Abstract
Native and chemically modified sugar beet pulp were used as a biosorbents for removal of uranium(VI) from the aqueous solution. The effects on the uranium(VI) uptake such as solution pH (2–9), adsorbent amount (50–500 mg), contact time (0–180 min), temperature (293–323 K) and initial concentration (20–60 mg U L−1) were studied. The obtained maximum adsorption capacities according to Langmuir isotherm model for native and modified sugar beet pulps were 20.45 and 19.80 mg g−1, respectively. Additionally, pseudo-second order kinetic model best fitted the experimental data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its radioactivity and chemical toxicity at very low concentrations uranium is extremely dangerous for the environment and human health. Increasing amounts of wastewater containing uranium are produced annually from various activities such as exploration and processing of uranium, mining industry, nuclear industry associated activities, industries that utilize radioisotopes and geological disposal of radioactive waste [1, 2]. In the environment uranium is mainly present in two oxidation states U(IV) and U(VI). Among this two uranium species, U(IV) is less soluble and occurs under strongly reducing conditions and U(VI) appears as a mobile, hydrated uranyl UO22+ ion [3, 4]. Some of conventional methods for removal of uranium from the wastewater are chemical precipitation, co-precipitation, electrochemical treatment, ion exchange, membrane processes, solvent extraction and adsorption [5,6,7,8,9,10,11,12]. All listed methods have a lot of limitations, such as incomplete metal removal, high energy depletion, limiting pH and concentrated liquid wastes [13, 14]. Among listed methods, the most promising method for uranium removal is adsorption due to its simplicity, applicability, high selectivity and capacity. Recently a lot of research is focused on development of new innovative adsorbents with special emphasis on biosorbents. Based on the use of biomass as an adsorbent, biosorption of different types of metal ions including uranium from aqueous solutions is considered as a very promising procedure. Compared to other methods, biosorption is more economical, eco-friendly, energy saving, has a wide working range of pH and temperature, minimal volume of disposable sludge, high efficiency, selectivity, moreover, once used biomass can be reused in multiple cycles depending on its capacity [15,16,17,18].
A lot of different adsorbents, such as pummelo peel [19], Eucalyptus citriodora distillation sludge [20], Citrobacter freudii, a bacterium [1], Catenella repens, a red alga [21], pollen pini [22], Bangia atropurpurea, a red alga [23], alginate beads [24], wood powder and wheat straw [4], polyaniline coated magnetic carboxymethylcellulose [25], silica particles grafted with polyacrylonitrile beads [26] etc. were considered for the removal of uranium ions from the aqueous solution, but there are only a few studies in which removal of uranium and other radionuclides were explore by adsorption on agricultural waste [27,28,29,30,31]. Agricultural waste as a low-cost biosorbent is of great interest for treatment and removal of toxic pollutants due to its several advantages such as specific chemical composition (i.e. hemicellulose, cellulose, lipids, lignin, sugar, pectins etc.) containing a lot of acidic functional groups (i.e. carboxylic and hydroxy) which can bind pollutant ions very effectively. Additional advantages are availability, high efficiency as well as easy to modify with different chemical reagents [13, 32]. Moreover, a numerous byproduct from industrial operations can be used as a potential inexpensive biosorbents [32]. Sugar beet pulp (Beta vulgaris) is a low-cost byproduct of the sugar industry and it is mainly used as animal feed. It is very rich in pectic substances which are complex heteropoly-saccharides containing galacturonic acid, arabinose, galactose and rhamnose as the major sugar constituents. It is also rich in cellulose, which main component is glucose [33,34,35,36]. Functional groups found in sugar beet pulp are associated to very strong heavy metal binding, specially carboxyl groups with a great biosorption and heavy metal removal potential [37]. According to its properties, sugar beet pulp has been widely used in many studies for heavy metal removal [34,35,36,37,38,39,40,41,42].
In this study sugar beet pulp was used as biosorbent in native and modified form for the removal of uranium(VI) from aqueous solution. The influence of adsorption conditions, such as pH, biosorbent dosage, contact time, initial metal ions concentration, and temperature on the biosorption process was investigated.
Experimental
Reagents and solutions
All used chemical reagents were of analytical grades. Arsenazo(III), UO2(NO3)2.6H2O and 60% HClO4 were obtained from Merck (Darmstad), 37% hydrocloric acid was purchased by Semikem (Sarajevo), 65% nitric was purchased by Carlo Erba (Milano) and sodium hydroxide by Alkaloid (Skopje). The stock solution of U(VI) (1000 mg/L) was prepared by weighting 2.1308 g (Metler Tolledo balance, ± 0.1 mg) of uranyl nitrate hexahydrate salt (A.R. grade) and dissolving it in 3 mol dm−3 perhloric acid. Working solution were prepared by diluting the stock solution.
Sugar beet pulp preparation and characterization
Sugar beet pulp was provided by Sunoko Sugar Factory (Novi Sad, Serbia) as a fraction which is remaining after the production of sugar. The sugar beet pulp was first washed by the tap water, then several times with distilled water, and then air-dried for 24 h. After 24 h it was dried in an oven at 100 °C to a constant weight and then divided into two equal parts. One part of it was blended and powder was sieved through a standard sieve (Ø 0.25 mm) then stored in hermetically sealed container and it was marked as a native sugar beet pulp (NSBP).
Second part of the remaining sugar beet pulp was modified with 0.25 mol L−1 nitric acid and 0.1 mol L−1 sodium hydroxide in the way it was first immersed in 0.25 mol L−1 HNO3 and occasionally stirred for 24 h. After 24 h it was rinsed with distilled water until pH of the filtrate wasn’t settled and then it was air-dried. The sugar beet pulp was then left 4 h in NaOH solution, washed several times with small quantities of distilled water until pH seetled, air-dried, blended then sieved through a standard steel sieve (Ø 0.25 mm) and stored in hermetically sealed container and marked as modified sugar beet pulp (MSBP).
