Abstract
A process hybridisation has been conceptualised in a three-stage process (a) Complexation, (b) Ultrafiltration (UF), and (c) Resin bed treatment. The system demonstration with nuclear plant generated waste was performed effectively by using a 5 L lab scale experimental capacity set-up. In complexation stage, water soluble polymers viz., Polyethylene imine (0.1%), Polyvinyl alcohol (0.1%), were used as the complexing agents either individually or in combination and around 0.12% total metal ions [that includes Uranium 1000 ppm, and other metals, pH-5.6] has been passed through UF membrane unit followed by a polyacrylamidehydroxamate resin bed, where outlet concentration was found to be in BDL level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Process Hybridisation (PH) is a combination of available processes for treatment of aqueous waste containing valuable and toxic metal ions. Hybrid process are gaining importance and has several advantages [1,2,3,4]. In the present study the hybridisation process is developed conceptually which involves three stage namely, complexing between metal with water-soluble polymer, Ultrafiltration to separate out the macromolecules, polishing of the permeate through a solid phase resin bed to separate any residual metal ions. The combination of the process steps makes the process selective, provides flexibility in design, each step has more efficiency and can be run for a longer period of time as the load on any one process is reduced.
Nuclear energy has emerged as a clean source [5] and the growth of nuclear science and technology has been significant. Aqueous nuclear waste contains several toxic metals, which are chemically and radiologically harmful. These wastes if disposed untreated will pose danger to living world. Nuclear industry produces (aqueous effluent) waste starting from mining, milling, fuel fabrication, operation of reactors to reprocessing of spent fuels [6,7,8].
The aqueous waste contains spectrum of elements; like uranium U(VI) with iron Fe(III), copper Cu(II), strontium Sr(II), aluminium Al(III), calcium, magnesium other transition metal ions, lanthanides, alkali metal etc., all of which has its source in the original ore. On investigating the nature of effluent and process of treatment; it was found that aqueous effluent contained several dissolved metal ions, colloidal particles, and radiotoxic valuable elements [9].
Various treatment methods can be deployed [10, 11] for the treatment of toxic metals which includes precipitation, adsorption, ion exchange, reverse osmosis (RO) to name a few. Some conventional processes viz., precipitation have disadvantages of consumption of other chemicals. Few operational procedures, viz., RO etc. Demands high pressure and capacity pumps etc. Hence, design criterion needs to be case specific; e.g., precipitation is effective for high concentration/metal ion loading even in percentage level contamination while processes like solvent extraction is suitable for ppm level concentration. Combination of such conventional processes may be utilised for removal of contaminants.
In the present study, actual waste generated during Uranium refining process has been treated with the PH technique. The PH is demonstrated with waste obtained from two sources generated in a Uranium refining facility. Initial study of polymer metal complexing was quantified using UV/Vis spectroscopy with synthetic solution and then ultrafiltration parameters was optimised on a 5 L scale. The initial study with synthetic solution was performed with representative metals from lanthanide Gd(III), transition Cu(II), alkaline earth metal Sr(II) and actinide U(VI) series. The sorption study with individual metal and PHA resin was carried out and metal resin was characterised by EDXRF and XRD. The process hybridisation was initially performed on a simulated solution containing mixed metal solution was used and is reported elsewhere [12].
Theory
The process is conceptualised with 3 stages, (a) Complexation and removal of any sediments formed by prefilter, (b) Ultrafiltration to remove any coagulates formed, (c) Sorption for final refinement of the effluent to remove even trace amount of metals.
Complexation
A polymer-metal complex is a coordination complex between a ligand function anchored on a polymer matrix that contains co-ordinating groups or atoms mainly N, O, S and a metal ion by a coordinate bond [13, 14]. Multivalent metal ions have affinity for macromolecular entity and if specific functional moieties are present then the metal ions are more prone to form stable chelate complexes. Figure 1 shows the schematic for the formation of metal polymer complex.
