction for uranium bioremediation
In recent times, many researchers have studied and discovered several kinds of treatment technologies for the efficient removal of uranium from waste effluent. Technologies include physicochemical, electrochemical and oxidation–reduction method. Membrane-filtration, ultra-filtration, chemical precipitation and ion-exchange are categorized under physicochemical method [134]. Electrochemical method consists of electro-dialysis, electro-dialysis reversal, electro-coagulation, flocculation and reverses osmosis. There are many floatation techniques for the removal of toxic heavy metals from waste effluents which comprise dissolved-air floatation, precipitate flotation and ion-floatation. Among these, ion-floatation technique is commonly used for the uranium-removal from the wastewaters. Filtration techniques include membrane-filtration, nano-filtration, ultra-filtration and out of which only membrane-filtration and ultra-filtration are used for the uranium removal. Electrocoagulation, chemical coagulation etc. are used for the heavy metal removal from wastewaters. Electrodialysis and electrodialysis reversal methods are used for the uranium remediation from the wastewater. A brief outline has been shown regarding the advantages of biosoption over conventional methods which are used for the uranium removal.
For complete and efficient bioremediation of uranium, many methods have been developed mainly biosorption, bioaccumulation, bioprecipitation and bioreduction. All these methods have some limitations and biosorption is the most suitable and environmental friendly process among all these methods. Biosorption is a metabolism-independent passive process in which the uranium ions simply binds with the active sites present on any biosorbent’s cell surface. It is a reversible process, while bioaccumulation is an irreversible process and depends on the cellular metabolism for the bioremediation of uranium. This process does not need any living biosorbents. Bioaccumulation process composed of much complex operating system while biosorption operating system is much simpler and there is no requirement of any additional nutrients as explained in Table 2. Considering bio precipitation and biosorption process, the later is again more suitable, cost effective method and it works on very small surface area, while, bioprecipitation requires large surface areaas as shown in Table 3. Also, in biosorption process, there is no involvement of uranium conversion from liquid to solid phase and that’s why this process is quick and bioremediate uranium completely.
While, comparing biosorption and bioreduction process for uranium bioremediation, the former is less time consuming than bioreduction process. Bioreduction involves enzymatic reduction of uranium ions, while biosorption simply involves adsorption of uranium ions onto the cell surface of any kind of biosorbents. Large operating space is not required in biosorption. This process is applicable even in harsh environmental conditions, since there is no risk of any reduction in microbial activites as explained in Table 4. Comparing both these processes with biosorption, it is clear that biosorption is the most simple, suitable, environment friendly and less time consuming process. Another important aspect of biosorption process is the possibility of regeneration of biosorbent which is not there in bioreduction and bioprecipitation processes.
Genetically modified/ engineered microorganisms and plants as novel biosorbents
In recent years, many researchers have reported more efficient biosorption of heavy metals (HMs) and radionuclides by modified/engineered microorganisms which can sustain harsh environmental conditions [135,136,137]. They can easily uptake large amount of metal ions as well as radionuclides along with other pollutants from waste effluents at a time. The Deino-flr-2 radiation–resistant genetically engineered bacterial strain have flr-2 fluorine-resistant gene, the recombinant plasmid pRADK-flr-2 transformed into Deinococcus radiodurans R1and it become fluoride-resistant genetically engineered bacteria, removed 90% of U(VI) ions from uranium-containing wastewater [138] as shown in Supplimentary Table 1. The Shewanella RCR17, MtrF gene is expressed in this strain, removed 97% of uranium from wastewater, while exppressing MtrC, OmcA genes in Shewanella RCR17 have much lower ability to remove uranium of higher concentration from uranium-containing wastewater [139].
For efficient or enhanced biosorption of uranium from all types of waste effluents, genetically engineered plants also can be used. So, to remove uranium from wastewater efficiently, we need to understand the molecular mechanism of radionuclide-tolerance in plants. Transgenic plants are used for uranium removal from wastewaters. Genetic engineering technique is used to transfer the desired trait’s genes from one organism to another. This technique include, transformation of genes which are uranium-tolerance, use of particle gun or electroporation, or by overproducing genes involved in the biosynthesis of chelating agents, use of transporter genes as it has an all-in-one quality, by overexpressing genes involved in metal-sorption and tolerance, by using regulatory genes. Brassica juncea, Chenopodium amaranticolor [140, 141] and Amoracia rusticana plants were engineered by using Agrobacterium rhizogenes which is a soil bacterium, the plants transformed by this soil bacterium contains Ri genes which is responsible for inducing hair root system (uptake more uranium and other radionuclide metals from waste effluents), thereby Ri genes can be transfer into transgenic plants [140]. Overexpressing of red alga Porphyra yezoensis containing CYTC6 (Cytochrome c6) genes in transgenic plants showed to be the best biosorbent for enhanced biosorption of uranium. HDG11 is also reported to be an ideal gene for phytoremediation of uranium and other radionuclides as well [140]. Recently, CRISPR/Cas9 genome editing technology has been used for the cadmium transporter gene OsNramp5 characterization in rice [140, 142]. Hence, it can be utilized in near future, for charaterization of uranium and other radionuclide biosorption or transporter genes. An Arabidopsis transgenic plant have MATE proteins, showed enhanced removal of uranium from waste effluents [140]. Construction of PhoN (Periplasmic enzyme) expressing recombinant Deinococcus radiodurans strain (DrPhoN), showed more than 90% biosorption capacity of uranium from nuclear waste effluents [143]. “Extremozymes” enzyme/proteins present in Extremophilic microorganisms such as Halophiles, Thermophiles, Psychrophiles, Acidophiles, Alkaliphiles, Basophiles showed enhanced biosorption of uranium ion in harsh environmental conditions [144]. Genetic engineering techniques are used to modify and improve different extremophilic bacterial or fungal strains or their extremophilic proteins for enhanced uranium and other radionuclide biosorption.
Novel bacterial strain Stenitrophomonas bentonitica BII-R7 have cell wall proteins CreD & OmpA; with phosphatase activity of PAP2 or ALP-like phosphatases; and RND transporters, thereby helps this bacterial strain to cope up with uranium for effective biosorption of this metal from various waste effluents [145]. Several studies reported that the native or genetically engineered microorganisms and plants contains some novel proteins or genes such as, DrPhoN, NiCoT, phoK, ChrR6, merA, czc, fccA, ctyc3 and PpcA which are involved in effective bioremediation of uranium on a large scale [146,147,148,149]. Phytochelatin are the small peptides with general structure (Glu-cys) which are rich in cysteine, while metallothioneins proteins are also cysteine rich and both of them have higher uranium binding and uptake capacity in the process of biosorption [135].
Chemical modifications on biosorbents and its importance
Recently, most of the biosorption process is carried out by chemically modified biosorbents, be it plant wastes, bacteria, algae or fungi. Several modifying agents like sodium hydroxide, formaldehyde, calcium hydroxide, sodium carbonate, hydrochloric acids, nitric acid, sulfuric acid, tartaric acid, citric acid, thioglycolicacid, ethylenediamine, methanol, acetic acid, hydrogen peroxide, few dyes like reactive orange 13 are involved in chemical modification of biosorbents for uranium biosorption [150, 151]. Tri-amidoxime [112], 2,5-diaminobenzene sulfonic acid, sodium-alginate, calcium-alginate, some flavonoids like Quercetin [25] has been used by many researchers for the modification of biosorbents. Now, the reason behind doing this modification is to increase the biosorption capacity of the biosorbents. It cause changes in functional groups as well as cell surface properties of biosorbents. This chemical modifications enriched structural polymers of cell wall of the microoganisms. Negative charge present on the cell surface of biosorbents also increases due to this chemical modifications. In this way, removal efficiency of uranium from waste effluents become enhanced. Many examples are given below showing the biosorption and removal efficiency of biosorbents after modifying them chemically.
Quercetin-NaOH modified Spirulina platensis for biosorption of uranium
The modified-dead biomass of microalgae such as, Spirulina platensis and some marine unicellular Cyanobacterium such as, Synechoccus elongatus has a greater biosorption capacity for the polyvalent metallic ions like U(VI) from the waste effluent. It has been observed that for increasing the adsorption efficiency of Spirulina platensis, these microalgae can be modified by using Quercetin [152] followed by NaOH [25]. The chemical modification of microalgae by using NaOH increases the negative charge on the cell surface of Spirulina platensis, while quercetin deprotonate in alkaline media and thus, the modified Spirulina platensis algal cell biomass surface becomes enriched and enhanced its biosorption capacity [153].
Biosorption of U(VI) by chemically modified marine-derived mangrove endophytic fungus Fusarium sp.#ZZF51
Mangrove endophytic fungus Fusarium sp. located in Chinese Zhanjiang sea area, are chemically modified by using formaldehyde, methanol and acetic acid. The reason behind its chemical modification is to enhance affinity of biosorption of U(VI) ion from wastewater. Through this modification, number of functional groups increased on the cell surface of Fusarium sp. #ZZF51 which induces more number of binding sites for U(VI) ions and thereby increases in removal efficiency in neutral pH at less temperature and time [154].
H3PO4 modified pomegranate peel as biosorbent
Pomegranate peel is the source of phenolic compounds like catechin, epicatechin, lignins, ellagic tannin, etc. [155]. The important constituents of pomegranate peel are carbon, oxygen and hydrogen, nitrogen along with the functional groups like carboxylic acid, phenol, carbonyl, etc. It is therefore used for the biosorption of various radioactive metal ions, mainly U(VI) and U(IV) ions from uranium-contaminated waste water due to its low-cost, easily available, reusuable and non-pollutant. For efficient bioremediation of uranium from waste water, some researchers has used H3PO4 chemical for modifying the cell surface properties of pomegranate peel. The activated carbon produced by pomegranate peel [156] make it as a good biosorbent for biosorption of uranium (VI) ions from all kinds of wastewater [157].
