Abstract
Anabaena flos-aquae, a typical species of cyanobacterial bloom, was employed as a useful biosorbent for uranium removal. Batch experiments were conducted to examine the effects of different parameters on the uranium uptake amount of Anabaena flos-aquae. The maximum adsorption capacity of 196.4 mg/g was obtained under the optimized experimental conditions. The calculations of kinetic and thermodynamic results proved the adsorption process was endothermic, chemisorption, and spontaneous. The adsorption of uranium onto Anabaena flos-aquae was better defined by the Langmuir model, which indicated the process was a monolayer sorption. In addition, the characterization of the biosorbent before and after uranium sorption implied that the dominant functional groups participated in the uranium adsorption process were hydroxyl, amino, and carboxyl. In conclusion, the environmentally friendly and biocompatible characteristics of Anabaena flos-aquae suggest that it can be a promising biosorbent for uranium removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the excessive amounts of heavy metal produced by anthropogenic activities discharged into the environment, the pollution of heavy metals has aroused wide concern. Among the major categories of heavy metals, radionuclides, known for its chemical toxicity and radiotoxicity, has gained wide public attention. Uranium, as the important source materials of nuclear energy, can be easily detected in the environment due to effluents from the smelting of uranium ore and normal discharge of radioactive waste from nuclear power plants (Ogar et al. 2014; Gudkov et al. 2016).Unfortunately, uranium is inhaled and ingested by human beings eventually through the food chain. Once consuming more than tolerated levels of uranium, it can lead to an increase risk of lots of diseases and physical deformities (ATSDR 2013; Soltani et al. 2019). Meanwhile, the non-renewable characteristics of uranium may prevent the sustainable utilize of nuclear energy (Li et al. 2016). Therefore, it is crucial to remove and recover of uranium from polluted water efficiently.
Traditional methods of uranium removal and recovery include solvent extraction (Cheira 2020; Mathuthu et al. 2019), chemical precipitation (Djedidi et al. 2009), reverse osmosis (Abdel-Khalek et al. 2011; Schulte-Herbrueggen et al. 2016), micellar ultrafiltration (Cojocaru et al. 2009), and adsorption. These methods mentioned above have been demonstrated effective in the field of uranium-contaminated wastewater treated. However, limitations of these methods, such as high energy consumption, secondary pollution, and incomplete removal, are unfavorable to the widespread popularization and promotion of these methods. Among these methods, adsorption gains an advantage over other methods owing to its high efficiency and environmentally friendly.
Biosorption is an adsorption process that utilizes biological materials as adsorbents (Ghasemi et al. 2011). Over the past decades, biosorption has attracted great attentions owe to its unparalleled advantage, such as simplicity, repeatability, eco-friendly, and so on (Yi et al. 2016). In recent years, many biosorbents are being widely used in uranium wastewater treatment, for instance, Citrus limon peels (Šabanović et al. 2019), Saccharomyces cerevisiae (Zheng et al. 2018), and Lemna sp. (Vieira et al. 2019). Among the biosorbents mentioned above, microalgae possess incomparable technical advantages. Firstly, the cultivation of algae is relatively simple (Hu et al. 2011). Secondly, it is easier be harvested because of widely distributed, locally abundant, and much larger size than bacteria (Yu et al. 2014). Finally, and most importantly, it has been confirmed that microalgae have a strong affinity for metals. The functional groups on the cell wall such as carboxyl and amino are important sites for the microorganism to bind metals (Vilar et al. 2008; He and Chen 2014). Recently, freshwater or marine algae, such as Spirulina platensis (Mohammed 2019), Gracilaria corticata (Dabbagh et al. 2018), Cystoseira sp. (Cem et al. 2017), Cladophora hutchinsiae (Bagda et al. 2017), and brown algae (Moghaddam et al. 2013), attracted a great deal of attention for uranium removal. However, according to our literature survey, rarely investigation on the biosorption of uranium by Anabaena flos-aquae has been reported.
Anabaena flos-aquae, a species of filamentous cyanobacteria, is known for its nitrogen-fixing abilities and is one of cyanobacterial species that produce toxins. If it could be harvested and played a vital role in the uranium-containing wastewater treatment, then we can realize a “win-win situation.” The influences of various experimental parameters on the biosorption of uranium were investigated. The characterization of raw and U-loaded Anabaena flos-aquae was performed by Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy dispersive X-ray fluorescence spectrometry (EDS).