Ultrafiltration
Ultrafiltration (UF) is a pressure-driven purification process that separates particulate matter from soluble compounds using an ultrafine membrane media. Ultrafiltration is an excellent separation technology for desalination pre-treatment, reverse osmosis pre-treatment, and wastewater reclamation, as well as for producing potable water [15,16,17,18].
Ultrafiltration (UF) is the process of separating extremely small particles and dissolved molecules from fluids. The primary basis for separation is molecular size—particles ranging from 1000 to 1,000,000 molecular weight are retained by ultrafiltration membranes. The nature of ultra-filtration is basically to treat colloids. The efficiency of a ultrafiltration set up is a trade off between the metal retention and the rejection ratio. From the concentrations of permeate and retentate the retention values are calculated as follows.
where Cp = concentration of metal ion in permeate and Cf is the concentration of metal ion in feed.
Solid phase extraction process
Sorption is a combination of both adsorption (which means accumulation of a substance at the surface of a solid or a liquid) and absorption (which means assimilation of a substance within the bulk of a solid or liquid). The equilibrium relationship is described by adsorption isotherms [19].
Reagents and samples collection
The water soluble polymers used were all analytical grade. Polyethyleneimine with average molecular weight 25,000. Polyvinyl Alcohol with average molecular weight 100,000. Polyacrylic acid with average molecular weight 125,000 were used as polymer. Solutions were prepared with deionized water using Millipore deionizer (Millipore’s milli-Q-ZRQSOP030).
The demonstration of the PH was carried out with 2 waste sources from nuclear refining plant.
Experimental procedure
The RAD-waste was diluted with demineralised water to maintain a total metal loading of around 1200 ppm and volume was made up to 5 L in the reaction tank as shown in figure 6.2 pH adjusted with NaOH to maintain the pH at 5–6. From 1% polymer stock solution, measured volume added to make a 0.1% 5 L polymer solution in the reaction tank. The mixture mixed for 1 h at 120 rpm for equilibration with the stirrer. The equilibrated solution is then passed through a polypropylene prefilter first and then through the UF membrane by a booster pump. The UF membrane is Hi-tech 1812 capillary type polysulphone based membrane with a pore size of 0.02 micron. The operational transmembrane pressure ranges from 5 to 25 psi and the maximum flow rate is 2gpm. The prefilter separates any impurities and also non-homogenised complex if any. Sample is collected at the prefilter outlet. PEI and PVA polymers are used sequentially and samples were collected on every step. Two polymers based on their anionic and cationic behaviour were chosen for the study of a multicomponent separation and the effectiveness of each polymer is studied in two separate experiments with same waste but different variations. Permeate sample from UF outlet after 1 h of recirculation. The samples are analysed in ICP-AES.
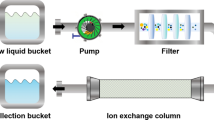
The permeate stream of UF was passed through the resin bed and the final outlets/samples were collected. A resin bed is a fixed bed with glass column having 3.8 cm diameter, 46 cm length loaded with 40 g polyacrylamide hydroxamate (PHA) resin and solution passed at a flow rate of 15 mL/min. The column outlet was also analysed in ICP-AES. Exhaustion of the resin bed was visible through transparent glass which is attributed to the special chromatographic properties of PHA resin. Figure 2 shows the experimental set-up for the hybrid process.
The demonstration of the PH was carried out with 2 waste sources from nuclear refining plant.
Lab waste from quality control lab of refining plant
Two lab waste sources from U refining facility were collected. Both the lab waste contains around U-9-10 g/L, along with other metals like Sr, Cu, Fe, Mg, Mn etc. free acid 1.2 N–1.5 N. The elemental characterisations of the waste solutions were carried out in ICP-AES. Solutions were diluted with demineralised water to bring the U concentration in the range of 1000 ppm. The Solutions were diluted about 10 times. Total metal ion concentration in the final diluted Solutions were around 1200 ppm. The pH of the solution was maintained at 5–6 with analytical grade NaOH. 30 g of NaOH flakes were added to the 5 L diluted solution. Currently, this waste solution is being treated by chemical precipitation method along with other waste in the effluent treatment plant. Figure 3 shows the polymer induced ultrafiltration set up for lab waste solution.