The morphology and composition of the milled native (NSBP) and modified sugar beet pulp (MSBP) were characterized by SEM/EDS using a JEOL JSM-6610LV Scanning Electron Microscope, at an accelerating voltage of 20 keV. In order to determine functional groups of NSBP and MSBP, infrared spectra of the Fourier transform (FT-IR) were recorded on a Perkin Elmer BX FT-IR spectrometer using KBr pellet technique in a region from 4000 to 400 cm−1.
Biosorption experiments
Batch techniques were conducted in closed polypropylene bottle at 95–100 movements per minute (mechanical shaker: Heidoplh Duomax 2030) to study the biosorption of U(VI) onto NSBP and MSBP. Experiments were performed by adding (50–500 mg) of NBP and MSBP biosorbents into a 50 mL solution which was having a particular concentration of uranium(VI) (20–60 mg L−1).
All experiments were performed in triplicate at 25 °C and the mean values have been used for data evaluation because the variation of the experimental data was within the measurement error (± 5%). The pH of each sample solution was adjusted with 3.0 mol L−1 NaOH and measured by digital pH meter (CG 841 Schott-GERÄTE GmBH). The concentration of uranium(VI) in solution was determined spectrophotometrically by Arsenazo-III method on a UV–Vis spectrophotometer (model: Varian Hary 50) [31, 43]. The maximum absorbance of the formed complex was on 650 nm, and the maximum absorbance of the Arsenazo(III) reagent was at 535 nm [31].
The amount of adsorbed uranium(VI) ions as adsorption capacity, q (mg g−1) onto NSBP and MSBP was calculated according to the equation:
where Ci and Cf are initial and final concentrations of uranium(VI) ions (mg L−1), V is the volume of the solution and m is the mass of adsorbent (g).
Removal efficiency (%) as the amount of uranium(VI) hold on the biosorbents was calculated through the following equation:
where Removal efficiency (%) is the amount of metal ion retained on the biosorbent.
Under the same experimental conditions but without biosorbent a control sample was subjected to all steps of the experiment to find out was there a loss of analyte during the process, and the concentration of control samples was taken as initial concentration of uranium U(VI) ions (Ci).
Biosorption kinetics
For biosorption kinetic modeling of U(VI) onto native sugar beet pulp (NSBP) and modified (MSBP) were used Lagergren pseudo-first-order model [44] (Eq. 3) and applied as follows:
where qe and qt are the amounts adsorbed at equilibrium and at time, t (mg g−1), respectively and k1 is the rate constant of the pseudo-first order adsorption (min−1) which can be obtained from the slope of the linear plot of ln (qe − qt) versus t.
The second used kinetic model was Lagergen pseudo-second-order model (Eq. 4) which assumes the limiting step is chemisorption [45,46,47] and for this study this model was applied in the form:
where k2 (g mg−1 min−1) is the rate constant of pseudo-second order kinetic model. The plot of t/qt versus t give a linear relation with a slope 1/qe and an intercept of 1/(k2q2e) [48].
Additionally, intraparticle diffusion (Weber–Morris) model (Eq. 5) was used [49] in the form:
where kin is the intraparticle diffusion rate constant (mg g−1 min−1/2) and C is the constant of the model in the function of the boundary layer thickness (mg g−1).
The applicability of these kinetic models were based on the coefficient of correlation (r2), because it is a measure of experimental data, compatible with the suggested model.
Equlibrium biosorption isotherms
The experimental data were analysed using Langmuir [50], Freundlich [51] and Temkin models [52].
The Langmuir model was applied in the linearized form:
where qe (mg g−1) is the equilibrated amount of analyte adsorbed per unit weight of biosorbent, ce (mg dm−3) is equilibrium concentration after biosorption, qmax (mg g−1) is maximum biosorption capacity and KL (dm3 g−1) is the Langmuir isotherm constant that is associated with biosorption free energy. A plot of 1/qe versus 1/ce gives the adsorption coefficients.
Affinity between the sorbate and sorbent using Langmuir isotherm is described by non-dimensional separating factor, RL given by the equation:
The value of RL indicates the nature of the biosorption mechanism [27]. The RL values are in the range of 0 < RL < 1, and if the value is between 0 and 1, the biosorption process is favorable [50].
The Freundlich model was also applied in the linear form as:
where KF (mg g−1) (dm3 mg−1)1/n) and n (g dm−3) are Freundlich’s constants of adsorption isotherms constants indicating adsorption capacity and intensity, respectively. The values of Freundlich’s constants (KF and exponent n) are obtained from a plot of log qe versus log ce [51].
The linear form of the applied Temkin’s isotherm equation is given as:
where ce is the concentration of adsorbate (mg L−1), qe is the amount of adsorbate adsorbed per unit mass of biosorbent (mg kg−1), AT (L g−1) is the equilibrium binding constant relating to the maximum binding energy and determines the Temkin adsorption potential, b (J mol−1) is indicator to the heat of biosorption, and T and R are the temperature (K) and gas constant (8.314 J mol−1 K−1), A plot of qe versus ln ce gives Temkin’s constants AT and b [52, 53].
Thermodynamics of uranium biosorption
Thermodynamic parameters, such as enthalpy change (ΔH), entropy change (ΔS) and change in Gibbs free energy (ΔG), were obtained from the following equations:
where ΔG (kJ mol−1) is change in Gibbs free energy, T is temperature in Kelvin (273 K), R is gas constant (8.314 × 10−3 kJ mol−1 K−1), Kc is the equilibrium constant, CAc is the equilibrium concentration (mg L−1) of the metal ion on adsorbent and ce is equilibrium concentration in the solution.
