Abstract
This study reports the surface interaction of the chemically modified marine unicellular cyanobacterium Synechococcus elongatus BDU130911 with uranium. The selective functional groups of the control (dead biomass) for binding with uranium in unicellular marine cyanobacteria were identified as carboxyl groups. The adsorption capacity of the biomass in a 1 mM uranium solution was found to be 92% in the control, 85% in the amine-blocked treatments, and 20% in the carboxyl-blocked treatments. The Langmuir isotherm provided a good fit to the data, suggesting a monolayer of uranium adsorption on all the tested biomass. The functional groups involved in the adsorption of uranium by the control and modified biomass were assessed by Fourier transform infrared spectroscopy, energy dispersive X-ray fluorescence and X-ray diffractive analysis. The results of this study identify, carboxyl groups as the dominant anionic functional group involved in uranium adsorption, which validates an ionic interaction between the biomass and uranium, a cationic metal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium occupies an important position in nuclear energy. This naturally occurring radionuclide contaminates the environment as a result of a variety of processes associated with the nuclear industry (Newsome et al. 2014). Finding an economic, sustainable bioremediation method for uranium contamination using microbial biomass is a challenging task for scientists across the globe (Hlihor et al. 2014).
Recovery or removal of heavy metals from the environment by living organisms through various physico-chemical interaction processes has been in vogue for the past two decades (Kyzas et al. 2013). Like other metals, biosorption of uranium from sea water, nuclear waste, and soils has recently gained the attention of nuclear scientists. There are several reports for recovery of uranium contamination using biomass in the forms of fungi (Liang et al. 2015), bacteria (Williamson et al. 2014), and algae (Lee et al. 2014). Although a number of microorganisms have potential for aiding in uranium remediation efforts, the combination of a large surface volume ratio, wide adaptability, and easy scalability from lab- to industrial-sized bioreactors suggests greater potential for cyanobacteria, the unique oxygen-evolving photosynthetic prokaryotes (Singh et al. 2017).
Marine cyanobacteria are considered to offer more advantages due to their high adaptability and applicability (Thangaraj et al. 2017). These organisms are also known to sequester radionuclides through an extracellular metal-binding process that involves ionic interaction, precipitation, complexation, co-ordination, etc. (Rashmi et al. 2013).
Metal adsorption to biomass occurs through interactions with functional groups present in proteins, lipids, and carbohydrates that compose the cell wall (Acharya and Apte 2013). To maximize the efficacy of the adsorption to biomass, it is important to identify the functional groups responsible for metal binding so that the biomass can be suitably modified. In this study, the surface of the marine cyanobacteria was chemically modified to elucidate the functional groups involved in uranium sequestration, suggesting new directions for cyanobacterial bioremediation.
Materials and methods
Chemicals
Analytical-grade chemicals were used throughout this study. Chemicals for biomass modifications and uranium estimation were from Sigma-Aldrich, USA. A filter-sterilized stock solution of uranyl acetate (Otto, USA) was used in the experiments.
Source of biosorbent
Synechococcus elongatus BDU130911, a unicellular marine cyanobacterium, was obtained from the repository of National Facility for Marine Cyanobacteria, Bharathidasan University, Tiruchirappalli, India. The criteria for selecting S. elongatus BDU130911 were its rapid growth and high efficacy of uranium adsorption as observed in our earlier studies (Rashmi et al. 2013). The organism was grown in ASN III marine synthetic medium (Rashmi et al. 2013) under white fluorescent light at an intensity of 20 µmol photon m2 s−1 with a 14:10 light: dark cycle at 25 ± 2 °C in a controlled culture room. All biosorption experiments were carried out in triplicates, and the data were expressed as the mean with SD using Origin 8 software, OriginLab Corp., Northampton.