Results and discussion
Metal polymer complex
UV studies: 10 mL of 0.1% polymer solution taken in beaker. To this solution metal solution was added in increasing volume. The concentration of metal solution taken was 1000 ppm. The resulting solution was mixed and tested in UV- Shimadzu double beam spectrometer using matched 10 mm quartz cells. The absorbance when plotted against volume of solution/metal loading. The absorbance of a series of mixture is measured at a suitable wavelength. The plot will give a maximum and the method is applicable which obeys Beer’s law. Table 1 shows the uptake of metals by two polymers PEI and PVA.
The interaction between metal ions and water-soluble polymer in solution is mainly the result of
-
a.
electrostatic forces
-
b.
coordination bonds
-
c.
covalent bonds
-
d.
some weaker interactions like trapping of respective metal ions in the polymeric bulk.
The bond stability depends on the type of interaction while the uptake is depends on the size and electro-negativity of the metal ions also. The differential uptake of metal ions is basically due to the nature of interaction. Cu(II) forms a predominantly co-ordinate bond with the lone pair of the nitrogen in PEI while forms a stable six membered ring type complex with PVA and hence uptake of Cu(II) by PVA is more. Due to the bulky size and f orbital contraction Gd(III) does not form a stable bond with the PVA polymer, while with the flexible polymer chain of PEI owing to its high co-ordination umber it is well entrapped. Sr(II) lacking d orbital does form stable complexes. The higher uptake of Sr(II) by PEI is due to the electrostatic force that may be existing. U(VI) having vacant f orbital shows good complex stability but owing to its bulky size the uptake is less.
Ultrafiltration study
The experiments were carried out to study the retention of metals by ultrafiltration on complexing it with polymers PEI, PVA. Effects of polymer to metal ratio variation on retention, were studied for different combination of metals and polymers. The performance of the membrane is a trade off between retention values and rejection ratios. Figure 4 shows the comparative retention of the metal polymer complexes and Fig. 5 shows the relative values of rejection ratios.
The retention trend was U(VI) ≫ Gd(III) > Cu(II) > Sr(II). With PVA the Trend observed was U(VI) ≫ Cu(II) > Gd(III) > Sr(II). With both the polymers Sr(II) shows a very low retention value less than 10% indicating a weak metal polymer bond. U(VI) on the other hand showed best performance with retention value exceeding 50% indicating a strong metal polymer bond. With PVA the retention of Cu(II) was more than Gd(III) due to better covalent bonding between Cu(II) and PVA forming a six membered ring.
It is observed that when the L/M is 10 we get the best rejection ratio. With the increase in concentration of metals the rejection ratio decreases.
Sorption of metals with PHA resin
The qm values (as Langmuir sorption is restricted to monolayer formation) of Langmuir model is 125 mg/g of PHA for Gd(III) while 111 mg/g of PHA for U(VI), 91 mg/g for Cu (II) and 11.4 mg/g for Sr(II). The uptake comparison for the four metals is Gd > U > Cu > Sr [19].
Metal PHA resin characterisation through EDXRF and XRD
The EDXRF characterisation is shown in Figs. 6,7,8, and 9.
A Jordon Valley energy dispersive X-ray florescence spectrophotometer, equipped with a Rh-X-ray source and Si(Li) semiconductor detector was used for the study. The samples were placed on a cylindrical plastic device of about 2 cm in height and 3 cm in internal diameter using an X-ray chemical resistant film. X-ray fluorescence (XRF) spectrometry is an instrumental technique used for multi-element analysis. When loaded individually, blank resin didn’t show presence of any metal, whereas other metal laden resin showed corresponding concentration of U, Gd, Cu and Sr metal ions in their respective samples. XRD Result for metal PHA loaded resin is shown in Fig. 10 a–e.