Materials and methods
Preparation of biosorbent
Anabaena flos-aquae strain was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Sciences. The cells of Anabaena flos-aquae were cultivated in BG-11 medium in a fermenter until the end of the exponential growth phase. The biomass was then harvested by centrifugation and washed twice with distilled water. Afterwards, the precipitate was freeze-dried, passed through a 100-size mesh, and finally preserved in desiccator for following experimental use.
Batch experiments
All adsorption experiments were duplicated at least to make reliable and repeatable results. The experiments were performed by setting the reaction mixture of Anabaena flos-aquae (10 mg) and 30 mL uranium solution of certain concentration into a conical flask, so as to characterize specific sorption parameters: initial pH, contact time, initial uranium concentration, and environmental temperature. The initial pH (2–8) was adjusted with trace amount of NaOH and HNO3. The effects of different gradient of Anabaena flos-aquae dose (10, 20, 30, 40, 50, 60, 80, 100 mg) on uranium biosorption were studied. Then, the mixture was rotated at 120 rpm in a rotary shaker. The uranium concentration in the supernatant was determined at 650 nm with UV-Vis spectrophotometer after centrifugation. The removal efficiency (R) and sorption amount (Q) of uranium were calculated by Eq. (1) and Eq. (2).
where C0 and Ce are the initial uranium concentration and equilibrium uranium concentration (mg/L), respectively; V is the volume of the solution (L); and m is the mass of the absorbent (g).
Characterization
The surface morphology of Anabaena flos-aquae was examined by SEM (GeminiSEM 300) coupled with EDS (operating conditions: probe current 45 nA, accelerating voltage 20 kV and counting time 60 s). The samples were gold-coated before observation to enhance the electrical conductivity. Functional groups on the surface of Anabaena flos-aquae were observed using FTIR (Bruker Vector 22). To perform FTIR analysis, sample disks were prepared by mixing 0.15 g potassium bromide (KBr) with 0.005 g dry Anabaena flos-aquae and pressed into tablet form. Then, the spectra were recorded over the 400–4000 cm−1 region with a resolution of 0.2 cm.
Results and discussion
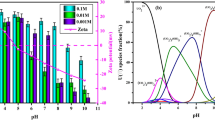
Effect of biomass dose
As presented in Fig. 1, the adsorption capacity decreased with the increase of biomass dose. The maximum biosorption capacity was 173.06 mg/g with 10 mg biomass dose. Similar results have been reported regarding the effect of biomass dose on biosorption capacity (Li et al. 2016). The negative relationship between biosorption capacity and biomass dose may result from the aggregate effect of sorbents (Deng et al. 2011). A “screening effect” that increased electrostatic interactions between cells, limited availability of binding sites, and reduced mixing generated by the higher sorbent dose results in a lower uranium sorption per unit of sorbent. In conclusion, 10 mg of Anabaena flos-aquae is appropriate for the following uranium biosorption experiments.
Kinetic studies
The influence of contact time on uranium biosorption was investigated. As shown in Fig. 2, the adsorption capacity increased sharply during the first 20 min and then reached equilibrium of maximum uranium sorption amount 196.4 mg/g at 50 min. Therefore, the reaction time of 1 h was employed in the following experiments.
The pseudo-first-order model and the pseudo-second-order model were employed to describe the adsorption kinetic characteristic. The two models can be expressed by the following Eq. (3) and Eq. (4).
where qe is the uranium uptake amount at equilibrium, and qt refers to the uranium adsorption capacity at any time “t”; k1 and k2 refer to the rate constant of the pseudo-first-order and pseudo-second-order sorption, respectively. The parameters obtained by the two models are presented in Table 1.
Table 1 shows the correlation coefficient (R2) of the pseudo-second-order model is higher than the value of the pseudo-first-order model. Meanwhile, the calculated qe value of pseudo-second-order model (197.71 mg/g) was approximate to the value measured by the experiment (Qe,exp = 196.4 mg/g). It indicated that the adsorption process is more favor of the pseudo-second-order model, which implying that adsorption process could be chemisorption (Idris et al. 2013; Humelnicu et al. 2011).