Enthalpy change (ΔH, in kJ mol−1) and entropy change (ΔS, in J mol−1 K−1) were obtained from the slope and intercept of Vant Hoff plots of log Kc versus 1/T using the following equation [54]:
The change in Gibbs free energy was calculated from the equation:
Results and discussion
Biosorbent characterization
Fourier transform infra-red (FT-IR) spectroscopy
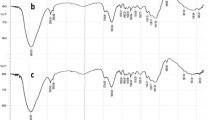
FTIR spectroscopy was used to determine which functional groups are present in the native and modified sugar beet pulp. Obtained spectra of native sugar beet pulp (NSBP) and modified sugar beet pulp (MSBP) are represented in Fig. 1.
The intense broad peaks at 3415 cm−1 for (NSBP) and (MSBP) are attributed to the O–H vibrations due to the stretching of the alcohols, phenols and carboxylic acids in cellulose, lignin and pectin. This is in accordance with the composition of the sugar beet pulp (lignin, cellulose, hemicellulose and pectin) and it indicates the presence of the “available” and associated O–H groups on which metal ions can bond [37, 40, 55,56,57,58]. In the spectra of (MSBP) appearance of low intensity peak at 2927 cm−1 is assigned to the symmetrical or asymmetrical C-H stretching vibrations of hydrocarbon chains [31, 40, 56,57,58]. This saturated C-H bond, probably originated from compounds formed by basic hydrolysis of ester group. The peak at ~ 2360 cm−1 for (NSBP) and (MSBP) indicates the presence of a triple C≡C bond. The bands at 1638 cm−1 for (NSBP) and 1636 cm−1 for (MSBP) are assigned to stretching of the C=O in the in the carboxyl groups of pectin and hemicellulose [59,60,61]. In the spectra of (MSBP), there is an overlap of the band by an intense band at ~ 1620 cm−1, and this can be attributed to asymmetrical stretching of the dissociated carboxyl groups (COO−) [31, 62]. A peak at 1618 cm−1 in the spectra of (NSBP) is assigned to the stretching vibrations of the carbonyl group in ester, because of their present in pectins which are obtained from the pulp residue of the sugar industry, and they have a high amount of methylester [40]. In the spectra of both peaks at 1400 cm−1 can be assigned to symetric stretching in carboxylate ions [61]. Additionally, in the spectra of (MSBP) a new peek at 1061 cm−1 can be observed, and it is attributed to the ether groups, which were formed in the presence of NaOH during the modification of the sugar beet pulp. The absorptions at 622 cm−1 in the spectra of (NSBP) and (MSBP) are assigned to the vibrations of the bonds between the carbon atoms and the halogens in the haloalkanes.
There can’t be observed significant differences in the spectra of native and modified sugar beet pulp, changes are only related to the intensities of absorption which were increased in the spectra of modified sugar beet pulp (MSBP) indicating the modification increased a number of the existing functional groups.
Scanning electron microscopy (SEM) and electron dispersion spectroscopy (EDS)
Scanning electron microscopy is mainly a non-destructive method of analysis, which provides information about the morphology, composition and other surface properties of the test sample in this study particulary, about biosorbents (NSBP and MSBP). Figure 2 shows scanning electron microscope picture of NSBP and MSBP. By comparing SEM micrographs (Fig. 2) differences in particle structure, shape and size between NBP and MBP are evident. As can be seen from SEM micrographs the heterogeneity of the surface of the particles on the native sugar beet pulp is noticeable. It has rough, irregular and non-uniform surface, particles are smaller and non-compact, while modified sugar beet pulp particles are larger and more compact.
The reason for the increase in MSBP particles may be the agglomeration of the particles due to the increase in the number of functional groups that interact (the most dominant are the electrostatic interactions as well as the hydrogen bond) which brings them closer to each other.
It is evident that the modification resulted in the removal of smaller particles of the native material, and it can be assumed that the acid-alkaline treatment of the sugar beet pulp resulted in the reduction of the “inactive” parts of the biomass, thus making the “active” biosorbent surface rich in functional groups more accessible for the binding of ions of interest. More precisely, after acid–base treatment, the structure of the MSBP is more homogeneous and smoother than the surface of NSBP particles indicating that the chemical treatment removed impurities from the surface (e.g. waxes and other fats) which were contributed to the roughness of the sugar beet pulp surface and was masking the functional groups available for biosorption [63]. The distribution of particles is less chaotic on the MSBP, and there is a noticeable decrease in the number of channels between the particles due to the agglomeration of particles. Based on the all mentioned above, modification of sugar beet pulp was successful and after it, functional groups are more accessible to water molecules and analyte ions. SEM analysis is an additional confirmation of the results obtained by FTIR spectroscopy, which means that the number of functional groups available for interaction with the analyte has increased.
In Table 1 are given results obtained by EDS analysis showing semi-quantitative composition of the utilized biosorbents surface.
The EDS analysis showed that the surface of NSBP and MSBP is mostly composed of carbon (C) and oxygen (O). Additionally, among alkali and alkaline earth metals magnesium (Mg2+), potassium (K+) and calcium (Ca2+) ions are also present on the surface. It is evident that the content of sulfur is higher compare to the content of potassium, which may indicate specific sulfur-containing amino acids in the protein structures of the biosorbent. Copper is also present, most probably as the part of metalloenzyme in the plant species. After modification, increased number of Na+ ions indicated successful modification. Additionally, a decrease in Ca2+ ions is evident, indicating that the mechanism of biosorption could take place via ion exchange. EDS analysis showed decreased number of silicon atoms. Namely, plants take silicon in the form of silica which is needed as a water resource in dry conditions. It is possible during the modification that the silica is converted into the form of sodium metasilicate or water glass, thus reducing the number of silicon atoms.