The XRD patterns of unloaded resin is basically amorphous but at 2Ɵ = 25° showed a raised crest, attributed to cross-linking; otherwise it is amorphous in nature. The loaded metal complexes were showed the crystalline region that shifted from the original virgin resin, e.g.,, for copper (21°) followed by uranium (20°) and Gadolinium (23.5°). However, strontium does not show a peak, instead a broad region (20–25)° which is attributed to its non-complexing nature.
PH with Rad-waste solution
Table 2 gives the feed concentration of the waste stream outlet concentration at various collection points for the PH on lab waste 1. Table 3 gives the results for Labwaste 2.
It is observed from Table 2 that the U concentration decreases by 97.9% during PIUF stage itself. A separation factor of 3.67 is observed for U(V) with respect to all other metals. Cu(II), Fe(III), Cr(III), concentration also reduces by 66.7%. The Al(III) concentration decreases by a significant 75% and that of Ni(II) is reduced by 50%. An overall 86.1% reduction in total metal concentrations observed in the first stage of hybridisation i.e. PIUF with a considerable reduction in metal content in the first primary stage of complexation itself. Sr(II) concentration is not decreased as Sr does not form strong covalent bonds with PEI like U(VI) and Cu(II). Mg(II) and Mn(II) also does not show a considerable decrease in concentration. Hence, the probable articulated fact can be envisioned as the complex cluster formed between metal and PEI is large enough to be separated at the primary filtration stage. In the second stage of hybridisation, the metal polymer complex is passed through the UF membrane. In the third and the final stage i.e. the solid phase extraction stage, all metal ions have been reached beyond BDL level and the water becomes suitable for reuse. The water is qualified for safe disposal or recycling back to the main process purpose. To qualify for the said purpose the BDL value is less than 1 ppm for all metal ions. This can also be used in plant as water is free of metal ions and also the pH is near to neutral. This demonstration also shows that even with use of only a single polymer i.e. PEI the PH is effective in removing all metal ions.
The second lab waste source is same as that of the first lab waste but from a different lot and hence shows different concentration of the elements. The second Lab-waste was treated first with PVA. PEI was found to be efficient in removing metal ions from waste solution. The second lab waste was treated with PVA polymer to study the efficiency of the polymer. 1000 ppm PVA was added as the complexing agent. It was observed that in the primary filter outlet i.e. after the primary filter 3% of U(VI) was retained. Metal retention at the primary outlet is low for all metals except for Al(III), Cr(III), Fe(II), Mn(II), Ni(II) around 50% reduction was observed. Earlier also we have observed these particular metals showed significant retention. This is due to the good bonding nature of these metals due to their favourable electronic structure. Cu(II) and Sr(II) concentration did not show any retention. The significant drop in metal concentration observed in first case where PEI was added is due to the flocking nature of PEI polymer. The PVA complexed metal solution when passed through UF membrane the U concentration was almost decreased by 50 from the previous stage and Cu(II) showed a 29% retention. Other metals did not show any significant decrease in concentration and thus not retained in this stage. From the experimental data it can be postulated that the U(VI) complex with PVA can be arrested by UF membrane and not primary filter. While the other metals were arrested in the first stage itself. This may be due to formation of precipitate at the said pH with PVA with the other metals and not U(VI). Addition of PEI into the solution and then passing through UF removed almost 97% of U(VI), Al(III), Cr(III) and Fe(II). Cu(II) was removed by 40%. Sr(II) did not show much retention even with PEI due to non-availability of “d” orbital as it belongs to alkaline earth metal. At this stage of UF with a combined polymer we observed almost 95% removal of all combined metals. Addition of 200 ppm of PAA did not have any effect on any of the metals. The final polishing with resin bed removed almost all metals and the water obtained was suitable for domestic use and can be safely recycled to plant operation. PAA forms complex with metal ions. The fact that in the 4th stage no significant retention was observed is due to the fact that most of the metals were complexed in the previous stages.
The major disadvantage of UF is the clogging of the pores over a time. To regenerate a Uranium metal loaded UF membrane it was washed with 1 N HCl solution for 1 h. The activity was found to reduce by 95% ensuring through clean up of the membrane. On reusing also, we obtained a flux near to a fresh membrane.