Preparation of biosorbents
Dead biomass
Synechococcus elongatus BDU130911 was harvested by centrifugation (7000×g for 15 min) from the stationary phase of its growth (10th day). The pellet was immediately rinsed with distilled water, followed by a second centrifugation, and inactivated by heating with a few drops of 0.1 N NaOH in an oven at 60 °C overnight (Bai et al. 2010). The heat-killed biomass was then washed with deionized water to remove the alkali and again centrifuged at 7000×g. The dried pellet was used as the control biomass for the uranium biosorption studies.
Preparation of functional group-blocked biomass
To identify the predominant functional group(s) involved in uranium binding, carboxyl and amine groups of S. elongatus BDU130911 biomass, initially prepared as described for the control biomass, were discretely blocked following the methods described below.
Carboxyl group blocking
Dried heat-killed biomass (0.5 g dry weight) of S. elongatus BDU130911 (control biomass) was suspended in a mixture of 20 ml of anhydrous methanol and 2 ml of concentrated hydrochloric acid. The mixture was agitated on a rotary shaker at 125 rpm for 6 h (Kapoor and Viraraghavan 1997). The treated cell suspension was harvested by centrifuging at 7000×g for 5 min, washed with distilled water, and then dried. This treatment was referred to as the carboxyl-blocked biomass.
Amine group blocking
Similar to the above, dried heat-killed biomass (0.5 g dry weight) of S. elongatus BDU130911 (control biomass) was suspended in 10 ml of formaldehyde and 5 ml of formic acid and then agitated at 125 rpm for 6 h (Kapoor and Viraraghavan 1997). The resulting pellet was consecutively washed by centrifugation and dried as described earlier. This treatment was referred to as the amine-blocked biomass.
Collectively, the carboxyl-blocked and amine-blocked dead biomass treatments were referred to as modified biomass.
Uranium adsorption studies
To identify the efficient functional group(s) involved in uranium adsorption, experiments were carried out using control and modified biomass. Control and modified biomass samples (0.1 g dry weight) were suspended in 10 ml of uranium solution with concentrations viz. 0.1, 0.5, 1, 5, and 10 mM. The biomass and uranium mixtures were incubated on a shaker at 150 rpm and 37 °C. The supernatant was separated by centrifugation (12,000×g for 5 min) at a regular interval of 30 min for 2 h. A sensitive colorimetric method for the determination of residual uranium concentration was performed with the reaction of dibenzoylmethane with uranium (V1), described by Rashmi et al. 2013. The reagent was alcoholic, colorless, and stable containing 1% dibenzoylmethane dissolved in 95%, distilled ethyl alcohol. To 1 ml of residual uranium solution 0.5 ml of 1% dibenzoyl methane solution was added (mole ratio of reagent is 2:1). A yellow complex forms instantaneously and had a maximum absorbance at 395 nm.
The amount of adsorbed uranium (qmax) was calculated using a linearized form of Langmuir isotherm (Langmuir 1918) as follows:
where ‘qmax’ is the maximum metal adsorbed (mg g−1) and ‘b’ is the ratio of adsorption rate to the energy of adsorption.
Uranium adsorption analysis
Fourier transform infrared spectroscopy (FTIR)
To perform FTIR analysis, sample disks were prepared from both control and modified biomass incubated with and without uranium. Disks were made by mixing 5 mg of dry biomass with 150 mg of potassium bromide (KBr) and then pressed into tablet form (Kannan et al. 2013). Infrared spectra were recorded over the 4000–400 cm−1 region with a resolution of 0.2 cm (Perkin Elmer Spectrum GX FTIR, U.S.A.).
Energy dispersive X-ray fluorescence (EDXRF)
Uranium adsorption by the control and modified biomass of S. elongatus BDU130911 was characterized with an energy dispersive X-ray fluorescence (EDXRF) spectrometer (Xenemetrix, EX-6600SDD). The X-ray spectrometer had a silicon drift detector with a beryllium window at 150 eV resolutions, which detects the characteristic radiation emitted by uranium. A spectroscopy amplifier was connected to a PC-based multi-channel analyzer. An acquisition time of 2000 cps was maintained for the tested biomass (Omale et al. 2014).