Effect of pH on U(VI) biosorption
The initial pH of the solution is a very significant parameter for biosorption of metal ions from aqueous solutions on the biosorbents, because it influences the charge of the binding sites present on the adsorbent surface and the solubility and speciation of metal ions [64, 65]. Removal of uranium(VI) from aqueous solution onto NSBP and MSBP was determined in the pH region between 2.0 and 9.0 and the results regarding adsorption capacity (mg g−1) and removal efficiency (%) are presented in Figs. 3 and 4. In order to investigate the pH at which the adsorbent surface has net electrical neutrality, pHpzc was also determined (Fig. 5). Determination of zero point of charge (pHzpc) of native (NSBP) and (MSBP) was performed according to Zou an Zhao [66], with a difference that in this study was applied pH value from 1 to 12.
As can be seen in Figs. 3 and 4 the obtained values for the adsorption capacity and efficiency are higher using native sugar beet pulp (NSBP), the maximum adsorption capacity (19.10 mg g−1) and removal efficiency (80.58%) are achieved at pH 8. Regarding the obtained value of pHpzc (5.1) the selected pH value for NSBP was well-chosen because the selected pH was above pHzpc, which means the surface is negatively charged, and more uranium(VI) ions should be removed from aqueous solution. As the pH increased, the biosorbent surface was in a less degree protonated and negatively charged groups appeared in a gradual increase in uranium biosorption.
The biosorption at pH 8 can be due to the surface complexation phenomenon, because of the dissociation of acidic functional groups (–COOH) which were found on the sugar beet pulp. It might be also to the negatively charged surface which bind the positively charged U(VI) cations by electrostatic attraction [4]. The obtained results for NSBP are similar with results obtained for removal of U(VI) onto pollen pini and wood powder [4, 22].
For MSBP highest adsorption capacity and removal efficiency was reached at pH 9 (20.62 mg g−1 and 87.41%), but because it was notice that at this pH uranium is starting to precipitate, as working pH for MSBP was selected pH 5, because of the stability of uranium species and generally solution (17.88 mg g−1 and 74.32%, respectively) [19, 47, 65]. The zero point of charge for MSBP (Fig. 5) was achieved at pH 7.1, which means at the pH < 7.1 surface of the biosorbent has more positively charged binding sites and because of that, the less amount of uranium(VI) will be adsorbed on the surface due to the repulsion effect between uranium(VI) ions and positive charge of the MSBP surface. However, in accordance with literature the chosen pH value can be lower or closer to pHpzc values [47, 66]. On the basis of all above, as optimal pH values for further experiments pH 8 was chosen for native sugar beet pulp, and pH 5 for modified sugar beet pulp, respectively.
Effect of biosorbent mass
The biosorbent mass is an important parameter to be considered for effective metal ions biosorption. It influences the density of available functional groups for metal biosorption and it controls the sorbate-sorbent equilibrium of the sorption system [67, 68]. The influence of biosorbent mass on biosorption was determined in the range from 0.05 to 500 g at the initial uranium(VI) concentration of 50 mg L−1 and the results are presented in Figs. 6 and 7.
It was found that the highest adsorption capacity of 18.32 mg g−1 and 17.33 mg g−1 was obtained for both used sugar beet pulps (NSBP and MSBP) with 0.1 g of added amount of biosorbent. As can be seen from Figs. 6 and 7 increase in biosorbent dosage decreased the adsorption capacity, for NSBP from 18.32 to 3.56 mg g−1 and for MSBP from 17.33 to 2.90 mg g−1. Obtained results are opposite due to the fact that the number and availability of the surface area of the biosorbent and biosorptive sites should be increased with an increase in the biosorbent dosage [63]. Decreased available surface area for biosorption of U(VI) ions can be due to partial aggregation and screening influence on the biosorbent surface which occurs at high biosorbent dosage [69]. Additionally, the mass exchange could be slow down, due to the increase of biosorbent amount mixing efficiency [31, 68]. Decreased biosorption capacity with increased amount of biosorbent dosage was also found in other similar studies in which removal of uranium(VI) from aqueous solution were performed using biomass [21, 27, 31, 70].
Biosorption kinetic modelling
Kinetics of uranium(VI) biosorption onto MSBP and NSBP from aqueous solution was determined using the pseudo-first-order, pseudo-second-order, and intraparticle diffusion models with the aim to determine the controlling mechanism of biosorption process. The applicability of three mentioned kinetic models was estimated through the kinetic constants (k), equilibrium biosorption capacity (qe) and correlation coefficients (r2) which are shown in Table 2.
Obtained results indicate very well fitting to the pseudo second order kinetic model for the uranium(VI) biosorption process. According to the obtained values, the calculated qe of the pseudo-second-order model for NSBP (20.37 mg g−1) and MSBP (19.16 mg g−1) were closest to the obtained experimental data for NSBP (19.78 mg g−1) and (MSBP (18.63 mg g−1) with correlation coefficients (0.998 and 0.999), respectively. The difference between calculated and experimental qe for the NSBP was 0.59 mg g−1 and for MSBP was 0.53 mg g−1. The very good agreement of the experimental and theoretical data for the pseudo-second-order indicated that the rate-limiting step of the reaction is controlled by the chemisorption mechanism. A lot of other studies reported that the pseudo-second-order kinetic model describes better uranium sorption data than the pseudo-first-order model [20, 22, 27, 47, 71]. Additionally, intraparticle diffusion model also indicated significant importance for the uranium(VI) biosorption process onto sugar beet pulps (NSBP and MSBP). The plots showed multilinearity in the biosorption which indicates that three steps are operational. The correlation coefficients (r2) for MSBP for the three steps were in the order: 0.991; 0.981; 0.847 and for NSBP were: 0.996; 0.946; 0.999. The first phase is assigned to the diffusion of U(VI) ions toward the outer surface of the native and modified sugar beet pulp or the boundary diffusion layer of the soluble molecules. The second phase is attributed to the sequential biosorption of the U(VI) ions for both used biosorbents, and the third phase is assigned to the final equilibrium stage due to the lower concentration of the uranium ions left in the solution [72,73,74]. As it is shown in the Table 2, according to the obtained data the intraparticle model fitted well for the native sugar beet pulp. For the modified sugar beet pulp first two stages fitted well indicating that the biosorption process occurred mostly on the biosorbent surface due to the better attainability of the active sites.