Figure 11 shows the colour change of the waste before and at different stages of treatment. Clear solution at the equilibrium stage indicates complete removal of metal ions.
Conclusion
Two waste streams from U refining plant were used for the demonstration of the conceptualised PH. The demonstration found that the hybrid process is useful and can produce water of domestic use quality even when the starting concentration of U(VI) in it was around 1000 ppm along with the presence of other metals in the transition, alkali earth metal and metals from various other groups in the periodic table were present. The study shows complexing initially with PEI removes the metal in the primary stage itself and hence the load on the subsequent UF stage and solid phase extraction stage decreases significantly. Nearly 100% removal of the metals from the aqueous waste was achieved using the hybrid process. The process is suitable at an pH of 5–6 and the fact that the pH can be easily adjusted with minimum quantity of NaOH is encouraging. The use of 3 stages diminishes the loading on any one stage and UF and resin bed can be used for longer life cycles. Hence reduction/minimisation in operation and waste immobilisation cost. Reusability of process-recovered treated water can be returned back to main stream metal extraction operation and thus ensuring water quality, recovery and reusability with safe handling by removal of chemical and radioactive metals, as well as wellness of heath monitoring and inspection. The regeneration of metals and the life cycle study for the PH system and techno-economic viability are in the scope for future study. Secondary waste generated from the process will be the exhausted resin and filters which may be disposed of as very low-level radioactive waste as the arrested metal can be easily recovered before disposal. The volume of waste generated per year will be in the tune of 500 L, which includes spent resin and UF filter along with other accessories.
References
Juang R-S, Shiau R-C (2000) J Membr Sci 165:159–167
Ahmadi S, Tseng LK, Batchelor B, Koseoglu SS (1994) Sep Sci Technol 29(18):2435–2450
Geckeler KE, Volchek K (1996) Environ Sci Technol 30(3):725–734
Llanos J, Perez A, Ca˜nizares P (2009) J Membr Sci 341:37–45
Gupta R (2010) Proceedings of the XI international seminar on mineral processing technology (MPT-2010)
Efremenkov VM (1989) Radioactive waste management at nuclear power plants. IAEA Bull 31(4):37–42
Jeswani H, Khelurkar N (2015) (ICTSD-2015), Feb. 04–06, 2015, Mumbai, India
Handling and Processing of Radioactive Waste from Nuclear Applications (2001) Technical reports series no. 402. International Atomic Energy Agency, Vienna
Abdel Rahman RO, Ibrahium HA, Hung Y-T (2011) Water 3:551–565
Bhagat M, Burgess JE, Paula A, Antunes M, Whiteley CG, Duncan JR (2004) Miner Eng 17(7–8):925–932
Shek TH, Ma A, Lee VKC, McKay G (2009) Chem Eng J 146(1):63–70
Singha M, Pal S, Meena SS (2019) Process hybridisation for nuclear effluent treatment. In: 2019, 64th DAE solid state physics symposium December 18–22. Indian Institute of Technology Jodhpur
Somasundaran P, Nagaraj DR (1984) Chemistry and applications of chelating agents in flotation and flocculation. Minerals and primary materials program of the National Science Foundation
Bisset W, Jacobs H, Koshti N, Stark P, Gopalan A (2003) React Funct Polym 55(2):109–119
Sikdar SK, Grosse D, Rogut I (1998) J Membr Sci 151:75–85
Ca˜nizares P, Perez A, Camarillo R (2002) Desalination 144:279
Geckler K, Volchek K (1996) Environ Sci Technol 30:725
Barakat MA, Schmidt E (2010) Desalination 256:90–93
Singha M, Pal S, Chowdhury S, Hareendran KN, Sahu ML (2020) J Radioanal Nucl Chem 323:795–803
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singha, M., Pal, S., Chowdhury, S. et al. Removal of hazardous metals from nuclear effluent using a process hybridisation technique. J Radioanal Nucl Chem 326, 1271–1279 (2020). https://doi.org/10.1007/s10967-020-07341-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07341-z