Sodium-alginate modified Bacillus megaterium as biosorbent
Bacillus megaterium is commonly used for the remediation of U(VI) and other heavy metals from all types of waste effluents such as, textile effluents, industrial waste effluents, nuclear waste effluents, mining or milling waste effluents etc. and the reasons behind using this bacteria for water treatment is due to its fast growth rate and presence of maximum metabolites. [158], modified this bacterial strain chemically by using sodium alginate. Sodium alginate is a linear anion copolymer having homopolymeric blocks which linked covalently to each other [159]. It contains carboxyl groups and pyranose oxygen atom which has the ability to form five membered chelates with metal ions and induces the efficiency of adsorption capacity of biosorbents [159,160,161] like Bacillus megaterium in a stable form. Sodium alginate includes surface grafting of functional groups on the biosorbent which increases the specific uranium ion uptake capacity [159]. Here, carboxyl groups plays an important role in providing binding sites for cationic uranium ions and another one is cross-linking, involved in building a 3D structure, it simply enhance the mechanical strength of biosorbents by changing its solubility [159]. Sodium-alginate was mixed with Bacillus megaterium for better biosorption of uranium ions on the cell surface of the bacterium [158]. This chemical modification enhances the binding sites for uranium ions for complete removal of uranium from aqueous solution. In this experiment, 8 mg of adsorbent is used for the biosorption of U(VI) ions at pH 3–9 and the adsorption capacity is 74.61 mg/g at 30 °C. Here, the adsorption capacity is not decreased even after reusing it for 5 times when Bacillus megaterium is chemically modified with more number of functional groups like C–H and C = O [158].
Factors affecting efficient biosorption of uranium from various waste effluents
There are many parameters influencing the biosorption process accurately. These factors play an important role in interaction between cationic uranium and anionic biosorbents. The fore most important factor among all the factors are pH and contact time, for an efficient biosorption of uranium ions, researchers are looking for genetically engineered biosorbents along with some chemical modifications. Presently, biosorption of uranium at neutral pH, in less time with less biosorbent dosage with more number of binding sites (functional group) is the main goal of all the researchers.
pH
The most vital factor for biosorption process is pH of the solution. Here metabolism of biosorbents play a significant role, as it determines optimum pH for efficient biosorption process. The behavior of functional groups present on the cell surface of biosorbents as well as the behavior or chemical nature of uranium metal ions are affected by pH. It is reported by many researchers that the value of pH is directly proportional to biosorption capacity of biosorbents [82, 162, 163]. The biosorption of U(VI) ions are usually reduced at low acidic pH level < 3.5. At low pH, the repulsion between uranium cationic ions and anionic charged cell surface (binding functional groups) of biosorbents occurs at high range which leads in less removal of uranium from wastewater. The optimum pH range for biosorption of uranium ion species is between pH 3.0–9.0. At this pH range, affinity between uranium species and biosorbents containing functional groups on cell surface enhanced and thereby leads to excessive removal of uranium from wastewater. Different predominant uranium species such as (UO2)2(OH)22+, UO22+, (UO2)3(OH)5+, (UO2)4(OH)7+, U3O8, UO2(NO3)2 have different affinity towards the functional groups of biosorbents that determines the biosorption process. In some cases, it is reported that higher pH leads to the development of negative or non-complexible uranium species due to hydrolysis of uranium [164]. Furthermore, formation of precipitate such as (4UO3. 9H2O) is also responsible for gradual decline in uranium biosorption capacity [165, 166].
Temperature
Temperature also plays an important role in biosorption process and most of the reactions are reported to be temperature dependent [167]. Increase in temperature induces increment of biosorbent pore size, active sites, and cell layer thickness reduction. Mostly, biosorptive remediation of uranyl ions are endothermic in nature and higher temperature mostly enhances the attachment of radionuclide ions onto the biosorbent surface [167].Efficient and complete biosorption of U(VI) ions occurs in the temperature range of 15–37 °C, above which biosorption capacity decreases. Beside, temperature affects the surface activity of the biosorbent and thereby the biosorption capacity. It has been observed that, thermodynamic study is the most important factor that determines the temperature for biosorption process [167]. Khani et al. [167] in the year 2010, reported that the spontaneous and exothermic nature of uranium biosorption process from industrial waste water by using Padina sp.The biosorption competency of the biosorbent shows retardation towards the uranium ion remediation which implies exothermic nature of the process. This negative impact may be due to the damage of biosorbent binding sites. Another reason may be due to desorption tendancy of the radionuclide ions from the biosorbent surface. Furthermore, higher temperature may cause physical disruption of biosorbent. For this reason, remediation of uranium through this process at room temperature is mostly beneficial [168]. Algal biomass at the temperature of 15 °C increased biosorption efficiency of uranium ions [168].
Contact time
Contact time is the third most imperative factor for effectual biosorption process. One of the important reasons of using of bacteria, fungi, algae, plant wastes, agricultural wastes, fruit wastes etc. as biosorbents because they have shown complete and efficient biosorption of uranium at less time. It’s important to mention that at maximum bisorption capacity, binding sites become fully saturated and at this point of time, increase in contact time doesn’t show any effects. For uranium biosorption by all kinds of biosorbents, range of contact time showed between 30 min and 48 h. In case of Sodium-alginate modified Bacillus megaterium biosorbent, 5 h sorption period showed good potentiality for uranium biosorption [158]. On the other hand, Deinococcus radiodurans bio-film biosorbed all uranium ions within 15–60 min [108] while, biosorption time for U(VI) ions by Ulva lactuca completed within 1 h [169].
Biosorbent dosage, initial and final uranium concentration
The dose of biomass also plays an important role during biosorption process. It is inversely proportional to the biosorptive capacity of uranium ions, but in case of highly contaminated water, this concept is not correct, as it is known that the cell surface of biosorbents are composed of a large number of functional groups that acts as a binding site for uranium ions. Hence, if less amount of biosorbent is supplied into the complex aqueous solution contaminated with uranium, then high competition occurs for these binding sites between the uranium ions and therefore less biosorption of uranium ion occurs. On the other hand, if the amount of biosorbent dosage is more supplied into this complex water contaminated with huge amount of uranium then, less or almost no competition occur for the binding sites of biosorbent and maximum biosorption of uranium takes place. The amount of biomass or biosorbent dosage used by most of the researchers was in between 0.1 and 20 g/L [170,171,172].It is important to record the initial uranium concentration before starting biosorption process. Thus after completion of biosorption process, it can easily determine the potentiality of novel biosorbents by comparing the final uranium concentration with initial one. Researchers showed that, the amount of initial uranium concentration was between 0.8 and 600 mg/L [71].
Sites (functional groups)
Surface area of the biosorbents plays a key role in biosorption process. It is directly proportional to the biosorption capacity of the biosorbent materials. Alternatively, more the number of functional groups that act as sites for binding of uranium ions, higher will be the biosorption capacity [173]. Anionic functional groups which are present on bacteria, fungi, algae and other biosorbent materials are hydroxyl, carboxyl, sulfhydryl, amino, amide, phosphate, alkyl and other aromatic compounds. These functional groups contribute negative charge to the cell surface of biosorbents due to which they easily involved in ion-exchange, physical adsorption and then forms complex with cationic uranium ions. Algal cell wall is composed of cellulose, hemicellulose, pectin, alginic acid, mucilage, fucin, fucoidin. In addition to this, cell wall of Chlamydomonas sp., Volvox sp., Ulothrix sp., Spirogyra sp., Spirulina platensis, Sargassum sp., are composed of polysaccharides and lignin. Cyanobacterial cell wall is composed of peptidoglycan, polymer of N-acetyl glucosamine and beta-1,4-N-acetylmuramic acid and therefore, they have carboxyl groups due to which they are involved in binding and uptake of uranium ions [174, 175].
Specifically, algal cell wall is composed of cellulose, hemicellulose, pectin, alginic acid, mucilage, fucin, fucoidin whereas cyanophycea cell wall composed of mucopeptide which consists of amino acids, glucosamine, muramic acid, The protoplast is surrounded by cell membrane (lipid and protein like other eukaryotic cell membrane), additionally some macro- and micro-algae chloroplast is present for example, Chlamydomonas sp., Volvox sp., Ulothrix sp., Spirogyra sp., Spirulina platensis, Sargassum, Catenellarepens. Furthermore, polysaccharides and lignin are also available. In case of bacteria, its cell structure is simple just like algal cell, bacterial cell is also consists of cell wall, cell membrane, capsule, slime layer, etc. Here slime layer contains functional groups such as, carboxyl, amino, sulfate, phosphate for biosorption of uranium ions. Gram-positive bacterial cell contains thick peptidoglycan layer connected by series of amino acids, their cell wall also contains teichoic acids which are connected to lipids of lipid bilayers and thereby forming lipoteichoic acids. Gram-negative bacterial cell contains outer-membrane and lipopolysaccharides, teichoic acids due to which they exhibit negative charges on them. Some bacteria also contain extracellular polysaccharides which also facilitate for uranium binding [80]. Bacteria such as, Bacillus sp., Pseudomonas sp., Deinococcus radiodurans (genetically engineered bacteria) are involved. Many Extremophiles, having extremozyme i.e. enzyme which help them to remain stable even in harsh environmental conditions acts as an excellent biosorbents for uranium biosorption. Thermophiles such as, Thermus thermophilus, Thermus scotuductus, Thermoanaerobacter sp., Psychrophiles such as, Arthrobacterpsychro lactophilus, Rhodobacteraceae, halophiles such as, Alcaligenes latus, Methylobacterium extorquens, Pseudomonas sp., recombinant Escherichia coli. Most of the halophiles contain Poly hydroxyl alkanoates (PHA) and Polyhydrobutyrate (PHB) [176]. Acidophiles namely Acidithiobacillus ferrooxidans, Acidithiobacillus caldus and basophiles such as, Natronocella acetinitrilica, Pseudomonas putida and Pseudomonas pseudoalcaligenes are involved in uranium ion biosorption from all types of wastewater.