X-ray diffraction (XRD)
The binding consistency of uranium adsorbed to homogenized cyanobacterial biomass was also analyzed by X-ray powder diffraction (XRD) using a high-precision, Bruker powder X-ray diffractometer (DS- advance, Germany) with a Ni filter and an exposure time of 2 h. The texture of the biomass was interpreted by its diffraction pattern recorded from 10 to 70, with the step length of 2θ being 0.02° (Acharya et al. 2009).
Results and discussion
The biosorption process involves the interaction of metallic ions with functional groups that make up the cell wall of certain microorganisms. This type of biosorbent consists of dead and metabolically inactive cells. Alkali-treated dead biomass of marine cyanobacteria is known to adsorb heavy metals, namely nickel, copper, zinc, cobalt (Goswami et al. 2014). Further, this biomass is reported to biodegrade a wide range of simple to complex compounds such as lignin, azo dye, and complex phenols (Singh et al. 2017) and to sequester radionuclides (Rashmi et al. 2013). Thus, the use of cyanobacteria as biosorbents provides a solution for two key issues: (i) increased use of chemicals and (ii) high energy costs of treatment processes.
The general goal for metal biosorption research is to identify biosorbents that can more effectively sequester heavy metals and provide a viable bioremediation technology (Ahad et al. 2017). The physical and chemical pretreatment process of biological material can affect properties of both the sorbate and biosorbent causing an increase in the biosorption rate (Newsome et al. 2014). Common physical treatments are heating, autoclaving, and freeze drying, whereas chemical treatments include the application of acids, alkalis, or organic chemicals, which results in modifications of specific functional groups (He and Chen 2014). The cell wall of cyanobacteria consists of polysaccharides, proteins and lipids, offering several functional groups, namely hydroxyl, carboxyl, and amino groups, which play a key role in the biosorption of cations from aqueous solutions (Acharya and Apte 2013). The objective of this study was to explicate the functional groups present on the surface of marine cyanobacterium S. elongatus BDU130911 and their roles in uranium adsorption.
Uranium biosorption experiments
The biosorption of heavy metal ions by microorganisms has often been observed to occur rapidly in two stages: the first stage is the transport of metals from aqueous solution to the surface of the adsorbent, and the second stage is the binding of the metals to available functional groups of adsorbent.
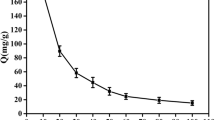
The results of uranium adsorption to control and modified biomass after 24 h of treatment with various uranium concentrations, namely 0.1, 0.5, 1, 5, and 10 mM, are shown in Fig. 1. The greatest percentage of uranium (92%) was found to be adsorbed to the control biomass incubated with 1 mM uranium, whereas amine-blocked biomass adsorbed 80% and carboxyl-blocked biomass adsorbed 20% of 1 mM uranium.
The higher proportion of uranium binding to the control biomass of S. elongatus BDU130911 may be due to the stronger negative charge on the biomass surface and associated slight alkalinity (Fig. 1). According to Dotto et al. (2012), binding sites for metal cations in biomass are more accessible above neutral pH (7.5–8.5). In contrast, at lower pH (< 6.0), the surface of cyanobacterial biomass acquires more H3O+ ions, which protonate amino groups to form \({\text{NH}}_{4}^{ + }\) and positively charge the surface, repelling metal cations. In other words, at an acidic pH, the cyanobacterial biomass surface is positively charged, and at a slight alkali pH, the surface is negatively charged, which is substantiated in the present study with uranium cations.
The present results (Fig. 1) also show a substantial decrease in uranium adsorption when carboxyl groups are blocked, denoting the predominance of negatively charged groups in the adsorption process. Less uranium adsorption are observed in the carboxyl group-blocked biomass compared to the control biomass, is similar to the findings of Xie et al. 2008, in which esterification of carboxyl groups through methanol treatment prevented uranium adsorption on the negative functional groups available on the biomass surface. Similar results have also been reported by Chen et al. (2007) for pronounced decrease in Cu2+ and Zn2+ binding after methanol esterification of biomass.