Effect of pH
pH was demonstrated one of the most important parameters influencing the metal adsorption (Ghorbani et al. 2008; Gok and Aytas 2009). The uranium removal efficiency (R) and uranium sorption amount (Q) increased dramatically with an increasing pH from 2 to 5 as can been seen in Fig. 3. The removal efficiency of uranium at pH 2 was nearly 3.8%, and corresponding uranium sorption amount was 8.06 mg/g. When pH ascent from 2 to 5, the maximum uranium removal efficiency (R) of 84.6% and uranium sorption amount of 177.6 mg/g were observed at pH 5. The increasing positivity of the adsorbents, the electrostatic repulsion between positively charged uranyl cations, and high concentrations of H+ in the reaction mixture result in the low adsorption capacity at lower pH (Ai et al. 2013; Cao et al. 2013). With increasing pH, an increase of negative charges owes to the deprotonated on the Anabaena flos-aquae, finally enhanced the biosorption of positively charge uranium on the cell surface of Anabaena flos-aquae. However, when the pH was above 5, the biosorption of uranium decreased gradually. The decline of the uranium sorption efficiency may result from the formation of precipitate (4UO3·9H2O) when the pH is higher than 5 (Ghorbani et al. 2008; Khani 2011). Therefore, the optimum pH (pH = 5) for subsequent uranium adsorption on Anabaena flos-aquae experiments was selected.
Adsorption isotherm
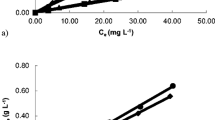
To illuminate the mechanism of the adsorption process, the Langmuir and Freundlich model analyses were conducted. Langmuir isotherm is commonly applied to describe monolayer adsorption (Foo and Hameed 2010; Ding et al. 2014). In contrast, the Freundlich model assumes multilayer sorption on the heterogeneous surface (Foo and Hameed 2010; Xiong et al. 2013). The two models can be expressed by Eq. (5) and Eq. (6).
where Ce refers to the equilibrium concentration (mg/L) and qe refers to the equilibrium adsorption capacity(mg/g), qm is the maximum adsorption capacity(mg/g), and KL is the constant related to the energy of adsorption; KF and nF denote the Freundlich constant and Freundlich exponent, respectively.
The value of qe increased firstly and then reached equilibrium state later with the concentration increased, which revealed that the initial concentration provides a driving force for uranium adsorbed onto Anabaena flos-aquae (Han et al. 2018; Gok and Aytas 2009). The parameters presented in Table 2 were calculated from the linear curves presented in Fig. 4. The higher R2 value of the Langmuir isotherm model and the sorption capacity calculated from the Langmuir model (qm = 190.1 mg/g) which was the approach to the value measured by the experiment (168.5 mg/g) both indicate that the biosorption of uranium onto Anabaena flos-aquae can be well described by the Langmuir model. Based on the isotherm analysis, it was confirmed that the uranium adsorption process onto Anabaena flos-aquae was likely monolayer coverage.
Notably, the biosorption capacity of adsorbents was always evaluated by the value of the maximum adsorption capacity (qm). For example, the qm for Dictyopteris polypodioides was 62.5 mg/g (Bampaiti et al. 2016), 113.5 mg/g for Saccharomyces cerevisiae (Faghihian and Peyvandi 2012), 152 mg/g for Cladophora hutchinsiae (Bagda et al. 2017), and 97.15 mg/g for Chlorella vulgaris (Amini et al. 2012). Evidently, a comparison of the maximum capacities among these adsorbents proved the Anabaena flos-aquae has a considerable high biosorption capacity for uranium. Besides, the low operational cost and maximization of benefits give the Anabaena flos-aquae more advantages over lots of adsorbents.
Adsorption thermodynamics
In order to figure out the influence of temperature on the adsorption reaction, the adsorption experiments at different temperatures (288.2 K, 298.2 K, and 308.2 K) were conducted. As presented in Fig. 5a, the amount of uranium adsorbed onto Anabaena flos-aquae increased gradually with the increase of temperature, which demonstrating the adsorption process may be endothermic. Thermodynamic parameters were calculated using the equations given below and listed in Table 3.
where Kd refers to the equilibrium constant and R represents gas constant (8.314 J mol−1 K−1); T refers to the absolute temperature in Kelvin (K). The enthalpy and entropy were calculated from the plot of lnKd against 1/T in Fig. 5b. The positive values of ΔH and ΔS proved the adsorption process was endothermic and the increased randomness at the solid-solution interface (Bozkurt et al. 2011; Saleem and Bhatti 2011; Donat 2009). Meanwhile, the negative value of ΔG suggests the adsorption process was spontaneous. In conclusion, the thermodynamic study revealed that the biosorption of uranium onto Anabaena flos-aquae was endothermic and spontaneous.