The results for biosorption kinetic modeling showed the mechanism of the uranium(VI) biosorption onto NSBP and MSBP is a complex process and the contributors to the process are surface biosorption, intraparticle diffusion and chemisorption.
Biosorption isotherms
Adsorption isotherm studies are very significant in the determination of the adsorption efficiency. Adsorption isotherms indicate the distribution of metal ion between the solid phase and liquid phase at equilibrium [75, 76]. In this study Langmuir, Freundlich and Temkin isotherm models were applied to experimental data in order to investigate the mechanism of uranium U(VI) adsorption onto modified and native sugar beet pulp. Models were used to calculate isotherm parameters (Table 3) and to estimate the experimental data.
Based on the obtained results the biosorption data for MSBP and NSBP as biosorbents for the uptake of U(VI) followed the Langmuir isotherm model. The high linear correlation coefficient values, with r2 = 0.966 (MSBP) and r2 = 0.981 (NSBP), indicate the monolayer biosorption process and that the sorption process occurs on homogenous surface of used biosorbents [77]. The maximum biosorption capacities, with qe,max = 19.80 mg g−1 (MSBP) and qe,max = 20.45 mg g−1 (NSBP) suggest very good agreement to the experimental data, qe,exp = 18.63 (MSBP) and qe,exp = 19.78 mg g−1, respectively. Additionally, separation coefficients, RL = 0.233 for modified sugar beet pulp and RL = 0.648 for native sugar beet pulp indicate favorable biosorption and applicability of the Langmuir isotherm model. Moreover, KL values fot both biosorbents are relatively low which again implies the high affinity of MSBP and NSBP for the uranium(VI). There is not significant differences for MSBP and NSBP for fitting in this model, but as can be seen from the results, NSBP showed better fiting. In most of the studies, the biosorption process of uranium was found to be in the best agreement with the Langmuir isotherm model [27, 31, 47, 78, 79].
Freundlich isotherm model didn’t show the agreement with data obtained for NSBP, with r2 = 0.727; 1/n = 0.444). Opposite, calculated data for MSBP showed a good agreement with this model. Value of n, obtained for MSBP, which indicates whether the biosorption process is favorable or not, suggests the biosorption of U(VI) onto MSBP is highy favorable.
Biosorption thermodynamics and temperature studies
Effect of temperature on the biosorption of uranium ions onto NSBP and MSBP from aqueous solution was estimated by variation of the temperature in the range from 293 to 323 K, while the other parameters (pH, bisorbent dosage, contact time, initial concentration, volume) were kept constant. As can be seen in Fig. 8 removal of uranium onto NSBP was slightly increased with increasing temperature, and then it was slightly decreased. On other side, for the uranium(VI) biosorption onto MSBP, which is shown in Fig. 9, decrease was even more obvious. This could be explained by the decreased surface activity, and moreover, due to the adsorption theory, molecules adsorbed on the surface of the biosorbents tend to desorb from the surface at higher temperatures [14, 20, 80]. Increased adsorption capacity at the beginning for both biosorbents could be due to increased energy of the system. From the obtained results it was established that bisorption of U(VI) onto NSBP and MSBP was temperature dependent process and the optimal temperature was 303 K for both biosorbents, with maximum adsorption capacities 24.23 mg g−1 and 23.84 mg g−1, respectively.
Thermodynamic parameters for biosorption of U(VI) onto NSBP and MSBP ECDS are shown in Table 4. The positive values of ΔH suggests that the process is endotermic, meaning the dehydration energy was partly compensated in the releasing of biosorption energy. Also, the magnitude of obtained ΔH indicates the physical nature of the biosorption process which is related to hydrophobic and/or electrostatic interactions [78, 81, 82] The negative values for biosorption entropy (ΔS) indicate decrease randomness at the solid-solution interface during biosorption. Moreover, negative values of obtained entropies are suitable results with the general information of decreasing entropy when U(VI) ions pass the solid phase from the solution. Fixation of uranium(VI) ions as a result of biosorption was assigned to a decrease in the degree of freedom of biosorbate ions which lead to a negative entropy change [83,84,85]. The positive ΔG values indicates non-spontaneous biosorption of uranium ions onto NSBP and MSBP. The confirmation of the non-spontaneous biosorption was further proven through the rise of ΔG° values with increase in temperature from 293 to 323 K (Table 4) The similar results for the thermodynamic parameters were obtained in the studies of Prashant et al. [86] and Nazal et al. [87].
Comparison of adsorption capacities
The comparison of maximum adsorption capacities (qmax) of native and modified sugar beet pulp with previous studies are shown in Table 5 [20, 27, 30, 31, 47, 64,65,66, 85,86,87,88,89,90,91,92,93,94,95,96,97]. After comparison with other adsorbents and biosorbents, sugar beet pulp in native and modified form can be considered as a potential biosorbent for removal of uranium(VI) from wastewaters. Additionally, sugar beet pulp represents a low cost, biodegradable and biosorbent with high biosorption effectiveness and it doesn’t need high consumption of chemicals.
Conclusions
Results obtained by the present study confirmed that sugar beet pulp in native/modified form can be used as a low cost industrial waste for the removal of uranium(VI) from the aqueous solution under the optimum conditions pH 5/8, sorbent dosage 0.1/0.1 g, initial uranium concentration 60/50 mg L−1, contact time 60/120 min, temperature 303/303 K. Equilibrium data for both biosorbents fitted best to Lagmuir isotherm model, it followed pseudo-second-order model and it is also related to intraparticle diffusion. The positive ΔG values at optimal temperature (22.87 kJ mol−1 and 93.93 kJ mol−1) indicated non-spontaneous biosorption of uranium(VI) ions onto NSBP and MSBP. From the obtained results it was established that bisorption of U(VI) onto NSBP and MSBP was temperature dependent process and the optimal temperature was 303 K for both biosorbents. Native sugar beet pulp showed slightly higher adsorption capacity (20.45 mg g−1) compared to modified sugar beet pulp (19.80 mg g−1). Considering obtained capacity values, biomass preparation of native sugar beet pulp is very fast and economic because it doesn’t require consumption of any toxic and expensive chemicals.