The reduction in uranium adsorption of the amine-blocked biomass compared to the control biomass is due to the formaldehyde treatment, which caused methylation of amino groups, reducing the number of positive charges on the biomass surface, thereby blocking \({\text{NH}}_{4}^{ + }\) groups (Bai et al. 2010). The methylation of amino groups made more carboxyl (negative) groups available on the biomass surface, facilitating the binding of uranyl cations to the surface. Overall, these results suggest that uranium adsorption to the biomass of S. elongatus BDU130911 is due to a strong electrostatic attraction that appears to be a selective process.
Adsorption isotherm analysis
The uranium adsorption process was assessed using the Langmuir isotherm, which was found to provide a good fit to the data and implies achievement of equilibrium in metal adsorption by the tested biomass. According to the Langmuir model, biosorption occurs at specific sites on the biomass surface, and once a metal ion occupies all the available binding site, no further adsorption occurs at that site, suggesting monolayer adsorption (Bulgariu and Bulgariu 2014). Biosorption data reveal that the rate of uranium adsorption in both the control and modified biomass was swift, attaining equilibrium within an hour, which indicates a good fit for the Langmuir adsorption isotherm (Table 1). The maximum metal biosorption capacity (qmax) estimated by this model was 208, 192 and 50 μgmg−1 dry weight, for control, amine-blocked and carboxyl group-blocked biomass, respectively (Table 1). The qmax value predicted from the Langmuir model is in agreement with the experimental absorption value. According to Akara et al. (2013), an initial rapid metal adsorption rate is due to the presence of instantaneous surface adsorption sites, and when these sites became gradually covered, the rate of adsorption attains a plateau. As the metal concentration increases, the adsorbent reaches a saturated state, and no further adsorption is observed. As explained by Kazy et al. (2009), high metal concentrations result in competition between the metal ions and available surface-binding sites, reducing the probability of metal binding. This explains the reduction in uranium adsorption observed in the present study at concentrations above 1 mM.
Adsorption analysis
FTIR of biomass pretreated with uranium
The cell surfaces of microorganisms consist of polysaccharides, proteins and lipids, offering several functional groups for the binding of heavy metal ions (Acharya and Apte 2013). The effects of the chemical treatments of S. elongatus BDU130911 biomass were evaluated according to the differences noted among the IR spectra. Careful scrutiny of FTIR spectra revealed the functional groups involved in the adsorption of uranium ions onto the cell wall of S. elongatus BDU130911. The characteristic troughs observed in the control biomass are at 1060–1065 cm−1 implying C–O stretching, at 3290 cm−1 implying –NH groups, and at 3575 cm−1 denoting –OH stretching. A strong intense peak at 1060 cm−1 confirms the presence of a carboxyl group in the control biomass (Fig. 2a).
In the FTIR spectrum of carboxyl-blocked biomass, the esterification of carboxyl groups is exhibited by a new shoulder at 1745 cm−1, and a corresponding broad trough from 1076 to 1078 cm−1, which implies a slight stretching of C–O group. These peak shifts seen in the carboxyl group-blocked biomass validate the esterification of carboxyl groups (Fig. 2b).
In the amine-blocked biomass, a major peak shift was observed from 3289 to 3783 cm−1, elucidating variation in the amino groups, which is in contrast to the control biomass, where a trough at 3290 cm−1 indicates –NH group. The peaks at 3289 to 3783 cm−1 are ascribed to the stretching vibration of N–H and O–H bands. Furthermore, a sharp bend in the amine-blocked biomass at 1394 cm−1 indicates the presence of methyl groups. Collectively, these changes in the amine group block biomass imply the methylation of amine groups. (Fig. 2c).
