Abstract
In a project sponsored by the NUCLEBRÁS, were mapped uraniferous anomalies in the municipalities of Pedra and Venturosa, in the rural region of Pernambuco, Brazil. As these municipalities are the main milk producers in this State, it was of interest in this work to investigate the levels of natural uranium in milk, curdled cheese and milk whey, produced by the cattle in these districts and nearby areas. Conventional uranium fluorimetry was used for analyses. The concentrations of uranium were higher in the milk whey. The milk and cheese produced in this area are insufficient to produce toxicity in the kidneys of humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk is an important source of nutrients for all ages. The presence of radionuclides in milk can be related to the use of phosphate fertilizers in herbaceous crops and forage grasses. Another important factor is the mineral supplement given to the cattle to improve reproductive efficiency and increase milk production. The mineral supplement is in most cases a mixture of common salt and concentrated mineral salt [1]. This mixture contains a significant amount of dicalcium phosphate which is the main source of phosphorus for cattle. This compound is known to have high concentrations of natural radionuclides due to its phosphate origin [2].
The presence of natural uranium in products of animal origin also depends on the animal species, the characteristics of the radionuclide, as well as its metabolic behavior in the animal and the incorporation process [3]. Ruminants are animals that consume considerable amounts of soil while grazing, so that soil intake is potentially important for the presence of radionuclides in the food chain. The amount of radionuclide ingested will depend in part on the amount of soil, which varies according to the climate and the animal species [4]. In the daily intake of pasture containing natural uranium, 1% is absorbed by the intestine and 0.014% is present in each liter of milk produced. The daily intake of 0.4 mg of uranium, can cause moderate effects (kidney toxicity) in dairy cattle [5]. Studies by Thornton and Abrahams [6] estimated that the intake of soil by the bovine could represent up to 18% of the daily intake of dry matter. Therefore, the involuntary ingestion of soil by animals becomes a potentially important factor to explain the presence of radionuclides, as in the case of natural uranium, in the human food chain.
Despite the concern to monitor the levels of radioactivity in milk and milk products in developed countries, data on the subject in Brazil are rare. In the northeast region, there are few studies focused on the subject [7,8,9]. In 1975, the Brazilian Nuclear Corporation (NUCLEBRÁS) together with the Geological Survey Company of Brazil (CPRM) carried out a study of radiological prospecting in the states of Pernambuco, Alagoas, and Sergipe [10, 11], this study identified 263 anomalies in which only Pernambuco showed a significant concentration of uranium.
IAEA [12] Technical Document TECDOC-1363 considers as one of the main characteristics in the identification of uranium anomalies that U(+III) concentration on the terrestrial surface is in the range of 4-20 ppm eU (equivalent to 49.4–247 Bq kg−1 uranium).
In 1977, NUCLEBRÁS supported a project to conduct geological and radiometric mapping in the municipalities of Pedra and Venturosa, in Agreste region of Pernambuco state, with the aim of identifying anomalous areas where the occurrence of natural uranium was higher than normal [11].
The discovery of higher than normal uranium in these municipalities raised interest in the occurrence of natural radioactivity since approximately 10% of the milk production in the state of Pernambuco is concentrated in these municipalities. The farms commercialize a portion of the milk produced and the other part is used as raw material for the dairy factories of the referred state. The feed crops used as feed for cattle is produced exclusively from vegetation grown on these farms. Part of the farms that breed cattle is in areas where there is the anomalous occurrence of natural uranium.
The main objective of this study was to evaluate the quantity of natural uranium in cow’s milk and in milk products, produced in the farms located close to areas where natural uranium is elevated. The results of the study will provide values for annual effective ingestion dose and the estimation of the uranium intake by the local population.