For instance, fungal cell wall is more rigid than bacterial cell wall. Fungal cell wall consists of various polysaccharides which forms complex with proteins, lipids and some pigments. The outer layer consists of glucans, mannans, galactans and inner layer consist of chitin chains, polymer of D-glycopyranose or sometime non-cellulosic glucan. It also contains polyphosphates and some inorganic ions. It consists of hexomine, lipid, phosphorus, carbohydrate. Chitin-chitosan and phosphate-glucoronic acid of fungal cell wall mediates binding of uranium ions through ion-exchange. Several functional groups such as, carboxyl, phosphate, uranic acids nitrogen-containing ligands on chitin or chitosan, protein facilitates effective biosorption of uranium from various kinds of waste effluents [177,178,179]. In recent times, plant wastes such as, wheat bran, pomegranate peels, pomelo peels, and human black hair contributes similar mechanisms for binding of uranium ions on their cell surface by modifying them using magnetic iron-oxide nano- or micro-particles or by using chemicals [89]. Thus, it is clear that functional groups of biosorbents are the most important factor for uptake of uranium ions from waste effluents.
Desorption
After the completion of biosorption process, the same biosorbent is reusable simply by removing the uranium ions and safely discarding them. Several desorbing agents such as sodium carbonate, sodium citrate, EDTA, dilute hydrochloric acid, dilute hydrogen sulphate, potassium hydroxide, citric acid, lactic acid, acetic acid, thiosulphate, sodium hydroxide are used in this process [119, 173]. The physical and chemical properties of biosorbents did not change during desorption process and thus, biosorbent materials can be reused and regenerated for further cycle. Scenedesmus obliqus was used for uranium removal in biosorption process followed adding 0.1 M NaOH and 2.0 M NaCl desorbing agents for the reuse of the biosorbent [180]. Akhtar et al. have reported that use of 0.1 M HCl desorbing agent for the removal of uranium ions from the Trichoderma harzianum biosorbent after the completion of biosorption process in order to reuse this biosorbent for the next biosorption cycles [181]. On the other hand, after completion of U(VI) ions biosorption by using novel graphene-oxide immobilized Saccharomyces cerevisiae gel beads, 0.1 M HNO3, 0.1 M HCl, 0.1 M NaOH desorbing agents were used for the desorption process. For removal of U(VI) ions from the amine and dithizidine-functionalized magnetite chitosan hybrid biosorbent material, two desorbing agents 0.3 M Na2CO3 and 0.1 M H2O2 were used [182]. Exact mechanism for desorption process is not yet understood vividly and therefore, more in depth research is highly required.
Equilibrium study
Generally biosorption isotherm study is an important curve that describes the phenomenon governing retention or mobility of uranium compounds from the liquid phase to a solid-phase at the same temperature and pH [108]. These are depicted graphically by plotting the solid-phase against its concentration. This model determines whether the adsorption remains constant or decline with increasing concentrations [183]. These isotherm models help us to know whether multilayer or single layer or no layer formed on biosorbent material [88, 145]. The reports showed that for uranium biosorption, Freundlich and Langmuir isotherm models are common compared to other models. Langmuir-type adsorption is a monolayer process, while Freundlich-type adsorption is a multilayer process in which the adsorbate amount per unit adsorbent mass increases gradually. Freundlich isotherm model is only applicable for adosorption process not for desorption [42, 184]. Freundlich isotherm can be formulated as follows [185],
Were, KF and n are the Freundlich rate constants designated as adsorption capacity and intensity respectively. It can be obtained from slope and intercept of the plot of logqe versus logCe. 1/n denotes whether the adsorption capacity remains constant or decline with increasing concentrations.
Langmuir isotherm model can be formulated as follows [186],
Were, Ce = concentration of sorbate or adsorbate at equilibrium (mg/g).
These models are used to determine the biosorption capacity of biosorbents.
In case of Langmuir isotherm model, adsorption is proportional to the fraction of the open adsorbent surface while, desorption is directly proportional to the fraction of the adsorbent covered surface [186, 187]. Dubinin-Radushkevich Isotherm model is referred as an empirical adsorption model which is only suitable for intermediate concentrations of adsorbate [188]. It is applicable only for physical adosorption mechanism qualitatively for the adsorption of gases and vapors on micro porous sorbents [189]. General application of this isotherm model is to differentiate between the physical and chemical adsorption of uranium metal ions. It is temperature-dependent [189].
This Dubinin-Radushkevich isotherm model can be formulated as follows [189, 190],
Here, beta is Dubinin-Radushkevich constant and E is mean adsorption energy.
Temkin isotherm model is applicable for an intermediate concentration of uranium ions. It is reported that due to increase in surface coverage, the adsorption heat of all molecules in the layer decreases linearly [191, 192].
Temkin isotherm model can be formulated as [193]:
Here, b is Temkin constant related to heat of sorption (J/mol) and KT = Temkin isotherm constant and its unit is L/g.
The R2 for uranium adsorption by H3PO4 modified pomegranate peel biosorbent biomass is well explained by Freundlich isotherm which is an indication of multilayer formation on biosorbent [92]. In short, it indicates that multilayer adsorption occurs on heterogeneous surface with a uniform energy. It also followed Temkin isotherm model which indicates adsorption heat of all molecules in the layer decrease linearly with the increase of the coverage of utilized H3PO4 modified pomegranate peel biosorbent [92]. Uranium adsorption by tea waste biosorbent is well explained by Freundlich’s isotherm model that means multilayer formation occurred on biosorbent [194]. Also, uranium adsorption by phosphonate functionalized ordered mesoporous silica (OMS-P) is well explained by Langmuir isotherm model which indicates that monolayer adsorption occur on the homogeneous surface of this adsorbent [195].
Adsorption kinetic and thermodynamic study
Adsorption kinetic plays an important role in controlling adsorption rate. It determines the time required for reaching equilibrium for the adsorption process. This model gives information about the pathways and mechanisms involved in adsorption process. To understand the adsorption mechanism, two famous models are used, Pseudo-first order and Pseudo-second order models. This adsorption kinetics is used to measure the diffusion of adsorbate into the pores of the adsorbent/biosorbent material.
When the kinetic model best fits for Pseudo-first order model where R2 value is approximately one then, it indicates that the reaction is physical adsorption. On the other hand if it fits best to Pseudo-second order model, then it indicates chemical adsorption reaction. In case of using Quercetin-NaOH modified Spirulina platensis for the biosorption of U(VI) ions from the waste effluent, Pseudo- first and second order model were best fitted which indicates that U(VI) ions adsorbed by chemical or surface complexion mechanism rather than mass transport and both physical and chemical adsorption occurred at the same time [25, 196].
The Pseudo-first order equation given by Lagergren model is given below,
Pseudo-second order equation is formulated as,
In both of these models, qe and qt = amounts of uranium adsorbed on adsorbent (mg/g).
t = time (minutes).
K2 = Pseudo-second order constant.
K1 = Pseudo-first order constant.
Other applications of biosorption
In search of a new inexpensive eco-friendly waste treatment technologies, lots of researches has been done on biosorption process to treat industrial as well as nuclear waste effluents containing toxic and radioactive heavy metals. Biosorption process is mainly concern for the bioremediation of toxic metal ions and in recovery of important metals such as gold, silver etc. Beside this, it has been reported that, biosorption process is also applicable for the remediation of dyes, antibiotics, antibiotic resistant genes, antibiotic resistant bacteria and polyaromatic hydrocarbons from the waste effluents.
Dye biosorption
Biosorbents such as, Terminalia catappa shell, Cassava root husks, water hyacinth, lichen such as, Pseudevernia furfuracea, apple pomace, wheat straw, fungi such as, Aspergillus japonica, Aspergillus niger, Rhizopus arrhizus, Saccharomyces cerevisiae, algal species such as, Spirogyra sp., Euchema spinosum, Chlorella vulgaris, Sargassum sp. have been used for the biosorption of dyes like, indigo carmine, black dye [197], methylene blue, malachite green, reactive blue 160 dyes from various kinds of waste effluents especially textile effluents [198,199,200,201]. It is significant to remediate textile industrial dyes, plastic manufacturing industrial dyes, paper industrial dyes and cosmetic industrial dyes as most of the dyes are carcinogenic and possess a serious problems to all the living organisms. Sulfonated tea waste has also been used as a biosorbent for complete biosorption of methylene blue dye from waste water and considered as an excellent eco-friendly biosorbent [202].
Antibiotic biosorption
In recent times, antibiotic resistance has become the most common problem. Rice husk, maize stalks, microorganisms such as, Cladophora sp., Spirulina sp., Chlorella sp., Sargassum sp. have been used for the biosorption of antibiotics from wastewater treatment plants [203,204,205]. Very few researches have been done for antibiotic remediation from wastewater especially from hospital and industrial wastewater by biosorption process. There should be more research in this field to prevent antibiotic resistance problem in order to save life of next generations.