FTIR of biomass after uranium treatment
After treatment with uranium, new peaks at wave lengths of 905–930 cm−1 were seen in all the three biomass treatments (control, carboxyl-blocked, amine-blocked). These peaks are due to the asymmetric stretching vibration of \({\text{UO}}_{2}^{2 + } ,\) which corroborates the findings of Frost et al. (2006). These peaks are more intense in the control biomass than in the modified biomass, suggesting the higher efficiency of uranium adsorption by control biomass. Further, the trough associated with the main functional groups, shifted with uranium treatment in the control biomass, where peak variation was observed from 1060 cm−1 in Fig. 2a to 1081 cm−1 in Fig. 3a; and in the carboxyl group-blocked biomass peak shifts were noticed from 1076 cm−1 in Fig. 2b to 1085 cm−1 in Fig. 3b. These shifts suggest major participation of carboxyl groups in uranium sorption. In the amine-blocked biomass, there was a small variation of the peak at 3289 cm−1 (Fig. 2c) to 3298 cm−1 in Fig. 3c, indicating less participation of amine groups in uranium adsorption.
As suggested by Bai et al. (2010), the peak shifts of the carboxyl group occur because the methanol treatment not only caused the esterification of the carboxylic acid groups of S. elongatus BDU130911 but also dissolved some soluble components in the cell membrane, which reflects on the peak shift. According to Cecal et al. (2012), ion exchange is the principal mechanism for uranium sorption, and this is supported by our results, suggesting a high electrostatic attraction between the negatively charged anionic surface of the S. elongatus BDU130911 and cationic uranium. Changes seen in the FTIR spectral analysis explicitly endorse the contribution of functional groups of the cell wall in binding uranium, with carboxyl groups being more responsible for binding than amino groups.
EDXRF
Energy dispersive X-ray fluorescence (XRF) compositional maps reveal the elemental selectivity for uranium associated with the control and modified biomass of S. elongatus BDU130911. EDXRF analysis is based on the fact that the X-rays emitted from an ionized atom have energies that are specific to the element involved in the analysis. X-ray intensity is proportional to both the elemental concentration and the strength of the ionizing source (Acharya et al. 2009). Hence, for the qualitative and quantitative assessment of uranium in the biomass, analysis can be very helpful. In the present study, EDXRF analysis of the tested biomass displayed clear, identifiable peaks of UL X-rays, i.e., ULα1 and ULβ1, in a range of 13.5–17 keV, which corroborates the report of Acharya et al. (2012). To assess the quantity of uranium adsorbed, maximum intensities in terms of counts per second (CPS) were evaluated and found to be highest in the control biomass (600 cps), followed by the amine-blocked biomass (475 cps) and then the carboxyl-blocked biomass (275 cps) (Fig. 4a, b, c). Thus, the EDXRF peaks support the assessment of the biosorption experiments and further confirm the superiority of the control biomass to the modified biomass for uranium sequestration. The high metal-binding preference of carboxyl groups is also supported by the lower uranium peaks seen with EDXRF in the carboxyl group-blocked biomass.
XRD
To elucidate the stability of uranium adsorbed to S. elongatus BDU130911, the tested biomass was subjected to X-ray diffraction analysis. The XRD profiles of the control and modified biomass exposed to uranium exhibited characteristic diffraction peaks at 2Ɵ 7.6°, 7.3° and 6.3° for the control, amine group-blocked, and carboxyl group-blocked biomass, respectively (Fig. 5). The XRD pattern of control biomass after uranium adsorption showed satisfactory correlation with the pattern of known uranium acetate compounds (JCPDS), with characteristic peaks at 2Ɵ 7.66° and 7.36°. The powder XRD pattern of uranium adsorbed to the control biomass revealed the biomass to be amorphous in nature with more characteristic crystallinity peaks. In contrast, while both the carboxyl-blocked and amine-blocked biomass also appeared amorphous, they showed poor crystallinity. Further XRD studies revealed that the control biomass is more stable based on its higher crystallinity, and there was a change in texture of the biomass after uranium adsorption. A similar pattern of phases for uranium-adsorbed biomass was observed by Acharya and Apte 2013, and Sarada et al. (2013), indicating a change in crystallinity after heavy metal adsorption. According to Kazy et al. (2009), crystalline uranium formations indicate the possible complexation of metals with cellular functional groups, namely carboxyls or phosphates, facilitating metal nucleation or precipitation in a crystalline state. The complexation of uranium with functional groups was also substantiated by the FTIR results in the present study.