Characterization of Anabaena flos-aquae
FTIR spectra of Anabaena flos-aquae
To characterize specific functional groups involved in biosorption, the FTIR patterns of Anabaena flos-aquae were recorded before and after sorption of uranium. For the Anabaena flos-aquae, the bands at approximately 3305.39 cm−1 corresponded to the O-H and N-H stretching vibrations. The peaks at 1654.62 cm−1 and 1544.7 cm−1 were ascribed to the N-H stretching of amino groups. The band at 1054.87 cm−1 were assigned to the C-OH stretching (Yi et al. 2016).
For the U-loaded Anabaena flos-aquae, the peaks at 1054.87, 1544.7, and 3305.39 cm−1 correspond to -COOH, -NH2, and -OH shifted to 1031.73, 1546.63, and 3313.11 cm−1, respectively (Fig. 6). It implied that the -COOH, -NH2, and -OH performed an important role in uranium biosorption. Furthermore, two new peaks at 1454.06 cm−1 and 916.02 cm−1 were observed. The two peaks represented the stretching frequency of the structure that formed by U atoms combined with oxygen-containing functional groups (Xie and Gao 2007; Zhang et al. 2014; Zhao et al. 2019).
SEM-EDS of Anabaena flos-aquae
The SEM images of Anabaena flos-aquae before and after uranium loaded were shown in Fig. 7. As can be seen from the micrographs of raw Anabaena flos-aquae with magnification of × 2000 and × 3000 in Fig. 7a, b, the morphology of pure Anabaena flos-aquae is smooth and flat. As presented in Fig. 7c, d, the surface became rough because of the formation of many deep grooves after exposure to uranium. These grooves may give the Anabaena flos-aquae more chance to accommodate considerable uranyl ions.
The element content of the sample before and after uranium sorption was analyzed by EDS. According to EDS analysis in Fig. 8a, b, the algae was mainly consisted of carbon (49%), oxygen (36%), and nitrogen (11%) as well as small quantities of sodium, magnesium, phosphorus, and calcium. After exposure to uranium, the uranium peak was detected (1.64%). Besides, the atomic percentage of nitrogen (3.87%) and oxygen (9.11%) decreased. The combination of SEM-EDS and FTIR data indicates that uranium has been successfully adsorbed by Anabaena flos-aquae.
Conclusion
As a common species of cyanobacteria bloom, Anabaena flos-aquae was utilized for the biosorption of uranium in this study. The maximum uranium sorption amount was found to reach the value of 196.4 mg/g. The biosorption of uranium onto Anabaena flos-aquae was well fitted to the Langmuir isotherm model and pseudo-second-order model, which proved the adsorption process was monolayer sorption and chemisorption. The results of thermodynamic analysis indicated that uranium uptake was endothermic and spontaneous. The FTIR results demonstrated that the -OH, -NH2, and -COOH performed an important role in uranium biosorption. In conclusion, the effective biosorption of uranium from aqueous solution by cyanobacterium Anabaena flos-aquae makes it a promising, environmentally friendly, and biocompatible biosorbent for the treatment of uranium contaminants.