Future perspectives
In this review, utilization of various biosorbents such as microorganisms, plant and fruit wastes, recombinant DNA technology utilization for biosorbent production for the biosorption and bioremediation of uranium ions have been illustrated. Based on the extensive literature survey, it is clear that biosorption is the cost effective, easiest process for uranium removal from waste waters. It can be used for the bioremediation of all types of radioactive as well as toxic heavy metals from the environment. Few researchers have started using genetically engineered microorganisms for efficient biosorption [138]. More research is needed in this field as almost no or limited research has been done on construction of genetically engineered algal species, till date. Through this biosorption process, one can remediate antibiotics, antibiotic resistant genes, antibiotic resistant bacteria from hospital and domestic waste effluents. Toxic dyes can also be removed in near future and it is observed that fewer studies are done in the field of remediation of toxic substances related to plastics [206].
Today’s research trend is in the direction of modification and engineering techniques to improve the biosorption capacity of biosorbents for various types of toxic pollutants. Regeneration of all types of biosorbents is the main goal of all the researchers working in this field. Recent studies showed that, novel immobilized gel beads are prepared by using graphene-oxide and other chemicals for uranium biosorption and its remediation from the environment [207]. Limited investigations have been done in nano-particles based biosorbent for uranium biosorption. In future, nanoparticle based biosorbent’s uptake capacity of uranium need to be studied and more research is required. Moreover, it is expected that biosorption technique will become further renowned and common techniques in near future. Recently, biosorbents based on microbial cells immobilized in magnetic nanoparticles has been used and proved to be the most innovative technique for efficient and complete biosorption of uranium and other toxic heavy metal ions from the public and industrial waste water. So, more research is needed to know whether these types of innovative biosorbents are reusable or not.
In this review, we have included the entire novel and innovative biosorbents that are commonly used, less studied by researchers, recent trends for biosorption of radioactive heavy metals especially U(IV) and U(VI) and their present and future impact in the field of innovative and science technology. Besides, possibility of using significant biosorbents in large scale are also highlighted, but, still more studies in this field are required. Most recently, plant wastes or fruit peels are used as a biosorbents for eco-friendly biosorption process. Further investigation needs to be carried out to know whether these plants wastes work for long hours for biosorption of all radioactive metals from the aqueous solution at a time or not. In future, modifications of biosorbents by two or more chemicals as well as two or more genes incorporation by using CRISPR/Cas9 technology are needed. For biosorption of uranium, little attention has received, though there are still several processes where more research needs to be carried out in order to make this biosorption process fully accessible. Many studies have now focused in thermodynamic aspects to determine the behavior of biosorption process. Apart from this, other properties of microorganisms such as, metal-tolerance, metal-resistance, radioactive-resistance, metal-binding proteins, functional groups, and enzymes for tolerating harsh environmental conditions should be evaluated in lab-scale. Also, biosorbents, illustrated here are mainly for uranium removal from waste effluent, but, other toxic radioactive metals needs to be tested as well with these biosorbents. More research should be done to find out other parameters that are responsible for mediating efficient biosorption of radioactive metals. Most importantly, no research has been done yet for biosorption of toxic radioactive metals as well as heavy metals by using combination of biosorbents. In near future, potable biosensors based on microbial fuel cells for monitoring water quality after completion of biosorption process needs to be prioritized to make this innovative eco-friendly biosorption process more approachable.
Conclusion
Biosorption is the best method for the bioremediation of uranium metal ions from all kinds of waste effluent efficiently than other methods. This method is feasible, less time consuming, eco-friendly and reusable. The biosorbent materials used in this method for the biosorption of uranium are all compatible and mediate efficient removal of uranium. The mechanism of biosorption suggest that pH of the process solution is one of the most important factor that influence the functional groups present on the cell surface of the biosorbent and helps in efficient biosorption and removal of uranium ions from the waste water. Recently, nano-based particles like magnetic iron oxide, graphene oxide, chitosan etc. showed an efficient biosorption and removal capacity. Extensive researches require for the removal of other pollutants like, antibiotics, reactive and toxic dyes, etc. Kinetic and thermodynamic studies played an important role in setting temperature during biosorption process. Plant wastes have also been used and proved to be an excellent biosorbent. Even, recent trends are in the direction of construction of genetically engineered microorganisms or transgenic plants for biosorption process. In order to increase more attention towards biosorption process, more strategies need to be developed for making biosorbents into usable form. Investigation in biosorption mechanism at molecular level will help to construct more genetically engineered biosorbents with improved biosorption capacity.
References
Banala UK, Das NPI, Toleti SR (2020) Microbial interactions with uranium: towards an effective bioremediation approach. Environ Technol Innov 21:101254
Ye T, Huang B, Wang Y, Zhou L, Liu Z (2020) Rapid removal of uranium (VI) using functionalized luffa rattan biochar from aqueous solution. Colloids Surf A Physicochem Eng Asp 606:125480
Merkel BJ (2006) Long term fate of uranium tailings in mountain areas. In: Merker BJ, Berger AH (eds) Uranium in the environment, 1st edn. Springer, Berlin HeCelberg
Hussain A, Dulay P, Rivera MN, Aramouni C, Saxena V (2019) Neoplastic pathogenesis associated with cigarette carcinogens. Cureus. https://doi.org/10.7759/cureus.3955
Calderon OAR, Abdeldayem OM, Pugazhendhi A, Rene ER (2020) Current updates and perspectives of biosorption technology: an alternative for the removal of heavy metals from wastewater. Curr Pollut Rep 6(1):8–27
Naseem Z, Bhatti HN, Sadaf S, Noreen S, Ilyas S (2016) Sorption of uranium (VI) by Trapa bispinosa from aqueous solution: effect of pretreatments and modeling studies. Desalin Water Treat 57(24):11121–11132
Khan F, Pattanayak SK, Verma PR, Dewangan PK (2020) Biofabrication of graphene QDs as a fluorescent nanosensor for detection of toxic and heavy metals in biological and environmental sample. In: Chaki J, Dey N, De D (eds) Smart biosensors in medical care, 1st edn. ScienceDirect
Verma S, Kuila A (2019) Bioremediation of heavy metals by microbial process. Environ Technol Innov 14:100369
Ledezma CZ, Bolagay DN, Figueroa F, Ledezma EZ, Ni M, Alexis F, Guerrero VH (2021) Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504
Singha M, Pal S, Chowdhury S, Hareendran KN, Sahu ML (2020) Removal of hazardous metals from nuclear effluent using a process hybridisation technique. J Radioanal Nucl Chem 326:1271–1279
Mahmoud MR, Othman SH (2018) Efficient decontamination of naturally occurring radionuclide from aqueous carbonate solutions by ion flotation process. Radiochim Acta 106(6):465–476
Montana M, Camacho A, Serrano I, Devesa R, Matia L, Valles I (2013) Removal of radionuclides in drinking water by membrane treatment using ultrafiltration, reverse osmosis and electrodialysis reversal. J Environ Radioact 125:86–92
Lupulescu M, Jula D (2012) Ultrafiltration system for treatment of the wastewater from uranium processing plant in Feldioara. Ann Univ of Petrosani Mech Eng 14
Zaheri A, Moheb A, Keshtkar A, Shirani A (2010) Uranium separation from wastewater by electrodialysis. J Environ Health Sci Eng 7(5):423–430
Shammas NK, Pouet MF, Grasmick A (2010) Wastewater treatment by electrocoagulation-flotation. In: Wang LK, Shammas NK, Selke WA, Aulenbach DB (eds) Flotation technology, 1st edn. Humana Press, Totowa, NJ