Overall, the techniques applied in this adsorption analysis clearly demonstrate that the adsorption of uranium by S. elongatus BDU130911 is primarily due to ionic interactions with carboxyl groups at the surface of the marine cyanobacterial biomass.
Conclusion
The present investigation addressed the surface interaction of the unicellular marine cyanobacterium S. elongatus BDU130911 with uranium. Surface adsorption of uranium was identified to be a monolayer, and the dominant functional groups involved were confirmed to be carboxyl groups. Thus, the present study substantiates the surface ionic interaction of marine cyanobacterial biomass with uranium radionuclides.
References
Acharya C, Apte SK (2013) Insights into the interactions of cyanobacteria with uranium. Photosynth Res 118:83–94
Acharya C, Joseph D, Apte SK (2009) Uranium sequestration by a marine cyanobacterium Synechococcus elongatus strain BDU/75042. Bioresour Technol 100:2176–2181
Acharya C, Chandwadkar P, Apte SK (2012) Interaction of uranium with a filamentous, heterocystous, nitrogen-fixing cyanobacterium, Anabaena torulosa. Bioresour Technol 116:290–294
Ahad RIA, Goswami S, Syiem MB (2017) Biosorption and equilibrium isotherms study of cadmium removal by Nostoc muscorum Meg 1: morphological, physiological and biochemical alterations. 3 Biotech 7:104
Akara T, Ozkarab E, Celikb S, Turkyilmazc S, Akar ST (2013) Biosorption of Acid Blue 25 by unmodified and CPC-modified biomass of Penicillium YW01: kinetic study, equilibrium isotherm and FTIR analysis. Colloids Surf B 101:307–314
Bai J, Yao H, Fan F, Lin M, Zhang ML, Ding H, Lei F, Wu X, Li X, Guo J, Qin Z (2010) Biosorption of uranium by chemically modified Rhodotorula glutinis. J Environ Radioactiv. 101:969–973
Bulgariu L, Bulgariu D (2014) Enhancing biosorption characteristics of marine green algae (Ulva lactuca) for heavy metals removal by alkaline treatment. J Bioprocess Biotech 4:146
Cecal A, Humelnicu D, Rudic V, Cepoi L, Ganju D, Cojocari A (2012) Uptake of uranyl ions from uranium ores and sludges by means of Spirulina platensis, Porphyridium cruentum and Nostok linckia alga. Bioresour Technol 118:19–23
Chen X, Shi J, Chen J, Csu X, Chen L, Wang H, Hu T (2007) Determination of copper binding in Pseudomonas putida CZ1 by chemical modifications and X-ray absorption spectroscopy. Appl Microbiol Biotechnol 74:881–889
Dotto GL, Esquerdo VM, Vieira MLG, Pinto LNN (2012) Optimization and kinetic analysis of food dyes biosorption by Spirulina platensis. Colloids Surf B 91:234–241
Frost RL, Musumeci AW, Kloprogge JT (2006) A Raman and infrared spectroscopic study of the uranyl silicates weeksite, soddyite and haiweeit. Biomol Spectrosc 64:308–315
Goswami S, Diengdoh OL, Syiem MB, Pakshirajan K, Kiran MG (2014) Zn (II) and Cu (II) removal by Nostoc muscorum: a cyanobacterium isolated from a coal mining pit in Chiehruphi, Meghalaya, India. Can J Microbiol 61:209–215
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78
Hlihor RM, Bulgariu L, Sobariu DL, Diaconu M, Tavares T, Gavrilescu M (2014) Recent advances in biosorption of heavy metals: support tools for biosorption equilibrium, kinetics and mechanism. Rev Roum Chim 59:527–538