References
Abdel-Khalek AA, Ali MM, Ashour RM, Abdel-Magied AF (2011) Chemical studies on uranium extraction from concentrated phosphoric acid by using PC88A and DBBP mixture. J Radioanal Nucl Chem 290:353–359
Ai L, Luo X, Lin X, Zhang S (2013) Biosorption behaviors of uranium (VI) from aqueous solution by sunflower straw and insights of binding mechanism. J Radioanal Nucl Chem 298:1823–1834
Amini M, Younesi H, Bahramifar N (2012) Biosorption of U (VI) from aqueous solution by Chlorella vulgaris: equilibrium, kinetic, and thermodynamic studies. J Environ Eng 139:410–421
ATSDR (Agency for Toxic Substances and Disease Registry) (2013) Toxicological profile for Uranium. Available online at: http://www.atsdr.cdc.gov/ToxProfiles/tp150.pdf
Bagda E, Tuzen M, Sari A (2017) Equilibrium, thermodynamic and kinetic investigations for biosorption of uranium with green algae (Cladophora hutchinsiae). J Environ Radioact 175:7–14
Bampaiti A, Yusan S, Aytas S, Pavlidou E, Noli F (2016) Investigation of uranium biosorption from aqueous solutions by Dictyopteris polypodioides brown algae. J Radioanal Nucl Chem 307:1335–1343
Bozkurt SS, Molu ZB, Cavas L, Merdivan M (2011) Biosorption of uranium (VI) and thorium (IV) onto Ulva gigantea (Kützing) bliding: discussion of adsorption isotherms, kinetics and thermodynamic. J Radioanal Nucl Chem 288:867–874
Cao Q, Liu Y, Wang C, Cheng J (2013) Phosphorus-modified poly (styrene-codivinylbenzene)-PAMAM chelating resin for the adsorption of uranium (VI) in aqueous. J Hazard Mater 263:311–321
Cem G, Aytas S, Sezer H (2017) Modeling uranium biosorption by Cystoseira sp. and application studies. Sep Sci Technol 52:792–803
Cheira F (2020) Solvent extraction of uranium and vanadium from carbonate leach solutions of ferruginous siltstone using cetylpyridinium carbonate in kerosene. Chem Pap 74:2247–2266. https://doi.org/10.1007/s11696-020-01073-w
Cojocaru C, Zakrzewska-Trznadel G, Miskiewicz A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach part 2: optimization of hydrodynamic conditions for a crossflow ultrafiltration module with rotating part. J. Hazard.Mater 169:610–620
Dabbagh R, Rojaee A, Heshmatipour Z (2018) Thermodynamics, kinetics and equilibrium studies of uranium sorption by Gracilaria corticata red alga. Environ Eng Manag J 17:1199–1208
Deng H, Lu J, Li G, Zhang G, Wang X (2011) Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem Eng J 172:326–334
Ding C, Cheng W, Sun Y, Wang X (2014) Determination of chemical affinity of graphene oxide nanosheets with radionuclides investigated by macroscopic, spectroscopic and modeling techniques. Dalton Trans 43:3888–3896
Djedidi Z, Bouda M, Souissi MA, Cheikh RB, Mercier G, Tyagi RD, Blais JF (2009) Metals removal from soil, fly ash and sewage sludge leachates by precipitation and dewatering properties of the generated sludge. J HazardMater 172:1372–1382
Donat R (2009) The removal of uranium (VI) from aqueous solutions onto natural sepiolite. J Chem Thermodyn 41:829–835
Faghihian H, Peyvandi S (2012) Adsorption isotherm for uranyl biosorption by Saccharomyces cerevisiae biomass. J Radioanal Nucl Chem 293:463–468
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Ghasemi M, Keshtkar AR, Dabbagh R, Safdari SJ (2011) Biosorption of uranium (VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: break through curves studies and modeling. J Hazard Mater 189:141–149
Ghorbani F, Younesi H, Ghasempouri SM, Zinatizadeh AA, Amini M, Daneshi A (2008) Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chem Eng J 145:267–275
Gok C, Aytas S (2009) Biosorption of uranium (VI) from aqueous solution using calcium alginate beads. J Hazard Mater 168:369–375
Gudkov SV, Chernikov AV, Bruskov VI (2016) Chemical and radiological toxicity of uranium compounds. Russ. J. General Chem 86:1531–1538
Han B, Zhang E, Cheng G, Zhang L, Wang D, Wang X (2018) Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chem Eng J 338:734–744
He J, Chen J (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresour Technol 160:67–78
Hu H, Li X, Yu Y, Wu Y, Sagehashi M, Sakoda A (2011) Domestic wastewater reclamation coupled with biofuel/biomass production based on microalgae: a novel wastewater treatment process in the future. J Water Environ Technol 9:199–207
