Abstract
Gatifloxacin (GTN) was derivatized to its dithiocarbamate derivative and its radiolabeling with technetium-99m (99mTc) using the [99mTc≡N]2+ core was investigated. The appropriateness of the 99mTcN–gatifloxacin dithiocarbamate (99mTcN–GTND) complex as a potential multi-drug-resistance Streptococcus pneumoniae (MRSP) infection radiotracer was evaluated in terms of stability in saline, serum, in vitro binding with MRSP and biodistribution in artificially MRSP infected Male Wistar Rats (MWR). In saline the 99mTcN–GTND complex showed more than 90% labeling yield up to 4 h with a maximum yield of 98.25 ± 0.20%, after reconstitution. In serum the 99mTcN–GTND complex showed stability up to 16 h of incubation with the appearance of insignificant 15.95% undesirable side products. The 99mTcN–GTND complex demonstrated saturated in vitro binding with MRSP with a maximum value of 75.50 ± 1.00% (at 90 min). In MWR model of group A, almost six times higher uptake of the labeled GTND was monitored in the muscle of MWR infected with live MRSP as compared to the inflamed and normal muscles. Based on the higher labeling yield in saline, in vitro stability in serum, saturated in vitro binding with live MRSP and promising biodistribution in MWR model we recommend 99mTcN–gatifloxacin dithiocarbamate complex as a potential MRSP infection radiotracer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diagnosis of infection and its segregation from inflammation is worldwide quandary in management of patients in early stages of the ailment. The Nuclear Medicine Imaging Technology (NMIT) had played a pivotal role in the early analysis of infectious foci, thereby minimizing the risk of erroneous diagnosis [1, 2].
In continuation to our recent studies, satisfactory in vivo and in vitro evaluation of a wide range of antibiotics radio-labeled with technium-99m (99mTc) have encouraged us to search for more appropriate and specific 99mTc radiotracer for imaging infection and its discrimination from inflammation [3–19].
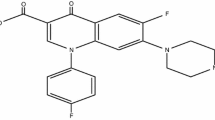
Gatifloxacin (GTN) [1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-quinoline-3-carboxylic acid] (Fig. 1a) is a new quinolone antibiotic of the fourth generation having a methoxy group at position C-8, showing broad spectrum activity against wide range of bacterial infections. Different investigations have confirmed the higher potency of the gatifloxacin against Gram positive bacteria including multi-drug-resistance Streptococcus pneumoniae (MRSP) [20, 21].
It is reported that bioactive molecules labeled with technetium-99m through [99mTV≡N]2+ core has shown superior radiochemical immovability for a longer time as compared to others direct and indirect methods. In continuation to our unrelenting investigations and to utilize the distinctive therapeutic characteristics of the GTN for diagnosis of deep tissue infection caused by MRSP at early stages, the current study is focused on the modification of GTN to its dithiocarbamate derivative (GTND) and subsequent its labeling with 99mTc using the [99mTcV≡N]2+ core. Thereafter, the 99mTc labeled GTND was radiobiologically characterized in terms of stability in saline, serum, in vitro binding with MRSP and biodistribution in artificially MRSP infected rabbit’s model.
Experimental
Materials
Gatifloxacin (GTN) was procured from Changzhou Kejia Chemical Co., Ltd. China, TLC plates from Merk. Germany, succinic dihydrazide (SDH), propylenediamine tetra-acetic acid (PDTA) and the rest of the chemicals and solvents of analytical grade from Sigma. RP-HPLC of Shimadzu, Japan, well counter, scalar count rate meter of Ludlum, USA, dose calibrator of Capintech, USA and Gamma camera GKS-1000 of GEADE Nuclearmedizine system, Germany, were used.
Method
Derivatization and radiolabeling of GTN
Gatifloxacin (GTN) was derivatized to gatifloxacin dithiocarbamate (GTND) using the previously reported scheme [19]. Subsequently, 2.0 mg of GTND was mixed with 74 MBq of sodium pertechnetate (Na99mTcO4 −), 100 μg of stannous fluoride (SnF2), 5.0 mg of PDTA and 5.0 mg of SDH in a sealed sterilized vial. After gently swirling, the vial containing the above mixture was incubated for 15 min at 27 °C followed by filtration through Millipore.
Partition coefficient (P) of 99mTcN–GTND complex
The P value of the 99mTcN–GTND complex was found by using the previously reported method [19]. In brief, an equal degree of the radiocomplex, phosphate buffer (PB) and octanol were vortexed at 27 °C for 5 min followed by centrifugation for 15 min at 5,000 rpm/min. Thereafter, a small amount (~100 μL) of the reaction mixture at different hiatus were withdrawn followed by counting for radioactivity in single well counter fitted with scalar count rate meter using the standard formula: P = (counts per min in octanol−counts per min in background)/(counts per min in buffer−counts per min in background).