Xiong ZW, Zhou SK, Lin GC (2007) Experimental study of treating radioactive waste water by UF, RO and ED. Nat Sci J Xiangtan Univ 29(2):100
Ladeira ACQ, Morais CAD (2005) Uranium recovery from industrial effluent by ion exchange-column experiments. Miner Eng 18(13–14):1337–1340
Misra SK, Mahatele AK, Tripathi SC, Vijayan K, Munshi SK (2005) Studies for the use of water soluble chelating polymer in ultra-filtration technique for the removal of uranium from aqueous solutions. Proc DAE-BRNS Symp Nucl Radiochem 49
Roig MG, Manzano T, Diaz M (1997) Biochemical process for the removal of uranium from acid mine drainages. Water Res 31(8):2073–2083
Clifford D, Zhang Z (1994) Modifying ion exchange for combined removal of uranium and radium. J Am Water Work Assoc 86(4):214–227
Lin KL, Chu ML, Shieh MC (1987) Treatment of uranium containing effluents with reverse osmosis process. Desalination 61(2):125–136
Tran TV (1985) Advanced membrane filtration process treats industrial wastewater efficiently. Chem Eng Prog US 81(3)
Tran TV, Shorr J (1985) Advanced membrane filtration process for treatment of uranium mill tailings wastewater. Trans Am Nucl Soc 50
Muller HM (1984) Decontamination by ultrafiltration of low radioactivity waste water from fuel element fabrication. CEC 78
Mohammed AAER (2020) Potentiality of quercetin-sodium hydroxide Spirulina platensis in uranium biosorption from waste effluent. Int J Environ Stud 77(1):48–60
Beni AA, Esmaeili A (2020) Biosorption, an efficient method for removing heavy metals from industrial effluents: a review. Environ Technol Innov 17:100503
Sun Y, Li Y (2021) Application of surface complexation modeling on adsorption of uranium at water-solid interface: a review. Environ Pollut 278:116861
Chen L, Liu J, Zhang W, Zhou J, Luo D, Li Z (2021) Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: a review. J Hazard Mater 413:125319
Yi ZJ, Yao J, Chen HL, Wang F, Yuan ZM, Liu X (2016) Uranium biosorption from aqueous solution onto Eichhornia crassipes. J Environ Radioact 154:43–51
Atar N, Olgun A, Colak F (2008) Thermodynamic, equilibrium and kinetic study of the biosorption of basic blue 41 using Bacillus maceran. Eng Life Sci 8(5):499–506
Kyzas GZ, Matis KA (2020) Biosorbents for heavy metal removal from dilute aqueous solution. Carbon Nanomater Agri Food Environ appl. https://doi.org/10.1016/B978-0-12-819786-8.00006-2
Ozudogru Y, Merdivan M (2020) Adsorption of U (VI) and Th (IV) ions removal from aqueous solutions by pretreatment with Cystoseira barbata. J Radioanal Nucl Chem 323:595–603
You W, Peng W, Tian Z, Zheng M (2021) Uranium bioremediation with U (VI)-reducing bacteria. Sci Total Environ 798:149107
Kulal DK, Loni PC, Dcosta C, Some S, Kalambate PK (2020) Cyanobacteria: as a promising candidate for heavy-metals removal. In: Singh PK, Kumar A, Shrivastava AK (eds) Advances in cyanobacterial biology, 1st edn. ScienceDirect
He D, Tan N, Luo X, Yang X, Ji K, Han J, Chen C, Liu Y (2020) Preparation, uranium (VI) absorption and reuseability of marine fungus mycelium modified by the bis-amidoxime-based groups. Radiochim Acta 108(1):37–49
Ezzati M, Yousefi B, Velaei K, Safa A (2020) A review on anti-cancer properties of quercetin in breast cancer. Life sci 248:117463
Ou Q, Zheng Z, Zhao Y, Lin W (2020) Impact of quercetin on systemic levels of inflammation: a meta-analysis of randomised controlled human trials. Int J Food Sci Nutr 71(2):152–163
Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L (2020) Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother 121:109604
Halevas E, Pekou A, Papi R, Mavroidi B, Hatzidimitriou AG, Zahariou G, Litsardakis G, Sagnou M, Pelecanou M, Pantazaki AA (2020) Synthesis, physicochemical characterization and biological properties of two novel Cu (II) complexes based on natural products curcumin and quercetin. J Inorg Biochem 208:111083
Babcsyani I, Tamas M, Szatmari J, Hambek-Olah B, Farsang A (2020) Assessing the impacts of the main river and anthropogenic use on the degree of metal contamination of oxbow lake sediments (Tisza River Valley, Hungary). J Soils Sediments 20(3):1662–1675
Semenova Y, Pivina L, Zhunussov Y, Zhanaspayev M, Chirumbolo S, Muzdubayeva Z, Bjorklund G (2020) Radiation-related health hazards to uranium miners. Environ Sci Pollut Res 27:1–15
Hashemi N, Dabbagh R, Noroozi M, Baradaran S (2020) Optimization of uranium biosorption in solutions by Sargassum boveanum using RSM method. Adv Environ Res 9(1):65–84
Virk HS (2020) Groundwater contamination in Punjab due to arsenic, selenium and uranium heavy metals. Res Rev J Toxicol 10(1):1–6
Dewar D (2019) Uranium mining: environmental and human health effects. In: Branch JLB, Fleck D (eds) Nuclear non-proliferation in international law, vol IV, 1st edn. TMC Asser Press, The Hague
Abshire ML, Romaniello SJ, Kuzminov AM, Cofrancesco J, Severmann S, Riedinger N (2020) Uranium isotopes as a proxy for primary depositional redox conditions in organic-rich marine systems. Earth Planet Sci Lett 529:115878
Bergmann M, Sobral O, Pratas J, Graca MAS (2018) Uranium toxicity to aquatic invertebrates: a laboratory assay. Environ Pollut 239:359–366
Lagauzere S, Terrail R, Bonzom JM (2009) Ecotoxicity of uranium to Tubifex tubifex worms (Annelida, Clitellata, Tubificidae) exposed to contaminated sediment. Ecotoxicol Environ Saf 72:527–537
Tang N, Liang J, Niu C, Wang H, Luo Y, Xing W, Ye S, Liang C, Guo H, Guo J, Zhang Y, Zeng G (2020) Amidoxime-based materials for uranium recovery and removal. J Mater Chem A 8(16):7588–7625
Wise SS, Thompson WD, Aboueissa AEM, Mason MD, Wise JP (2007) Particulate depleted uranium is cytotoxic and clastogenic to human lung cells. Chem Res Toxicol 20(5):815–820
Gangwar S, Singh VP, Tripathi DK, Chauhan DK, Prasad SM, Maurya JN (2014) Plant responses to metal stress: the emerging role of plant growth hormones in toxicity alleviation. In: Ahmad P, Rasool S (eds) Emerging technologies and management of crop stress tolerance, 1st edn. ScienceDirect
Elbaz A, Wei YY, Meng Q, Zheng Q, Yang ZM (2010) Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicol 19(7):1285–1293
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Li X, Yang Y, Jia L, Chen H, Wei X (2013) Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol Environ Saf 89:150–157
Xu C, Li T, Hu C, Guo H, Ye J, Li L, Liu W, Niu L (2021) Waterborne uranium causes toxic effect and thyroid disruption in zebrafish larvae. Ecotoxicol Environ Saf 208:111585
Redvers N, Chischilly AM, Warne D, Pino M, Colbert AL (2021) Uranium Exposure in American Indian communities: health, policy, and the way forward. Environ Health Perspect 129(3):035002
Motum MH (2021) Radiation exposure levels and their potential effects on domesticated animals in West Pokot Kenya. UOE
Case MJ, Halford D (2018) Analysis of radiological impacts to terrestrial biota in support of environmental assesment for use of DOE-Owned high-assay low-enriched uranium stored at Idaho National laboratory. Idaho National Lab. (INL)l, Idaho Falls, ID (United States)
Xie Z, Askari A (2002) Na+/ K+-ATPase as a signal transducer. Eur J Biochem 269:2434–2439
Kumar A, Ali M, Ningthoujam RS, Gaikwad P, Kumar M, Nath BB, Pandey BN (2016) The interaction of actinide and lanthanide ions with hemoglobin and its relevance to human and environmental toxicology. J Hazard Mater 307:281–293
Bucher G, Mounicou S, Simon O, Floriani M, Lobinski R, Frelon S (2016) Insights into the nature of uranium target proteins within zebrafish gills after chronic and acute waterborne exposures. Environ Toxicol Chem 35(3):736–741
Ma M, Wang R, Xu L, Xu M, Liu S (2020) Emerging health risks and underlying toxicological mechanisms of uranium contamination: lessons from the past two decades. Environ Int 145:106107
Amaral RDS, Junior JADS, Aquino FDS, Amaral BDA, Fernandez ZH, Bezerra MBCF, Silva ANCD, Santos DCD, Santos JMDN, Silva AAD, Correia FLDB (2019) Exposure risk assessment of uranium intake of the milk products from the region of Pernambuco, Brazil. J Radioanal Nucl Chem 321:927–933
Arrudo-Neto J, Tavares M, Filadelfo M (1997) Concentrations of uranium in animal feed supplements: measurements and dose estimates. J Radioanal Nucl Chem 221:97–104
Redha AA (2020) Removal of heavy metals from aqueous media by biosorption. Arab J Basic Appl Sci 27(1):183–193
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 84(1):13–28
Gupta NK, Sengupta A, Gupta A, Sonawane JR, Sahoo H (2018) Biosorption- an alternative method for nuclear waste management: a critical review. J Environ Chem Eng 6(2):2159–2175
Wei W, Wang Q, Li A, Yang J, Ma F, Pi S, Wu D (2016) Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: Adsorption behavior and mechanism assessment. Sci Rep 6(1):1–10