References
Xie S, Yang J, Chen C, Zhang X, Wang O, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99:126–133
Benedict M, Pigford TH (1957) Nuclear chemical engineering. McGraw-Hill, New York
Bayramoglu G, Arica MY (2017) Polyethylenimine and tris(2-aminoethyl) amine modified p(GA–EGMA) microbeads for sorption of uranium ions: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 312:293
Bagherifam S, Lakzian A, Ahmadi SJ, Rahimi MF, Halajnia A (2010) Uranium removal from aqueous solutions by wood powder and wheat straw. J Radioanal Nucl Chem 283:289–296
Mellah A, Chegrouche S, Barkat M (2007) The precipitation of ammonium uranyl carbonate (AUC): thermodynamic and kinetic investigations. Hydrometallurgy 85(2–4):163–171
Sodayea H, Nisanb S, Poletikoc C, Prabhakara S, Tewaria PK (2009) Extraction of uranium from the concentrated brine rejected by integrated nuclear desalination plants. Desalination 235:9–32
Donia AM, Atia AA, Moussa MM, Sherif AM, Magied MO (2009) Removal of uranium(VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296:434–441
Jyothi A, Rao GN (1990) Solvent extraction behaviour of lanthanum (III), cerium (III), europium (III), thorium (IV) and uranium(VI) with 3-phenyl-4-benzoyl-5-isoxazolone. Talanta 37:431–433
Prasadaao TR, Metilda P, Gladis JM (2006) Preconcentration techniques for uranium(VI) and thorium (IV) prior to analytical determination—an overview. Talanta 68(4):1047–1064
Kumar JR, Kim JS, Lee JY, Yoon HS (2011) A brief review on solvent extraction of uranium from acidic solutions. Sep Purif Rev 40(2):77–125
Stucker V, Ranville J, Newman M, Peacock A, Cho J, Hatfield K (2011) Evaluation and application of anion exchange resins to measure groundwater uranium flux at a former uranium mill site. Water Res 45(16):4846–4876
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Bhatti HN, Bajwa II, Hanif MA, Bukhari IH (2010) Removal of lead and cobalt using lignocellulosic fiber derived from Citrus reticulata waste biomass. Korean J Chem Eng 27:218–227
Boddu VM, Abburi K, Talbott JL, Smith ED (2003) Removal of Cr(VI) from wastewater using a new composite chitosan biosorbent. Environ Sci Technol 37:4449–4456
Chojnacka K (2010) Biosorption and bioaccumulation—the prospects for practical applications. Environ Int 36(3):299–307
Nadeem R, Hanif MA, Mahmood A, Jamil MS, Ashraf M (2009) Biosorption of Cu (II) ions from aqueous effluents by blackgram bran (BGB). J Hazard Mater 168(2–3):1622–1625
Bai J, Wu X, Fan F, Tian W, Yin X, Fan F, Li Z, Tian L, Qin Z, Zhao L, Guo J (2012) Biosorption of uranium by magnetically modified Rhodotorula glutinis. Enzyme Microb Technol 51:382
Li Q, Liu Y, Cao X, Pang C, Wang Y, Zhang Z, Liu Y, Hua M (2012) Biosorption characteristics of uranium(VI) from aqueous solution by pummelo peel. J Radioanal Nucl Chem 293:67–73
Bhatti HH, Hamid S (2013) Removal of uranium(VI) from aqueous solutions using Eucalyptus citriodora distillation sludge. J Environ Sci Technol 11:813–822
Bhat SV, Meloa JS, Chaugule BB, D’Souza SF (2008) Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Wang F, Tan L, Liu Q, Li R, Li Z, Zhang H, Hu S, Liu L, Wang J (2015) Biosorption characteristics of Uranium (VI) from aqueous solution by pollen pini. J Environ Radioact 150:93
Bayramoglu G, Akbulut A, Acıkgoz-Erkaya I, Yakup Arica MY (2017) Uranium sorption by native and nitrilotriacetate-modified Bangia atropurpurea biomass: kinetics and thermodynamics. J Appl Phycol 30(1):1–13
Gok C, Aytas S (2009) Biosorption of uranium(VI) from aqueous using calcium alginate beads. J Hazard Mater 168:504
Arica MY, Bayramoglu G (2016) Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chem 310:711
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: modification into amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater 226:117
Kausar A, Bhatti HN, MacKinnon G (2013) Equilibrium, kinetic and thermodynamic studies on the removal of U(VI) by low cost agricultural waste. Colloids Surf B 111:124
Anirudhan TS, Bringle CD, Rijith EtS (2009) Removal of uranium(VI) from aqueous solution and nuclear industry effluents using humic acid-immobilized zirconium-pillared clay. Desalin Water Treat 12:16
Ahmed SH, El Sheikh EM, Morsy AMA (2014) Potentiality of uranium biosorption from nitric acid solutions using shrimp shells. J Environ Radioact 134:120
Saleem N, Bhatti HN (2011) Adsorptive removal and recovery of U(VI) by citrus waste biomass. BioResources 6:2522
Šabanović E, Muhić-Šarac T, Nuhanović M, Memić M (2018) Biosorption of uranium(VI) from aqueous solution by Citrus limon peels: kinetics, equlibrium and batch studies. J Radioanal Nucl Chem 319:425–435
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
Kuhnel S, Schols HA, Gruppen H (2011) Aiming for the complete utilization of sugar-beet pulp: examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol Biofuels 4:14
Dronnet VM, Renard CMGC, Axelos MAV, Thibault JF (1996) Characterisation and selectivity of divalent metal ions binding by citrus and sugar-beet pectins. Carbohydr Polym 30:253–263