Radiochemical stability in normal saline
High pressure liquid chromatographic (HPLC) technique was employed for the determination of the radiochemical stability of the 99mTcN–GTND complex at room temperature. Shimadzu (SCL-10 AVP) fitted with SDP-10 AVP, UV detector (254 nm), Packard 500 TR, flow scintillation analyzer, 4.6 × 150 mm C-18 column, binary pump and online degasser was used for separation and measurement of various components of the 99mTcN–GTND complex. Using a sterilized injector 5 μL of the freshly prepared 99mTcN–GTND complex was injected to the device and the mobile phase (water:methanol (W:M)) was permitted for 15 min (1 mL/min). The elution stricture was at 0–2 min (100:00), 2–5 min (70:30), 5–7 min (60:40), 7–10 (40:60), 10–12 (30:70), and 12–15 (50:50), respectively. The eluent 1 mL/min was amassed separately using sterilized vial and measured for activity in a single well counter fitted with scalar count rate meter. The overall scheme was replicated at 30, 60, 90, 120, and 240 min after reconstitution of the 99mTcN–GTND complex.
Stability in serum
Thin layer chromatographic (TLC) technique was used for the determination of the stability of the 99mTcN–GTND complex in serum at 37 °C. Using the method already been reported, 200 μL of the freshly prepared 99mTcN–GTND complex was incubated at 37 °C C for 16 h with 1.8 mL of fresh serum. During incubation process small quantity of the reaction mixture was introverted and spotted on the TLC strip at 0, 2, 4, 6, 8, 10, 12, 14, and 16 h. Thereafter, the strips were developed instantly in normal saline and CH2Cl2:CH3OH (9:1) (v/v). The developed strips were then equally divided into two parts and measured for activity using single well counter fitted with scalar count rate meter.
MRSP in vitro binding
The in vitro binding of the 99mTcN–GTND complex with MRSP was investigated using the previously reported method [22]. Briefly, 0.1 mL sodium phosphate buffer (SPB) and 10 MBq 99mTcN–GTND was taken in a sterilized and pyrogen free test tube and thereafter, 0.01 M (0.8 mL 50%, v/v) acetic acid including 1 × 108 colony forming units (CFU) of MRSP. The amalgamation was nurtured for 60 m at 4 °C at pH 5. The nurtured concoction was then centrifuged for 10 min at 3,000 rpm. After removing the supernatant the pellets were socked followed by centrifugation for 10 min at 3,000 rpm. Thereafter, the supernatant was discarded once again and the bacterial pellets were counted for percent radio-uptake using single well counter fitted with scalar count rate meter.
Biodistribution in rats
In vivo distribution of the 99mTcN–GTND complex freshly prepared was investigated in Male Wistar Rats (MWR) artificially infected with live and heat killed MRSP. Eight healthy MWR (weight 150–180 g) were selected and divided into two groups (A and B). All MWR were inflamed by injecting 0.2 mL germ-free turpentine oil into the left thighs. Thereafter, 0.2 mL of live MRSP were injected into the right thighs of the MWR of group A and 0.2 mL of heat killed MRSP were injected to the group B. After 18 h, 0.2 mL of the 99mTcN–GTND complex was injected intravenously to the group A and B MWR. Subsequently, the MWR of group A and B were sacrificed in accordance with the rules stipulated in the manual of Nuclear Medicine Research Laboratory (NMRL) University of Peshawar part-I and II. Thereafter, one gram each of the blood, liver, spleen, stomach, intestines, kidneys, infected, inflamed, and normal muscles from the MWR of each group were accurately obtained and measured for the percent uptake of the 99mTcN–GTND complex using single well counter fitted with scalar count rate meter.
Results and discussion
Derivatization and radiolabeling of GTN
Gatifloxacin GTN as shown in Fig. 1a was modified to its dithiocarbamate derivative by reacting equimolar amount of the GTN, sodium hydroxide (NaOH) and carbon disulphide as shown in Fig. 1b. Thereafter, the GTND was radiolabeled with technetium-99m (99mTc:radiometal) through 99mTcV≡N core using the method described earlier to give the 99mTcNGTND complex [19]. The main structural properties of 99mTcNGTND can be accounted for by comparison with the reported properties of TcN core [23].
Technetium can exist in a number of oxidation states but +V state is the most common in TcN complexes. The two sets of molecular orbital (MO) will be nonbonding highest occupied MO (HOMO) and the two degenerate π* anti-bonding lowest unoccupied orbital’s (LUMO). The electronic arrangement of these orbital’s can account for the structural configuration of the TcN complexes as square pyramidal.