Modak JM, Natarajan KA (1995) Biosorption of metals using nonliving biomass:a review. Mining Metall Explor 12(4):189–196
Ubando AT, Africa ADM, Maniquiz-Redillas MC, Culaba AB, Chen WH, Chang JS (2021) Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J Hazard Mater 402:123431
Cheng J, Gao J, Zhang J, Yuan W, Yan S, Zhou J, Zhao J, Feng S (2021) Optimization of hexavalent chromium biosorption by shewanella putrefaciens using the box-behnken design. Water Air Soil Pollut 232(3):1–14
Vieira LC, Araujo LGD, Ferreira RVDP, Silva EAD, CanevesiMarumo RLSJT (2019) Uranium biosorption by Lemna sp. and Pistia stratiotes. J Environ Radioact 203:179–186
Priyadarshini E, Priyadarshini SS, Pradhan N (2019) Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl Microbiol Biotechnol 103(8):3297–3316
You W, Peng W, Tian Z, Zheng M (2021) Uranium bioremediation with U (VI)-reducing bacteria. Sci Total Environ 103:149107
Spain O, Plohn M, Funk C (2021) The cell wall of green microalgae and its role in heavy metal removal. Physiol Plant 173(2):526–535
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69(9):1775–1787
Keryanti K, Mulyono EWS (2021) Determination of optimum condition of lead (Pb) biosorption using dried biomass microalgae aphanothece sp. Period Polytech Chem Eng 65(1):116–123
Ginisty P (1999) Study of the mechanisms of biosorption of metal cations by biological materials. Brest
Choi J, Lee JY, Yang JS (2009) Biosorption of heavy metals and uranium by starfish and Pseudomonas putida. J Hazard Mater 161(1):157–162
Sar P, Kazy SK, D’Souza SF (2004) Radionuclide remediation using a bacterial biosorbent. Int Biodeterior Biodegradation 54(2–3):193–202
Hussien SS (2021) New studies of uranium biosorption by extracellular polymeric substances (EPSs) of Aspergillus clavatus, using isotherms, thermodynamics and kinetics. Int J Environ Stud 78(2):228–246
Yoon JY, Nam IH, Yoon MH (2021) Biosorption of Uranyl ions from aqueous solution by Parachlorella sp. AA1. Int J Environ Res Public Health 18(7):3641
Hufton J, Harding J, Smith T, Romero-Gonzalez ME (2021) The importance of the bacterial cell wall in uranium (VI) biosorption. Phys Chem Chem Phys 23(2):1566–1576
Banala UK, Das NPI, Toleti SR (2021) Uranium sequestration abilities of Bacillus bacterium isolated from an alkaline mining region. J Hazard Mater 411:125053
Xu X, He S, Wang Z, Zhou Y, Lan J (2013) Biosorption of uranium by Bacillus sp. FB12 isolated from the vicinity of a power plant. Adv Environ Res 2(24510):12989
Hosomomi Y, Baba Y, Kubota F, Kamiya N, Goto M (2013) Biosorption of rare earth elements by E.coli. J Chem Eng Japan 13we031
Dai Y, Zhou L, Tang X, Xi J, Ouyang J, Liu Z, Huang G, Adesina AA (2020) Macroporous ion-imprinted chitosan foams for the selective biosorption of U (VI) from aqueous solution. Int J Biol Macromol 164:4155–4164
Wu W, Chen D, Li J, Su M, Chen N (2018) Enhanced adsorption of uranium by modified red muds: adsorption behavior study. Environ Sci Pollut Res 25(18):18096–18108
Noubactep C (2006) Effect of selected ligands on the U (VI) immobilization by zerovalent iron. J Radioanal Nucl Chem 267:13–19
Saini AS, Melo JS (2015) Biosorption of uranium by human black hair. J Environ Radioact 142:29–35
Wang H, Ji Y, Tian Q, Horska K, Shao X, Maderova Z, Miao X, Safarikova M, Safarik I (2014) Biosorption of uranium by magentically modified wheat bran. Sep Sci Technol 49(16):2534–2539
Nuhanovic M, Smjecanin N, Curic N, Vinkovic A (2021) Efficient removal of U (VI) from aqueous solution using the biocomposite based on sugar beet pulp and pomelo peel. J Radioanal Nucl Chem 328:347–358
Moghadam M, Salami M, Mohammadian M, Khodadadi M, Emam-Djomeh Z (2020) Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll 104:105735
Ighalo JO, Adeniyi AG, Eletta OAA, Arowoyele LT (2020) Competitive adsorption of Pb (II), Cu (II), Fe (II) and Zn (II) from aqueous media using biochar from oil palm (Elaeis guineensis) fibers: a kinetic and equilibrium study. Indian Chem Eng 1–11
Rani S, Gunjyal N, Ojha CSP, Singh RP (2021) Review of challenges for algae-based wastewater treatment: strain selection, wastewater characteristics, abiotic and biotic factors. J Hazard Toxic Radioact Waste 25(2):03120004
Sharma NK, Arivalagan AR (2021) Algae or bacteria–the future of biological wastewater treatment. In: Rahman ROA, Hussain CM (eds) Handbook of Advanced approaches towards pollution prevention and control, 1st edn. Elsevier
Kroger N, Poulsen N (2008) Diatoms- from cell wall biogenesis to nanotechnology. Annu Rev Genet 42:83–107
Hoham RW, Remias D (2020) Snow and glacial algae: a Review. J Phycol 56(2):264–282
Aytas S, Gunduz E, Gok C (2014) Biosorption of uranium ions by marine macroalga padina pavonia. Clean Soil Air Water 42(4):498–506
Viola R, Nyvall P, Pedersen M (2001) The unique features of starch metabolism in red algae. Proc R Soc B Biol Sci 268(1474):1417–1422
Dabbagh R, Rojaee A, Heshmatipour Z (2018) Thermodynamics, kinetics and equilibrium studies of uranium sorption by gracilaria corticata red alga. Environ Eng Manag J 17(5):1199
Lee KY, Kim KW, Baek YJ, Chung DY, Lee EH, Lee SY, Moon JK (2014) Biosorption of uranium (VI) from aqueous solution by biomass of brown algae Laminaria japonica. Water Sci Technol 70(1):136–143
Thakare M, Sarma H, Datar S, Roy A, Pawar P, Gupta K, Pandit S, Prasad R (2021) Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr Res Biotechnol 3:84–98
Emenike CU, Agamuthu P, Fauziah SH (2016) Blending Bacillus sp., Lysinibacillus sp. and Rhodococcus sp. for optimal reduction of heavy metals in leachate contaminated soil. Environ Earth Sci 75(1):1–8
Kilinc E, Ozdemir S, Yalcin MS, Soylak M (2021) A new method for the preconcentrations of U (VI) and Th (IV) by magnetized thermophilic bacteria as a novel biosorbent. Anal Bioanal Chem 413(4):1107–1116
Zhang C, Malhotra SV, Francis AJ (2014) Toxicity of ionic liquids to Clostridium sp. and effects on uranium biosorption. J Hazard Mater 264:246–253
Vijayaraghavan R, Ellappan V, Dharmar P, Lakshmanan U (2018) Preferential adsorption of uranium by functional groups of the marine unicellular cyanobacterium Synechoccocus elongatus BDU130911. 3 Biotech 8(3):1–9
Zhao C, Liu J, Li X, Li F, Tu H, Sun Q, Liao J, Yang J, Yang Y, Liu N (2016) Biosorption and bioaccumulation behavior of uranium on Bacillus sp. dwc-2: investigation by box- Behenken design method. J Mol Liq 221:156–165
Manobala T, Shukla SK, Rao TS, Kumar MD (2021) Kinetic modelling of the uranium biosorption by deinococcus radiodurans biofilm. Chemosphere 269:128722
Manobala T, Shukla SK, Rao TS, Kumar MD (2019) A new uranium bioremediation approach using radio-tolerant deinococcus radiodurans biofilm. J Biosci 44(5):1–9
Kazy SK, Sar P, D’Souza SF (2008) Studies on Uranium removal by the extracellular polysaccharide of a Pseudomonas aeruginosa strain. Bioremed J 12(2):47–57
Shukla SK, Hariharan S, Rao TS (2020) Uranium bioremediation by acid phosphatase activity of Staphylococcus aureus biofilms: can a foe turn a friend. J Hazard Mater 384:121316
Han J, Hu L, He L, Ji K, Liu Y, Chen C, Luo X, Tan N (2020) Preparation and uranium (VI) biosorption for tri-amidoxime modified marine fungus material. Environ Sci Pollut Res 1–11
Wang JS, Hu XJ, Liu YG, Xie SB, Bao ZL (2010) Biosorption of uranium (VI) by immobilized aspergillus fumigatus beads. J Environ Radioact 101(6):504–508
Yang H, Tan N, Wu F, Liu H, Sun M, She Z, Lin Y (2012) Biosorption of uranium (VI) by a mangrove endophytic fungus Fusarium sp. #ZZF51 from the South China Sea. J Radioanal Nucl Chem 292:1011–1016
Liang J, Liu L, Song W (2021) Enhancement of U (VI) biosorption by Trichoderma harzianum mutant obtained by a cold atmospheric plasma jet. J Radioanal Nucl Chem 327:1325–1333
Viraraghavan T, Srinivasan A (2011) Fungal biosorption and biosorbents. In: Kotrba P, Mackova M, Macek T (eds) Microbial biosorption of metals, 1st edn. Springer, Switzerland
Guibal E, Roulph C, Cloirec PL (1992) Uranium biosorption by a filamentous fungus Mucor miehei pH effect on mechanisms and performances of uptake. Water Res 26(8):1139–1145
Giese EC, Silva DDV, Costa AFM, Almeida SGC, Dussan KJ (2020) Immobilized microbial nanoparticles for biosorption. Crit Rev Biotechnol 40(5):653–666
Pandey P, Pandey M, Pandey PK (2021) Uranium contamination removal from water by an orchid (Vanda tessellata) based biosorbent. J Radioanal Nucl Chem 328:89–101
Stopa LCB, Yamaura SM (2010) Uranium removal by chitosan impregnated with magnetite nanoparticles: adsorption and desorption. Int J Nucl Energy Sci Technol 5(4):283–289