Dronnet VM, Renard CMGC, Axelos MAV, Thibault JF (1997) Binding of divalent metal cations by sugar-beet pulp. Carbohydr Polym 34:73–82
Pehlivan E, Yanık BH, Ahmetli G, Pehlivan M (2008) Equilibrium isotherm studies for the uptake of cadmium and lead ions onto sugar beet pulp. Bioresour Technol 99:3520–3527
Reddad Z, Gerente C, Andres Y, Ralet MC, Thibault JF, Le Cloirec P (2002) Ni (II) and Cu (II) binding properties of native and modified sugar beet pulp. Carbohydr Polym 49:23–31
Mata YN, Blázquez ML, Ballester A, González F, Muñoz JA (2009) Sugar-beet pulp pectin gels as biosorbent for heavy metals: preparation and determination of biosorption and desorption characteristics. Chem Eng J 150(2–3):289–301
Mata YN, Blázquez ML, Ballester A, González F, Muñoz JA (2010) Studies on sorption, desorption, regeneration and reuse of sugar-beet pectin gels for heavy metal removal. J Hazard Mater 178(1–3):243–248
Castro L, Blázquez ML, González F, Muñoz JA, Ballester A (2017) Biosorption of Zn (II) from industrial effluents using sugar beet pulp and F. vesiculosus: from laboratory tests to a pilot approach. Sci Total Environ 598:856–866
Pehlivan E, Cetin S, Yanık BH (2006) Equilibrium studies for the sorption of zinc and copper from aqueous solutions using sugar beet pulp and fly ash. J Hazard Mater B 135:193–199
Altundogan HS, Arslan NE, Tumen F (2007) Copper removal from aqueous solutions by sugar beet pulp treated by NaOH and citric acid. J Hazard Mater 149:432–439
Khan MH, Warwick P, Evans N (2006) Spectrophotometric determination of uranium with arsenazo-III in perchloric acid. Chemosphere 63:1165–1169
Lagergren SY (1898) Zur Theorie der sogenannten Adsorption gelöster Stoffe, Kungliga Svenska Vetenskapsakad. Handlingar 24(4):1
Zhou LM, Shang C, Liu ZR, Huang GL, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366:165–172
Sureshkumar MK, Das D, Mallia MB, Gupt PC (2010) Adsorption of uranium from aqueous solution using chitosan-tripolyphosphate (CTPP) beads. J Hazard Mater 184:65–72
Limin Z, Zhiwei H, Taian L, Yuyan J, Zhirong L, Adesoji AA (2015) Biosorption of uranium(VI) from aqueous solution using phosphate-modified pine wood sawdust. J Radioanal Nucl Chem 303:1917–1925
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451
Weber W Jr, Morris JC (1963) Kinetics of adsorption on carbon from solutions. J Sanit Eng Div 89:31
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1362
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem US 57(4):385
Tempkin MI, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR 12:327
Babaeivelni K, Khodadoust AP (2013) Adsorption of fluoride onto crystalline titanium dioxide: effect of pH, ionic strength, and co-existing ions. J Colloids Interface Sci 394:419–427
Meenaa AK, Mishra GK, Rai PK, Rajagopal C, Nagar PN (2005) Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J Hazard Mater B 122:161–170
Wahab MA, Jellali S, Jedidi N (2010) Amonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour Technol 101:5070–5075
Ahmed MJK, Ahmaruzzaman M (2016) A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J Water Process Eng 10:39
Malekbala MR, Hosseini S, Kazemi YS, Soltani SM (2012) The study of the potential capability of sugar beet pulp on the removal efficiency of two cationic dyes. Chem Eng Res Des 90(5):704–712
Iqbal M, Saeed A, Zafar SI (2009) FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J Hazard Mater 164(1):161–171
Arief VO, Trilestari K, Indraswati N, Ismadji S (2008) Recent progress on biosorption of heavy metals from liquid using low cost adsorbents: characterization, biosorption parameters and mechanism studies. Clean Soil Air Water 36(12):937–962
Hassan ML, Kassem NF, Abd El-Kader AH (2010) Novel Zr (IV)/sugar beet pulp composite for removal of sulfate and nitrate anions. J Appl Polym Sci 117:2205–2221
Feng N, Guo X, Liang S (2009) Adsorption study of copper (II) by chemically modified orange peel. J Hazard Mater 164:1286
Schiewer S, Balaria A (2009) Biosorption of Pb2+ by original and protonated citrus peel: equilibrium, kinetics, and mechanism. Chem Eng J 146:211
Akar ST, Yilmazer D, Celik S, Balk YY, Akar T (2015) Effective biodecolorization potential of surface modified lignocellulosic industrial waste biomass. Chem Eng J 259:286–292
Yi Z, Zhu JYM, Chen H, Wang F, Liu X (2017) Biosorption characteristics of Ceratophyllum demersum biomass for removal of uranium(VI) from an aqueous solution. J Radioanal Nucl Chem 313(1):19–27
Yi Z, Yao J, Wang F, Chen H, Liu H, Yu C (2012) Removal of uranium(VI) from aqueous solution by apricot shell activated carbon. J Radioanal Nucl Chem 295:2029–2034
Zou W, Zhao L (2012) Removal of uranium(VI) from aqueous solution using citric acid modified pine sawdust: batch and column studies. J Radioanal Nucl Chem 292:585–595
Nour TAG, El-Chaghaby GA (2014) Biosorption for metal ions removal from aqueous solution: a review of recent studies. Int J Latest Res Sci Technol 3(1):24–42