Crystal structural studies on TcN complexes, showed that when the four coordinated atoms are π-donor Lewis bases, then square pyramidal (sp) geometry is preferred [24, 25]. The cogitated structure of TcN–GTND (Fig. 1c) with tetradentate ligand (two sites of bidentate) will have a square pyramidal geometry with TcN:GTND ratio of 1:1. Intermolecular complexation could be symmetrical (H–Tc–H and T–Tc–T), unsymmetrical (H–Tc–T and T–Tc–H) and mixed complexes with the ligand.
HPLC analysis and radiochemical stability
The HPLC profile of the 99mTcN–GTND complex is given in Fig. 2 showing two distinctly erratic peaks at 3.2 and 11.7 min of retention. The signal at 3.2 min represent the free [99mTcV≡N]2+ intermediate and 11.7 m the 99mTcN–GTND complex.
Figure 3 showed the radiochemical stability profile of the 99mTcN–GTND complex in the normal saline up to 240 min. The maximum radiochemical stability was observed at 30 min (98.25 ± 0.20%) after reconstitution. The radiochemical stability decreased with time and it reached to 90.55 ± 0.24% within 240 min.
Stability in serum at 37 °C
The 99mTcN–GTND complex at 0, 2, 4, 6, 8, 10, 12, 14, and 16 h after reconstitution showed a stable behavior up to 4 h at 37 °C with the appearance of side radio-products. Increase in the radio-side products was observed and it reached to a maximum value of 15.95% within 16 h as shown in Fig. 4.
MRSP in vitro binding
Figure 5 demonstrated saturated in vitro binding of the 99mTcN–GTND complex with MRSP. The maximum in vitro uptake of the radiolabeled GTND by MRSP observed was 75.50 ± 1.00% (at 90 min).
Biodistribution in rats
Table 1 demonstrated the percent absorption of the 99mTcN–GTND complex in the blood, liver, spleen, stomach, intestines, kidneys, infected, inflamed, and normal muscles of the group A and B MWR. Initially the activity of the 99mTcN–GTND complex in the blood, liver, spleen, stomach, and intestines was 19.50 ± 0.26, 18.15 ± 0.20, 9.00 ± 0.00, and 7.80 ± 0.20%, respectively. In the uptake profile of the 99mTcN–GTND complex in the blood, liver, spleen, stomach, and intestines a plodding decrease was observed. The activity the blood, liver, spleen, stomach, and intestines decreased within 120 min to 3.50 ± 0.00, 4.85 ± 0.26, 4.00 ± 0.24, and 4.25 ± 0.00%, respectively. However in kidneys repeal in uptake profile was noted wherein the activity went up from 9.55 ± 0.22 to 24.25 ± 0.24% within 120 min. No significant difference in the uptake of the 99mTcN–GTND complex was observed in group A and B MWR with regards to the blood, liver, spleen, stomach, intestines and kidneys. However, a significant difference in the uptake of the complex was noted in the MWR of group A as compared to group B. Almost six fold higher uptake of the labeled GTND was seen in the infected muscle as compared to normal and inflamed of the MWR of group A. While in group B animal no significant difference in the uptake of the complex in infected, inflamed and normal muscle was observed. Different percent uptake of the 99mTcN–GTND complex in infected, inflamed and normal muscle of the MWR models is given in Fig. 6 showing ratio wise biodistribution. Both the circulatory and urinary system distribution profile suggested normal excretion route.
Conclusion
The derivatized gatifloxacin was radiolabeled with 99mTc using [99mTcV≡N]2+ core and its suitability as potential MRSP infection radiotracer was investigated. Based on the higher labeling yield in saline, in vitro stability in serum, saturated in vitro binding with live MRSP and promising biodistribution in MWR model we recommend 99mTcN–gatifloxacin dithiocarbamate complex as a potential MRSP infection radiotracer.