Experimental
Study area
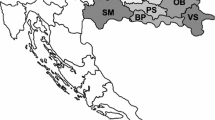
The studied area is located in the semi-arid region of the municipalities of Pedra and Venturosa, in the northeast region of Brazil, where the occurrence of uranium is higher than normal. Farms selected for this study had the highest milk production with approximately six thousand liters in the period of the research. A representative map (Fig. 1) identify all the farms classified by codes from F01 to F09. During the collection procedure, F07 farm was identified as one of the places included in the 1977 NUCLEBRÁS survey project. Control locations were used as a reference for natural uranium background and samples from these locations (F10 and F11 points in Fig. 1), were collected samples in the municipalities of São Bento do Una and Arcoverde, which are 70 km and 35 km from the anomalous natural uranium occurrences, respectively.
The region of these municipalities presents a warm and dry climate. Average annual temperatures range from 24 to 26 °C. However, in the warmer period, months of December to February, the temperature varies from 26 to 33 °C and, in the coldest months (June to August), it varies between 16 °C and 26 °C.
The geology of the region is characterized by the presence of amphibolitic calcium-silicate rocks. This type of rock usually does not have associated uranium, however, the average uranium content determined by NUCLEBRÁS was 10,000 ppm, much higher than those found in other locations [9].
Sample collection and preparation
Twenty-three samples of milk and milk products that were produced on farms and dairy factories located in the region highlighted in Fig. 1 were analyzed. In addition to milk, samples of curdled cheese and whey were collected from ten dairy factories that were supplied by some of the farms in this study. Whey was also collected since it is used to produce yogurt and ricotta cheese and it is also widely used by farmers to feed various animals (young and adult calves, heifers and cows) as well to fatten pigs. The same amounts of samples were collected in June 2002 (rainy season), December 2003 (dry period) and May 2004 (rainy season).
Samples of cheese were weighed, divided into small parts and dried in an oven at 80 °C for 24 h to determine dry weight. After drying, samples were placed in a muffle where the temperature was raised gradually over a period of 48 h to 450 °C. The samples remained in the furnace for an additional 48 h period to destroy all microorganisms. After determining the final ash weight, samples were sealed in polyethylene containers. All processing was performed at Radioecology Laboratory and Environmental Control (LARCA) belonging to the Department of Nuclear Energy of the Federal University of Pernambuco.
The milk samples were transferred to 2000 mL glass beakers and evaporated on a heating plate at a temperature of 70 °C for 96 h. After drying, the milk samples were baked following the procedure described above. Aliquots of two grams of ash were dissolved in 10 mL of HNO3, 3 mL of HClO4 and 5 mL of 8 M HNO3. Once dissolved, the solution was raised to 50 mL with distilled water.
Radioactivity measurement
For uranium determination, conventional fluorimetry was used. This technique involves the extraction of natural uranium with an organic solvent followed by a fluoride fusion and measuring the uranium fluorescence with a Jarrel-Ash Mod. 27-000 fluorimeter. Uranium fluorimetry was performed at the Department of Nuclear Energy.
Uranium extraction was performed in 100 mL polyethylene tubes using 20 mL aliquots of each sample with the addition of 1.0 mL of saturated Al(NO3)3 and 1.0 mL of trioctylphosphine oxide (TOPO). Extraction was also performed using samples containing a known amount of uranium. The polyethylene tubes contained the samples were agitated on a mechanical table for 10 min and the solution was transferred to glass tubes with pre-defined volumes, which were produced specifically for that study. After transfer, the samples were allowed to stand for 1 h to separate the organic phase. Thereafter, a LiF/NaF mixture was prepared.
The pellets used to melt the natural uranium are generally composed by a high concentration of fluorides and/or compounds with a high concentration of carbonates. As a result of 1% LiF a 2% creep composition with 98% NaF was found to be an ideal composition for fluorimetry. One way to deal with this issue was sensitivity and precision [13]. Fluorides with organic aliquots containing extracted natural uranium is a uranium fluorescence compound which, once excited by the ultraviolet light of the fluorimeter, produces a fluorescence that is picked up by the fluorimeter, where a uranium concentration reading occurs.
For analysis, 0.1 mL of the organic phase the standards and the deionized water were transferred to the platinum crucibles containing the fluoride mixture, which were oven dried at 130 °C for 30 min and melted in a standard specific oven at 1000 °C for 6 min.