Ismail L, Khalil F, Orabi FMA (2020) Modification of silica nanoparticles with cysteine or methionine amino acids for the removal of uranium (VI) from aqueous solution. Silicon 12(11):2647–2661
Hamza MF, Fouda A, Elwakeel KZ, Wei Y, Guibal E, Hamad NA (2021) Phosphorylation of guar gum magnetite/chitosan nanocomposites for uranium sorption and antibacterial applications. Molecules 26(7):1920
Khalil U, Shakoor MB, Ali S, Ahmad SR, Rizwan M, Alsahli AA, Alyemeni MN (2021) Selective removal of hexavalent chromium from wastewater by rice husk: kinetic, isotherm and spectroscopic investigation. Water 13(3):263
Deng Y, Zhang T, Wang Q (2017) Biochar adsorption treatment for typical pollutants removal in livestock wastewater: a review. In: Huang WJ (ed) Engineering applications of biochar, 1st edn. Books on Demand
Ferreira RVDP, Araujo LGD, Canevesi RLS, Silva EAD, Ferreira EGA, Palmieri MC, Marumo JT (2020) The use of rice and coffee husks for biosorption of U (total), 241 Am, and 137 Cs in radioactive liquid organic waste. Environ Sci Pollut Res 27(29):36651–36663
Ye G, Wu Y, Wang L, Tan B, Shen W, Li X, Liu Y, Tian X, Zhang D (2021) Comparison of six modification methods on the chemical composition, functional properties and antioxidant capacity of wheat bran. LWT 149:111996
Xiao Y, Liu Y, Wang X, Li M, Lei H, Xu H (2019) Cellulose nanocrystals prepared from wheat bran: characterization and cytotoxicity assessment. Int J Biol Macromol 140:225–233
Singh A, Saini P (2020) Biosorption: a novel biotechnological application for removal of hazardous pollutants. In: Mishra P, Mishra RR (eds) Innovations in food technology, 1st edn. Springer, Singapore
Ahmed TAE, Wu L, Younes M, Hincke M (2021) Biotechnological applications of eggshell: recent advances. Front Bioeng Biotechnol 9:548
Haddad B, Mittal A, Mittal J, Paolone A, Villemin D, Debdab M, Mimanne G, Habibi A, Hamidi Z, Boumediene M, Belarbi E (2021) Synthesis and characterization of eggshell (ES) and eggshell with membrane (ESM) modified by ionic liquids. Chem Data Collect 33:100717
Mittal A, Teotia M, Soni RK, Mittal J (2016) Applications of egg shell and egg shell membrane as adsorbents: a review. J Mol Liq 223:376–387
Xuan S, Zhang Bo, Xiao L, Li G, Zhang Y, Zhang Y, Li J (2021) Facile carboxylated of natural eggshell membrane for highly selective uranium (VI) adsorption from radioactive wastewater. Environ Sci Pollut Res 1–10
Nuhanovic M, Grebo M, Draganovic S, Memic M, Smjecanin N (2019) Uranium (VI) biosorption by sugar beet pulp: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 322:2065–2078
Krishnan S, Zulkapli NS, Kamyab H, Taib SM, Din MFBM, Majid ZA, Chaiprapat S, Kenzo I, Ichikawa Y, Nasrullah M, Chelliapan S, Othman N (2021) Current technologies for recovery of metals from industrial wastes: an overview. Environ Technol Innov 22:101525
Srivastava N (2021) Remediation of heavy metals through genetically engineered microorganism. Environ Pollut Remediat 315–366
Dave D, Sarma S, Parmar P, Shukla A, Goswami D, Shukla A, Saraf M (2020) Microbes as a boon for the bane of heavy metals. Environ Sustain 3(3):233–255
Gahlout M, Prajapati H, Tandel N, Patel Y (2021) Biosorption: An eco-friendly technology for pollution removal. In: Panpatte DG, Jhala YK (eds) Microbial rejuvenation of polluted environment, 1st edn. Springer
Luo J, Zhu Q, Li S, Chen F, Xiao F, Peng G (2021) Construction of Deino-flr-2 radiation-tolerant genetically engineered strain containing flr-2 fluoride-tolerant gene and its enrichment behavior for U (VI). J Radioanal Nucl Chem 328:1265–1278
Ghasemi R, Fatemi F, Mir-Derikvand M, Zarei M (2020) Evaluation of mtr cluster expression in Shewanella RCR17 during uranium removal. Arch Microbiol 202(10):2711–2726
Reddy PCO, Raju KS, Sravani K, Sekhar AC, Reddy MK (2019) Transgenic plants for remediation of radionuclides. In: Prasad MNV (ed) Transgenic plant technology for remediation of toxic metals and metalloid, 1st edn. Academic press
Eapen S, Suseelan KN, Tivarekar S, Kotwal SA, Mitra R (2003) Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and chenopodium amaranticolor. Environ Res 91(2):127–133
Tang L, Mao B, Li Y, Lv Q, Zhang LP, Chen C, He H, Wang W, Zeng X, Shao Y, Pan Y, Hu Y, Peng Y, Fu X, Li H, Xia S, Zhao B (2017) Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci Rep 7(1):1–12
Appukuttan D, Seetharam C, Padma N, Rao AS, Apte SK (2011) PhoN-expressing, lyophilized, recombinant deinococcus radiodurans cells for uranium bioprecipitation. J Biotechnol 154(4):285–290
Tapadar S, Tripathi D, Pandey S, Goswami K, Bhattacharjee A, Das K, Palwan E, Rani M, Kumar A (2021) Role of extremophiles and extremophilic proteins in industrial waste treatment. In: Shah MP (ed) Removal of emerging contaminants through microbial processes, 1st edn. Springer
Pinel-Cabello M, Jroundi F, Lopez-Fernande M, Geffers R, Jarek M, jauregui R, Link A, Vilchez-Vargas R, Merroun ML, (2021) Multisystem combined uranium resistance mechanisms and bioremediation potential of Stenotrophomonas bentonitica BII-R7: Transcriptomics and microscopic study. J Hazard Mater 403:123858
Koul B, Adlakha K (2021) Bioremediation of radionuclides by plant-microbe system: current progress and challenges. In: Kumar A, Singh VK, Singh P, Mishra VK (eds) Microbe mediated remediation of environmental contaminants, 1st edn. Sciencedirect
Upadhyay KH, Vaishnav AM, Tipre DR, Dave SR (2021) Metal bioremediation, mechanisms, kinetics and role of marine bacteria in the bioremediation technology. In: Joshi SJ, Deshmukh A, Sarma H (eds) Biotechnology for Sustainable Environment, 1st edn. Springer Verlag, Singapore
Datta S, Sengupta D, Saha I (2020) Bacterial Metabolites for removal of toxic dyes and heavy metals. In: Inamuddin S, Ahamed MI, Lichtfouse E, Asiri AM (eds) Methods for bioremediation of water and wastewater pollution, 1st edn. Springer
Nedelkova M, Merroun ML, Rossberg A, Hennig C, Selenska-Pobell S (2007) Microbacterium isolates from the vicinity of a radioactive waste depository and their interactions with uranium. FEMS Microbiol Ecol 59(3):694–705
Ngah WSW, Hanafiah MAKM (2008) Biosorption of copper ions from dilute aqueous solutions on base treated rubber (Hevea brasiliensis) leaves powder: kinetics, isotherm, and biosorption mechanisms. J Environ Sci 20(10):1168–1176
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd (II) and Pb (II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105(1–3):121–142
Zang X, Cheng M, Zhang X, Chen X (2021) Quercetin nanoformulations: a promising strategy for tumor therapy. Food Funct 12(15):6664–6681
Gurisik E, Arica MY, Bektas S, Genc O (2004) Comparison of the heavy metal biosorption capacity of active, heat-inactivated and NaOH-treated Phanerochaete chrysosporium biosorbents. Eng Life Sci 4(1):86–89
Chen F, Tan N, Long W, Yang SK, She ZG, Linn YC (2014) Enhancement of uranium (VI) biosorption by chemically modified marine-derived mangrove endophytic fungus Fusarium sp. #ZZF51. J Radioanal Nucl Chem 299:193–201
Argaman AN, Cohen-Zinder M, Leibovich H, Yishay M, Eitam H, Agmon R, Hadaya O, Mesilati-Stahy R, Miron J, Shabtay A (2020) Dietary pomegranate peel improves milk quality of lactating ewes: Emphasis on milk fat globule membrane properties and antioxidative traits. Food Chem 313:125822
Ben-Ali S (2021) Application of raw and modified pomegranate peel for wastewater treatment: a literature overview and analysis. Int J Chem Eng. https://doi.org/10.1155/2021/8840907
Prahas D, Kartika Y, Indraswati N, Ismadji SJCEJ (2008) Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chem Eng J 140(1–3):32–42
Li D, Yang Y, Zhang P, Liu J, Li T, Yang J (2021) U (VI) adsorption in water by sodium algiate modified Bacillus megaterium. R Soc Open Sci 8(2):202098
Gao X, Guo C, Hao J, Zhao Z, Long H, Li M (2020) Adsorption of heavy metal ions by sodium alginate based adsorbent- a review and new perspectives. Int J Biol Macromol 164:4423–4434
Ghimire KN, Inoue K, Ohto K, Hayashida T (2008) Adsorption study of metal ions onto crosslinked seaweed Laminaria japonica. Bioresour Technol 99(1):32–37
Paudyal H, Pangeni B, Inoue K, Kawakita H, Ohto K, Ghimire KN, Harada H, Alam S (2013) Adsorptive removal of trace concentration of fluoride ion from water by using dried orange juice residue. Chem Eng J 223:844–853
Sheikh Z, Amin M, Khan N, Khan MN, Sk Sami, Khan SB, Hafeez I, Khan SA, Bakhsh EM, Cheng CK (2021) Potential application of Allium Cepa seeds as a novel biosorbent for efficient biosorption of heavy metals ions from aqueous solution. Chemosphere 279:130545
Chen C, Hu J, Wang J (2020) Biosorption of uranium by immobilized Saccharomyces cerevisiae. J Environ Radioact 213:106158