References
Qaiser SS, Khan AU, Khan MR (2010) Synthesis, biodistribution and evaluation of 99mTc–sitafloxacin kit: a novel infection imaging agent. J Radioanal Nucl Chem 284:189
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biodistribution of 99mTc–rifampicin: a novel radiotracer for in vivo infection imaging. Appl Radiat Isot 68:2255
Shah SQ, Khan AU, Khan MR (2011) 99mTc–novobiocin: a novel radiotracer for infection imaging. Radiochim Acta 99:53
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis, biodistribution and scintigraphy of the 99mTc–teicoplanin complex in artificially infected animal models. J Label Compd Radiopharm 54:145
Shah SQ, Khan AU, Khan MR (2011) Radiosynthesis and biological evaluation of 99mTcN–sitafloxacin dithiocarbamate as a potential radiotracer for Staphylococcus aureus infection. J Radioanal Nucl Chem 287:827
Shah SQ, Khan AU, Khan MR (2011) Radiosynthesis and biodistribution of 99mTcN–garenoxacin dithiocarbamate complex a potential infection imaging agent. J Radioanal Nucl Chem 288:59
Shah SQ, Khan AU, Khan MR (2011) Radiosynthesis and biological evolution of 99mTc(CO)3–sitafloxacin dithiocarbamate complex: a promising Staphylococcus aureus infection radiotracer. J Radioanal Nucl Chem 288:131
Shah SQ, Khan AU, Khan MR (2011) 99mTc(CO)3–garenoxacin dithiocarbamate synthesis and biological evolution in rats infected with multiresistant Staphylococcus aureus and penicillin-resistant Streptococci. J Radioanal Nucl Chem 288:171
Shah SQ, Khan AU, Khan MR (2011) Synthesis, biological evaluation and biodistribution of the 99mTc–garenoxacin complex in artificially infected rats. J Radioanal Nucl Chem 288:207
Shah SQ, Khan MR (2011) Radiocomplexation and biological characterization of the 99mTcN-trovafloxacin dithiocarbamate: a novel methicillin-resistant Staphylococcus aureus infection imaging agent. J Radioanal Nucl Chem 288:215–220
Shah SQ, Khan MR (2011) Synthesis of the 99mTc(CO)3–trovafloxacin dithiocarbamate complex and biological evaluation in artificially methicillin-resistant Staphylococcus aureus infected rats model. J Radioanal Nucl Chem 288:297
Shah SQ, Khan MR (2011) Radiolabeling of gemifloxacin with technetium-99m and biological evaluation in artificially Streptococcus pneumoniae infected rats. J Radioanal Nucl Chem 288:307
Shah SQ, Khan MR (2011) Radiocharacterization of the 99mTc–rufloxacin complex and biological evaluation in Staphylococcus aureus infected rat model. J Radioanal Nucl Chem 288:373
Shah SQ, Khan MR (2011) Radiosynthesis and biodistribution of 99mTc–tricarbonyl temafloxacin dithiocarbamate complex: a potential Streptococci pneumoniae infection radiotracer. J Radioanal Nucl Chem 288:411
Shah SQ, Khan MR (2011) Synthesis of techentium-99m labeled clinafloxacin (99mTc–CNN) complex and biological evaluation as a potential Staphylococcus aureus infection imaging agent. J Radioanal Nucl Chem 288:423
Shah SQ, Khan MR (2011) Synthesis of 99mTcV≡N-pazufloxacin dithiocarbamate complex and biological evaluation in Wister rats artificially infected with Escherichia coli. J Radioanal Nucl Chem 288:511
Shah SQ, Khan MR (2011) Radiosynthesis and biological evaluation of 99mTc–prulifloxacin in artificially infected animals. Nuklearmedizin (in press)
Shah SQ, Khan MR (2011) Radiosynthesis and characterization of the 99mTc–fleroxacin (99mTc–FXN) complex: a novel Escherichia coli infection imaging agent. Transit Met Chem 36:283
Shah SQ, Khan MR (2011) Evaluation of 99mTcN–moxifloxacin dithiocarbamate, as a potential radiopharmaceutical for scintigraphic localization of infectious foci. J Radioanal Nucl Chem 288:357
Pankey GA, Ashcraft DS (2005) In vitro synergy of ciprofloxacin and gatifloxacin against ciprofloxacin-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:2959
Hershberger E, Rybak MJ (2000) Activities of trovafloxacin, gatifloxacin, clinafloxacin, sparfloxacin, levofloxacin, and ciprofloxacin against penicillin-resistant Streptococcus pneumoniae in an in vitro infection model. Antimicrob Agents Chemother 44:598
Welling MM, Paulusma-Annema A, Batler HS, Pauwels EKJ, Nibbering PH (2000) Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med 27:292
Boschi A, Duatti A, Uccelli L (2005) Development of technetium-99m and rhenium-188 radiopharmacueticals containing a terminal metal-nitrido multiple bond for diagnosis and therapy. Top Curr Chem 252:85
Bandoli G, Dolmella A, Porchia M, Tisato E, Refosco P (2001) Structural overview of technetium compounds (1993–1999). Coord Chem Rev 214:43
Schwochau K (2001) Technetium chemistry and radiopharmaceutical applications. Wiley-VCH, Weinheim
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S.Q., Khan, M.R. 99mTcN–gatifloxacin dithiocarbamate complex: a novel multi-drug-resistance Streptococcus pneumoniae (MRSP) infection radiotracer. J Radioanal Nucl Chem 289, 903–908 (2011). https://doi.org/10.1007/s10967-011-1180-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1180-1