The uranium standards were produced in the LARCA using uranyl nitrate and standardized according to the IRD Intercomparison Program [14]. Quality control samples included three duplicate samples of milk ash with and without addition of uranium.
Calibration model
For the calibration of the fluorimeter, secondary uranium standards were used, with concentrations certified by the Institute of Radioprotection and Dosimetry of the National Nuclear Energy Commission in Brazil.
The mathematical models and their respective calibration functions were determined by correlating the uranium contents in the standards and the values of the electric current recorded in the scales of the equipment, considering that there is direct proportionality between these two measurements.
Due to the differences in the concentrations of the standards, the scales of 0.01 μA and 0.1 μA were used. This method has a detection limit for natural uranium of 0.1 μg L−1 (1.24 mBq L−1).
For the construction of the calibration model with low concentrations, secondary patterns of 2, 3, 4, 5 and 6 ppb were used. In the model with higher concentrations the standards of 30, 45, 50 and 60 ppb were used. From the data obtained in the calibration, it was possible to calculate the results of the present study and formulation in the units of interest.
Results and discussion
Statistical analysis
For the statistical treatment of the data, some of the main descriptive parameters and non-parametric test were analyzed, which was the Kolmogorov–Smirnov test with Lilliefors correction. The Kolmogorov–Smirnov adhesion test showed that the results obtained for uranium in milk and its milk products present significant dispersions, whose distribution tendency does not obey a homogeneous behavior of the environment.
To quantify the natural uranium ingested, the annual per capita household food consumption of milk and cheese from residents of the study area was used [8, 15].
Concentration of natural uranium in milk samples and milk products
The concentrations of natural uranium in milk samples obtained from farms F01 to F09 were represented in a bar chart (Fig. 2). A large variation occurred in the results, values ranged from 12 to 1835 mBq L−1, with a median of 99.5 mBq L−1.
The farms with the highest concentration of natural uranium in milk were farms F01 and F09 and are within close proximity to the main location of elevated natural uranium. These farms also grow vegetation that is fed to milk-producing animals.
The cattle herd of the region also consume mineral supplements and water of the studied region. The chemical composition of milk probably facilitated the absorption of natural uranium.
According to Sheppard et al. [16], uranium is easily adsorbed by organic matter. Primordial studies demonstrated that the mechanism of fixation at concentrations of uranium occurring in vivo involves an ionic exchange between uranium in solution and some group (probably Ca) on the surface of the bone crystals [17].
Froidevaux et al. [18], studied the concentration of 90Sr, 238U and other radionuclides in Emmental milk produced in Europe, and claims that uranium contaminates milk mainly through the water that dairy cows consume and less through fodder.
The results of the concentrations of natural uranium, from samples collected from ten dairy factories, ranged from 2.0 to 3.0 mBq kg−1 (dry weight) and from 99 to 3102 mBq kg−1 (dry weight), for cheese and whey, respectively (Table 1). For whey samples it was only possible to collect in five dairy factories.
The leached of natural uranium occurred into the whey during the cheesemaking process (Table 1). The dairy factories present in the region use the milk marketed by the producing farms. Almost all farms that market milk to the dairy factories is located close to the occurrence of natural uranium.
In previous studies [19], analyzes performed in the area revealed a maximum value of U3O8 of 22,000 mg kg−1. This may have given rise to the high values obtained for uranium in the milk samples in the current investigations, much above the range reported by other studies (Table 2).
Temporal behavior of uranium content in milk
In order to evaluate the seasonal influence on uranium behavior in milk, investigations were carried out during periods with different seasons. Three consecutive years were studied, 2002, 2003 and 2004 (Fig. 3). For the milk samples produced on the control farms F10 and F11, a variation of 9–33 mBq L−1 was observed, due to changes in pasture location of the cattle herd, since the two properties are very extensive and acquire foods from different regions.