Humelnicu D, Dinu MV, Drăgan ES (2011) Adsorption characteristics of UO22+ and Th4+ ions from simulated radioactive solutions onto chitosan/clinoptilolite sorbents. J Hazard Mater 185:447–455
Idris S.A., Alotaibi K.M., Peshkur T.A., Anderson P., Morris M., Gibson L.T (2013) Adsorption kinetic study: effect of adsorbent pore size distribution on the rate of Cr (VI) uptake. Micropor Mesopor Mater 165:99–105
Khani MH (2011) Statistical analysis and isotherm study of uranium biosorption by Padina sp. algae biomass. Environ. Sci. Pollut. Res 18:790–799
Li F, Li D, Li X, Liao J, Li S, Yang J, Yang Y, Tang J, Liu N (2016) Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem Eng J 284:630–639
Mathuthu M, Mokhine ND, Stassen E (2019) Organic solvent extraction of uranium from alkaline nuclear waste. J Radioanal Nucl Chem 319:687–693
Moghaddam MR, Fatemi S, Keshtkar A (2013) Adsorption of lead (Pb2+) and uranium (UO22+) cations by brown algae: experimental and thermodynamic modeling. Chem Eng J 231:294–303
Mohammed AAER (2019) Potentiality of quercetin-sodium hydroxide modified Spirulina platensis in uranium biosorption from waste effluent. Int J Environ Stud 77:1–13
Ogar A, Grandin A, Sjoberg V, Turnau K, Karlsson S (2014) Stabilization of uranium (VI) at low pH by fungal metabolites: applications in environmental biotechnology. APCBEE Procedia 10:142–148
Šabanović E, Muhić-Šarac T, Nuhanović M, Memić M (2019) Biosorption of uranium (VI) from aqueous solution by Citrus limon peels: kinetics, equlibrium and batch studies. J Radioanal Nucl Chem 319:425–435
Saleem N, Bhatti HN (2011) Adsorptive removal and recovery of U(VI) by citrus waste biomass. BioResources 6:2522–2538
Schulte-Herbrueggen HMA, Semiao AJC, Chaurand P, Graham MC (2016) Effect of pH and pressure on uranium removal from drinking water using nf/ro membranes. Environ Sci Technol 50:5817–5824
Soltani M, Zarei MH, Salimi A, Pourahmad J (2019) Mitochondrial protective and antioxidant agents protect toxicity induced by depleted uranium in isolated human lymphocytes. J Environ Radioact 203:112–116
Vieira LC, de Araujo LG, de Padua Ferreira RV, da Silva EA, Canevesi RLS, Marumo JT (2019) Uranium biosorption by Lemna sp. and Pistia stratiotes. J Environ Radioact 203:179–186
Vilar VJP, Botelho CMS, Boaventura RAR (2008) Lead and copper biosorption by marine red algae Gelidium and algal composite material in a CSTR (“Carberry” type). Chem Eng J 138:249–257
Xie X, Gao L (2007) Characterization of a manganese dioxide/carbon nanotube composite fabricated using an in situ coating method. Carbon 45:2365–2373
Xiong Y, Xu J, Shan W, Lou Z, Fang D, Zang S, Han G (2013) A new approach for rhenium (VII) recovery by using modified brown algae Laminaria japonica adsorbent. Bioresour Technol 127:464–472
Yi Z, Yao J, Chen H, Wang F, Yuan Z, Liu X (2016) Uranium biosorption from aqueous solution onto Eichhornia crassipes. J Environ Radioact 154:43–51
Yu J, Zhao W, Yang G, Zeng S (2014) Research on biological materials with uranium biosorption by microalgae: a review. Appl Mech Mater 508:290–296
Zhang S, Shu X, Zhou Y, Huang L, Hua D (2014) Highly efficient removal of uranium (VI) from aqueous solutions using poly (acrylic acid)-functionalized microspheres. Chem Eng J 253:55–62
Zhao C, Liu J, Deng Y, Tian Y, Sun Q (2019) Uranium (VI) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J Clean Prod 236:117624
Zheng X, Shen Y, Wang X, Wang T (2018) Effect of pH on uranium (VI) biosorption and biomineralization by Saccharomyces cerevisiae. Chemosphere 203:109–116
Funding
This work is financially supported by Natural Science Foundation of Jiangxi Province (No. 20192BAB214005; No. 20181BAB214005) and National Natural Science Foundation of China (Grant No. 41867063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yijun Yuan and Nana Liu should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Yuan, Y., Liu, N., Dai, Y. et al. Effective biosorption of uranium from aqueous solution by cyanobacterium Anabaena flos-aquae. Environ Sci Pollut Res 27, 44306–44313 (2020). https://doi.org/10.1007/s11356-020-10364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10364-4