Kostić M, Radović M, Mitrović J, Antonijević M, Bojić D, Petrović M, Bojić A (2014) Using xanthated Lagenaria vulgaris shell biosorbent for removal of Pb(II) ions from wastewater. J Iran Chem Soc 11:565
Boota R, Bhatti HN, Hanif MA (2009) Removal of Cu(II) and Zn(II) using lignocellulosic fiber derive from Citrus reticuolate (Kinnow) waste biomass. Sep Sci Technol 44:4000–4022
Li F, Li D, Li X, Liao J, Li S, Yang J, Yang Y, Tang J, Liu N (2016) Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem Eng J 284:630–639
Wang X, Zhu G, Guo F (2013) Removal of uranium(VI) ion from aqueous solution by SBA-15. Ann Nucl Energy 56:151–157
Ortaboy S, Atun G (2014) Kinetics and equilibrium modeling of uranium(VI) sorption by bituminous shale from aqueous solution. Ann Nucl Energy 73:345–354
Ofomaja AE (2010) Intraparticle diffusion process for lead (II) biosorption onto mansonia wood sawdust. Bioresour Technol 101:5868
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897
Gonte R, Balasubramanian K (2013) Heavy and toxic metal uptake by mesoporous hypercrosslinked SMA beads: isotherms and kinetics. J Saudi Chem 20(1):579–590
Kannamba B, Reddy KL, Apparao BV (2010) Removal of Cu (II) from aqueous solutions using chemically modified chitosan. J Hazard Mater 175:935–948
Kumar J, Balomajumder C, Mondal P (2011) Application of agro based biomasses for zinc removal from wastewater—a review. Clean Soil Air Water 39:641
Bayramoglu G, Yakup MA (2016) Amidoxime functionalized Trametes trogii pellets for removal of uranium(VI) from aqueous medium. J Radioanal Nucl Chem 307(1):373–384
Al-Masri MS, Amin Y, Al-Akel B, Al-Naama T (2010) Biosorption of cadmium, lead, and uranium by powder of poplar leaves and branches. Appl Biochem Biotechnol 160:976–987
Horsfall M, Spiff AI (2005) Effect of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (wild cocoyam) biomass. Electr J Biotechnol 8:162–169
Gupta NK, Sengupta A, Gupta A, Sonawane JR, Sahoo H (2018) Biosorption-an alternative method for nuclear waste management: a critical review. J Envrion Chem Eng 6(2):2159–2175
Bayramoglu G, Akbulut A, Acıkgoz-Erkaya I, Arica MY (2017) Uranium sorption by native and nitrilotriacetate-modified Bangia atropurpurea biomass: kinetics and thermodynamics. J Appl Phycol 30:649
Khan SA, Rehman R, Khan MA (1995) Adsorpton of chromium (III), chromium (VI) and silver (I) on bentonite. Waste Manag 15:271–282
Gok C, Turkozu DA, Aytas S (2011) Removal of Th(IV) ions from aqueous solution using bi-functionalized algae-yeast biosorbent. J Radioanal Nucl Chem 287:533–541
Donat R (2009) The removal of uranium(VI) from aqueous solutions onto natural sepiolite. J Chem Thermodyn 41:829–835
Prashant R, Balasubramanian K, Gonte RR (2014) Uranium (VI) remediation from aqueous environment using impregnated cellulose beads. J Environ Radioact 136:22–29
Nazal MK, Al-Bayyari M, Khalili FI (2019) Salvadora Persica branches biomass adsorbent for removal of uranium(VI) and thorium (IV) from aqueous solution: kinetics and thermodynamics study. J Radioanal Nucl Chem 321(3):985–996
Li X, Li F, Jin Y, Jiang C (2015) The uptake of uranium by tea wastes investigated by batch, spectroscopic and modeling techniques. J Mol Liq 209:413
Nie X, Dong F, Liu M, Sun S, Yang G, Zhang W, Qin Y, Ma J, Huang R, Gong J (2016) Removel of uranium from aqueous solutions by spirodela punctata as the mechanism of biomineralization. Proc Environ Sci 31:382
Yu J, Wang J, Jiang Y (2017) Removal of uranium from aqueous solution by alginate beads. Nucl Eng Tecnol 49(3):534–540
Aly Z, Luca V (2013) Uranium extraction from aqueous solution using dried and pyrolyzed tea and coffee wastes. J Radioanal Nucl Chem 295:889–900
Das D, Sureshkumar MK, Koley S, Mithal N, Pillai CGS (2010) Sorption of uranium on magnetite nanoparticles. J Radioanal Nucl Chem 285:4477
Shuibo X, Chun Z, Xinghuo Z, Jing Y, Xiaojian Z, Jingsong W (2009) Removal of uranium(VI) from aqueous solution by adsorption of hematite. J Environ Radioact 100:162–166
Wang G, Wang X, Chai X, Liu J, Deng N (2010) Adsorption of uranium(VI) from aqueous solution on calcined and acid-activated kaolin. Appl Clay Sci 47:448–451
Fasfous II, Dawoud JN (2012) Uranium (VI) sorption by multiwalled carbon nanotubes from aqueous solution. Appl Surf Sci 259:433–440
Song S, Huang S, Zhang R, Chen Z, Wen T, Wang S, Hayat T, Alsaedi A, Wang X (2017) Simultaneous removal of U(VI) and humic acid on defective TiO2-x investigated by batch and spectroscopy techniques. Chem Eng J 325:576–587
Incili GK, Aycik GA (2014) Adsorption thermodynamic and desorption studies of U(VI) on modified silica gel (SiAPMS-HL). J Radioanal Nucl Chem 302:79–85
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nuhanović, M., Grebo, M., Draganović, S. et al. Uranium(VI) biosorption by sugar beet pulp: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 322, 2065–2078 (2019). https://doi.org/10.1007/s10967-019-06877-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06877-z