The chart of Fig. 3 demonstrates that there is no uniform behavior. In farm F10, the highest concentration was obtained in the year 2002 with a value of 19 mBq L−1 and in farm F11, the highest result was obtained in the year 2004 with a value of 33 mBq L−1. As can be observed, there is a greater similarity of the results for F11 sample with respect to the others, such as F02, F04, F06 and F08, once this sample is closer to these points (35 km), when compared with the F10 (70 km from the anomalous natural uranium occurrences). In addition, the geological formation of each studied area could have influenced the results obtained.
Analysis of natural uranium intake
The data that defined the average annual intake of natural uranium through milk and cheese were based on annual consumption per person in the cities of Pedra and Venturosa, with a value of 183 L and 4.0 kg, respectively [8, 15]. Considering the median values obtained in the milk and cheese samples, 99.5 mBq L−1 and 2.0 mBq kg−1, the annual activity ingested was 18,208 and 8.0 mBq, respectively.
According to the studies conducted by Wrenn et al. [22], gastrointestinal absorption of uranium by standard man is about 1.4%. Based on studies by this author, the daily absorption of 10–30 mg day−1 uranium is sufficient to produce toxicity in the kidneys of adult humans.
Converting the value of the activity ingested in mBq shown above, for mass unit, we have that the level of intake per person was 1.47E−3 mg y−1 and 6.48E−4 mg y−1 of natural uranium obtained for milk and cheese, respectively. Thus, based on the levels established by Wrenn et al. [22], this value is not sufficient to produce toxicity in the kidneys of an adult.
Dose assessment
In order to calculate the annual effective ingestion dose of natural uranium (Eing), the value of 0.045 µSv Bq−1 was used as the dose conversion factor (e), considering the study made by Giri et al. and ICRP Publication 119 [19, 20] (Eq. 1).
where As is the specific activity (Bq kg−1) and m the annual intake of milk and cheese per individual (kg y−1).
Figure 4 presents these values for farms F03, F05 and F11 where samples of milk and cheese were collected in the respective dairy factories (LT1-LT4).
The annual effective ingestion dose per each farm show that the highest values were obtained for milk, mainly in farm F 05 with a result of 5.08 μSv y−1. Concerning cheese samples, doses in the range of 3.60E−4 to 5.4E−4 μSv y−1 were obtained.
Studying the risk assessment of the ingestion of radionuclides and heavy metal from samples of milk of twelve villages around an rich area on uranium ore Giri and cols [23], found the ingestion dose of 0.49 μSv y−1. In this work, the lowest values for milk were found in F11 farm. This may be because it is more distant from the occurrence of natural uranium, as shown in Fig. 1.
Conclusions
According to the results obtained for the concentrations of natural uranium in bovine milk and its milk products, consumed in the municipalities of Pedra and Venturosa, the highest values showed that there is not significant risk to cause damage to the kidneys of the residents. Particularly as regards the marketing of its milk products, since the local dairy industries take as production basis the mixture of milk produced daily, which minimizes uranium concentrations. However, given the local uranium anomaly, guidelines should be established to analyze the influence of other activities in the context of radioecological impact, especially agriculture, rock extraction, minerals, as well as water use, mainly from tubular wells.