Kannan C, Muthuraja K, Devi MR (2013) Hazardous dyes removal from aqueous solution over mesoporous aluminophosphate with textural porosity by adsorption. J Hazard Mater 244:10–20
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Bioresour Technol 61:221–227
Kazy SK, D’Souza SF, Sar P (2009) Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater 163:65–72
Kyzas GZ, Kostoglou M, Lazaridis NK, Bikiaris DN (2013) N-(2-Carboxybenzyl) grafted chitosan as adsorptive agent for simultaneous removal of positively and negatively charged toxic metal ions. J Hazard Mater 244:29–38
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lee KY, Kim KW, Baek YJ, Chung DY, Lee EH, Lee SY, Moon JK (2014) Biosorption of uranium (VI) from aqueous solution by biomass of brown algae Laminaria japonica. Water Sci Technol 70:136–143
Liang X, Hillier S, Pendlowski H, Gray N, Ceci A, Gadd GM (2015) Uranium phosphate biomineralization by fungi. Environ Microbiol 17:2064–2075
Newsome L, Morris K, Lloyd JR (2014) The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem Geol 363:164–184
Omale PE, Okeniyi SO, Faruruwa MD, Ngokat AB (2014) Determination for levels of radionuclides of uranium, thorium and potassium in water, sediments and algae samples from selected coastal areas of Lagos, Nigeria; using Energy dispersive X-ray fluorescence. Global J Pure Appl Chem Res 2:1–24
Rashmi V, ShylajaNaciyar M, Rajalakshmi R, D’Souza SF, Prabaharan D, Uma L (2013) Siderophore mediated uranium sequestration by marine cyanobacterium Synechococcus elongatus BDU130911. Bioresour Technol 130:204–210
Sarada B, Prasad MK, Kumar KK, Murthy CHVR (2013) Potential use of leaf biomass, Araucaria heterophylla for removal of Pb+2. Int J Phytoremediat 15:756–773
Singh M, Pant G, Hossain K, Bhatia AK (2017) Green remediation. Tool for safe and sustainable environment: a review. Appl Water Sci 7:2629–2635
Thangaraj B, Rajasekar DP, Vijayaraghavan R, Garlapati D, Devanesan AA, Lakshmanan U, Dharmar P (2017) Cytomorphological and nitrogen metabolic enzyme analysis of psychrophilic and mesophilic Nostoc sp.: a comparative outlook. 3 Biotech 7:107
Williamson AJ, Morris K, Law GT, Rizoulis A, Charnock JM, Lloyd JR (2014) Microbial reduction of U (VI) under alkaline conditions: implications for radioactive waste geodisposal. Environ Sci Technol 48:13549–13556
Xie S, Yang J, Chen C, Zhang X, Wang Q, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99:126–133
Acknowledgements
The study was financially supported by Department of Atomic Energy (Govt of India) Grant No: (2007/37/29/BRNS/1906) and Department of Biotechnology (Govt of India) for funding National Facility for Marine Cyanobacteria (Grant no: BT/IS/MAIN/1/98). The authors express their earnest gratitude to Dr. V. Venugopal, Dr. Rajesh Kumar, and Mr. Sabapati (Department of Atomic Energy, Govt. of India), BARC Facilities, Kalpakkam, Tamil Nadu, for providing EDXRF facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Rights and permissions
About this article
Cite this article
Vijayaraghavan, R., Ellappan, V., Dharmar, P. et al. Preferential adsorption of uranium by functional groups of the marine unicellular cyanobacterium Synechococcus elongatus BDU130911. 3 Biotech 8, 170 (2018). https://doi.org/10.1007/s13205-018-1167-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-018-1167-5