Bai Z, Liu Q, Zhang H, Liu J, Yu J, Wang J (2020) High efficiency biosorption of Uranium (VI) ions from solution by using hemp fibers functionalized with imidazole-4, 5-dicarboxylic. J Mol Liq 297:111739
Yuan Y, Liu N, Dai Y, Wang B, Liu Y, Chen C, Huang D (2020) Effective biosorption of uranium from aqueous solution by cyanobacterium Anabaena flos-aquae. Environ Sci Pollut Res 27(35):44306–44313
Silva JIR, Ferreira ACDM, Costa ACAD (2009) Uranium biosorption under dynamic conditions: preliminary tests with Sargassum filipendula in real radioactive wastewater containing Ba, Cr, Fe, Mn, Pb, Ca and Mg. J Radioanal Nucl Chem 279:909–914
Yi ZJ, Yao J, Zhu MJ, Chen HL, Wang F, Liu X (2017) Uranium biosorption from aqueous solution by the submerged aquatic plant Hydrilla verticillata. Water Sci Technol 75(6):1332–1341
Khani MH (2011) Uranium biosorption by Padina sp. algae biomass: Kinetics and thermodynamics. Environ Sci Pollut Res 18(9):1593–1605
Gad NS, Saamaan JM, Hashem IM (2021) Biosorption capacity for uranium and thorium by some microalgae species. Egypt J Microbiol 56(1):53–60
Abozaid SM, Shetaia YM, Rabie KA, Ahmed BM, Soliman ERS (2021) Mohamed SS (2021) Recovery of uranium from solutions using Aspergillus nidulans isolated from monazite mineral. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.1939022
Naeem H, Bhatti HN, Sadaf S, Iqbal M (2017) Uranium remediation using modified Vigna radiata waste biomass. Appl Radiat Isot 123:94–101
Sohbatzadeh LH, Keshtkar AR, Safdari J, Fatemi A, Ghasemi TM (2018) Screening of significant factors in uranium biosorption from aqueous solutions using Pseudomonas putida immobilized on chitosan. J Nucl Sci Technol 38(4):45–47
Cheng W, Sun Y, Fan M, Li Y, Wang L (2021) Qian H (2021) Wheat bran, as the resource of dietary fiber: a review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.1913399
Cerri R, Niccolai A, Cardinaletti G, Tulli F, Mina F, Daniso E, Bongiorno T, Zittelli GC, Biondi N, Tredici MR, Tibaldi E (2021) Chemical composition and apparent digestibility of a panel of dried microalgae and cyanobacteria biomasses in rainbow trout (Oncorhynchus mykiss). Aquaculture 544:737075
Yin J, Fan W, Du J, Feng W, Dong Z, Liu Y, Zhou T (2020) The toxicity of graphene oxide affected by algal physiological characteristics: A comparative study in cyanobacterial, green algae, diatom. Environ Pollut 260:113847
Mitra R, Xu T, Xiang H, Han J (2020) Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb Cell Fact 19(1):1–30
Orabi AH, Abdelhamid AE, Salem HM, Ismaiel DA (2021) Uranium removal using composite membranes incorporated with chitosan grafted phenylenediamine from liquid wastes solution. Cellulose 28(6):3703–3721
Elsalamouny AR, Desouky OA, Mohamed SA, Galhoum AA, Guibal E (2017) Uranium and neodymium biosorption using novel chelating polysaccharide. Int J Biol Macromol 104:963–968
Tong K (2017) Preparation and biosorption evaluation of Bacillus subtilis/alginate-chitosan microcapsule. Nanotechnol Sci Appl 10:35
Yu JY, Zhao WY, Yang GW, Zeng SS (2014) Research on biological materials with uranium biosorption by microalgae: a review. Appl Mech Mater 508:290–296
Akhtar K, Akhtar MW, Khalid AM (2007) Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res 41(6):1366–1378
Elwakeel KZ, Hamza MF, Gulbal E (2021) Effect of agitation mode (mechanical, ultrasound and microwave) on uranium sorption using amine- and dithizone-functionalized magnetic chitosan hybrid materials. Chem Eng J 411:128553
Liao J, Zhang Y, He X, Zhang L, He Z (2021) The synthesis of a novel titanium oxide aerogel with highly enhanced removal of uranium and evaluation of the adsorption mechanism. Dalton Trans 50(10):3616–3628
Bakather OY, Zouli N, Abutaleb A, Mahmoud MA, Daher A, Hassan M, Eldoma MA, Alasweda SO, Fowad AA (2020) Uranium (VI) ions uptake from liquid wastes by Solanum icanum leaves: Biosorption, desorption and recovery. Alex Eng J 59(3):1495–1504
Ganguly P, Sarkhel R, Das P (2020) Synthesis of pyrolyzed biochar and its application for dye removal: batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surf Interfaces 20:100616
Azizian S, Eris S, Wilson LD (2018) Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem Phys 513:99–104
Czepirski L, Balys R, Czepirska EK (2000) Some generalization of langmuir adsorption isotherm. Int J Chem 3(14):1099–8292
Hu Q, Zhang Z (2019) Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: a theoretical analysis. J Mol Liq 277:646–648
Dada AO, Olalekan AP, Olatunya AM, Dada OJIJC (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR JAC 3(1):38–45
Kiran I, Akar T, Ozcan AS, Ozcan A, Tunali S (2006) Biosorption kinetics and isotherm studies of acid red 57 by dried Cephalosporium aphidicola cells from aqueous solutions. Biochem Eng J 31(3):197–203
Rajahmundry GK, Garlapati C, Kumar PS, Alwi RS, Vo DVN (2021) Statistical analysis of adsorption isotherm models and its appropriate selection. Chemosphere 276:130176
Chen L, Li Z, Li W, Chen Z, Chen G, Yang W, Zhang X, Liu X (2021) Investigation of adsorption kinetics and the isotherm mechanism of manganese by modified Diatomite. ACS Omega 25(6):16402–16409
Gok C, Aytas S, Sezer H (2017) Modeling uranium biosorption by Cystoseira sp. and application studies. Sep Sci Technol 52(5):792–803
Yang A, Zhu Y, Li P, Huang CP (2019) Preparation of a magnetic reduced-graphene oxide/ tea waste composite for high-efficiency sorption of uranium. Sci Rep 9(1):1–12
Giannakoudakis DA, Anastopoulas I, Barczak M, Antoniou E, Terpilowski K, Mohammadi E, Shams M, Coy E, Bakandritsos A, Katsoyiannis IA, Colmenares JC, Pashalidis I (2021) Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J Hazard Mater 413:125279
Zinicovscaia I, Safanov A, Zelenina D, Ershova Y, Boldyrev K (2020) Evaluation of biosorption and bioaccumulation capacity of cyanobacteria Arthrospira (spirulina) platensis for radionuclides. Algal Res 51:102075
Scheufele FB, Staudt J, Ueda MH, Ribeiro C, Stefeen V, Borba CE, Modenes AN, Kroumov AD (2020) Biosorption of direct black dye by cassava root husks: Kinetics, equilibrium, thermodynamics and mechanism assessment. J Environ Chem Eng 8(2):103533
Bagchi M, Bera D (2021) Adhikari S (2021) Biosorption of an azo dye reactive blue 4 from aqueous solution using dead and CMC immobilized biomass of Rhizopus oryzae (MTCC 262). Bioremediat J. https://doi.org/10.1080/10889868.1884526
Semiao MA, Haminiuk CWI, Maciel GM (2020) Residual diatomaceous earth as a potential and cost effective biosorbent of the azo textile dye reactive blue 160. J Environ Chem Eng 8(1):103617
Hevira L, Ighalo JO, Zein R (2020) Biosorption of indigo carmine from aqueous solution by Terminalia catappa shell. J Environ Chem Eng 8(5):104290
Kumar KV, Ramamurthi V, Sivanesan S (2006) Biosorption of malachite green, a cationic dye onto Pithophora sp., a fresh water algae. Dyes Pigments 69(1–2):102–107
Ahsan MA, Katla SK, Islam MT, Hernandez-Viezcas JA, Martinez L, Diaz-Moreno CA, Lopez J, Singamaneni SR, Banuelos J, Torresdey JG, Noveron JC (2018) Adsorptive removal of methylene blue, tetracycline and Cr (VI) from water using sulfonated tea waste. Environ Technol Innov 11:23–40
Abd IN, Mohammed-Ridha MJ (2021) Tetracycline antibiotic removal from aqueous solution using Cladophora and Spirulina algae biomass. Iraqi J Agric Sci 52(2):336–347
Balarak D, Bandani F, Shehu Z, Ahmed NJ (2020) Adsorption properties of thermally treated rice husk for removal of sulfamethazine antibiotic from pharmaceutical wastewater. J Pharm Res Int 84–92
Angulo E, Bula L, Mercado I, Montano A, Cubillan N (2018) Bioremediation of Cephalexin with non-living Chlorella sp., biomass after lipid extraction. Bioresour Technol 257:17–22
Golveia J, Santiago MF, Silva LB, Campos LC, Schimidt F (2021) Utilization of the corncob agro-industrial residue as a potential adsorbent in the biosorption of bisphenol-A. J Braz Chem Soc 32:1396–1404
Fan L, Huang G, Yang S, Xie Y, Liu W, Shi J (2021) Preparation of phosphate-functionalized biopolymer/ graphene oxide gels for enhanced selective adsorption of U (VI) from aqueous solution. J Radioanal Nucl Chem 329:555–564
Acknowledgements
The authors thank Techno India University, West Bengal, India for support and encouragement during this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest. No financial support has been taken from any agencies to carry out this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Banerjee, S., Kundu, A. & Dhak, P. Bioremediation of uranium from waste effluents using novel biosorbents: a review. J Radioanal Nucl Chem 331, 2409–2435 (2022). https://doi.org/10.1007/s10967-022-08304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08304-2