References
Coppock CE (2004) Ração total: nutrição certa com economia. Rev Balde Branco 77:32–35
UNSCEAR (2010) Sources, effects and risks of ionizing radiation. United Nations
Arruda-Neto JDT, Tavares MV, Filadelfo M (1997) Concentrations of uranium in animal feed supplements: measurements and dose estimates. J Radioanal Nucl Chem 221:97–104
Cooke AI, Green N, Rimmer DL et al (1995) Development of an in vitro method to assess the avail-ability of soil-associated radionuclides for uptake by ruminants. J Environ Radioact 28:191–207
WHO HWG (2012) World health statistics: a snapshot of global health. WHO, Geneva
Thornton I, Abrahams P (1983) Soil ingestion-a major pathway of heavy metals into livestock grazing contaminated land. Sci Total Environ 28:287–294
Silva CM, Alcoforado ES, Amaral RS et al (2010) Stable lead in milk and derivates. Food Anal Methods 3:85–89
Silva CM, Amaral RS, Amaral A et al (2006) 226Ra in milk of the dairy cattle from the rural region of Pernambuco, Brazil. J Radioanal Nucl Chem 270:237–241
Silva CM, Amaral RS, Tabosa JN et al (2010) Estimation of dose due to ingestion of 210Pb in milk from dairy cattle in the semi-arid region of Pernambuco, Brazil. Bull Environ Contam Toxicol 85:103–108
Santos JA, Amaral RS, Silva CM, Menezes RSC (2010) Radium equivalent and annual effective dose from geological samples from Pedra - Pernambuco - Brazil. Radiat Meas 45:861–864
Calheiros MEV (1977) Projeto Venturosa: Estudos Geológicos e Radiométricos na Folha Venturosa – área I, Sub-área E. Recife, Brasil
IAEA (2003) Guidelines for radioelement mapping using gamma ray spectrometry data, IAEA-TECDOC-1363, Vienna-Austria
Centanni FA, Ross AM, Desesa MA (1956) Fluorimétric determination of uranium. Anal Chem 2:1651–1657
IRD/CNEN (1990) Programa de Intercomparação de Resultados de Análises Radioquímicas para Determinação de Radionuclídeos em Matrizes Artificiais. Amostra 01IU085/1190. Determinações de urânio natural, Ra-226 e Ra-228. Rio de Janeiro, Brasil
Carvalho MP, Martins P do C, Wright JTC, Spers RG (2007) Cenários para o leite no Brasil em 2020, 1ra ed. MG
Sheppard SC, Sheppard MI, Gallerand MO, Sanipelli B (2005) Derivation of ecotoxicity thresholds for uranium. J Environ Radioact 79:55–83
Neuman WF (1948) The deposition of uranium in bone. [Part] IV, In vitro adsorption studies. U.S. Atomic Energy Commission, Technical Information Division, Oak Ridge, Tennessee
Bosset J-O, Froidevaux P, Valley J-F et al (2003) 90Sr, 238U, 234U, 137Cs, 40 K and 239/240Pu in Emmental type cheese produced in different regions of Western Europe. J Environ Radioact 72:287–298
Costa AC, Pedrosa IL, Mendes VA (1977) Projeto Agreste de Pernambuco. Convênio DNPM/CPRM. Relatório Final. Recife, Brasil
Giri S, Jha VN, Singh G, Tripathi RM (2013) Estimation of annual effective dose due to ingestion of natural radionuclides in foodstuffs and water at a proposed Uranium mining site in India. Int J Radiat Biol 89:1071–1078
Garnier–Laplace J, Colle C, Morello M (2010) Natural Uranium and the environment, France
Wrenn ME, Durbin PW, Howard B, Lipsztein J, Rundo J, Still ETWD (1985) Metabolism of ingested U and Ra. Health Phys 48:601–633
Giri S, Singh G, Jha VN, Tripathi RM (2011) Risk assessment due to ingestion of natural radionuclides and heavy metals in the milk samples: a case study from a proposed uranium mining area, Jharkhand. Environ Monit Assess 175:157–166
Acknowledgements
The authors express their gratitude to the Laboratory of Radioecology and Environmental Control of the Department of Nuclear Energy (UFPE) for the provided infrastructure; the Pro-Rectorate for Research and Graduate Studies (PROPESQ) of UFPE for the financial assistance granted by Qualis A 2018; the Foundation for Support to Science and Technology of the State of Pernambuco (FACEPE) for the postdoctoral fellowship granted to one of the co-authors (project BFP-0009-3.09/17); the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq) for doctoral fellowships awarded to co-authoring students (140513/2018-3, 145566/2014-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos Amaral, R., dos Santos Júnior, J.A., da Silva Aquino, F. et al. Exposure risk assessment of uranium intake of the milk products from the region of Pernambuco, Brazil. J Radioanal Nucl Chem 321, 927–933 (2019). https://doi.org/10.1007/s10967-019-06645-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06645-z