Abstract
Garenoxacin (GXN) was modified to its dithiocarbamate followed by radiolabeling with technetium-99m (99mTc) through [99mTc-N]2+ core. The suitability of the 99mTcN–Garenoxacin dithiocarbamate (GXND) complex as a potential multiresistant Staphylococcus aureus (MDRSA) and penicillin-resistant Streptococci (PRSC) infection radiotracer was assessed in artificially infected rats (AFRT). The radiolabeled complex was investigated for its radiochemical purity (RCP), permanence in serum using HPLC and TLC methods. In vitro binding with MDRSA and PRSC was performed at 37 °C. The 99mTcN–GXND showed maximum RCP of 98.00 ± 0.22% and remained more than 90% stable up to 4 h. The 99mTcN–GXND showed saturated in vitro binding with living MDRSA and PRSC, respectively. The complex showed normal biodistribution in healthy rats (HRT), however in AFRT, seven fold uptakes was observed in infected muscle as compared to inflamed and normal muscles. Based on the high RCP, stability in serum, better in vitro binding with bacteria, biodistribution behavior and the target to non-target (infected to inflamed muscle) ratio, we recommend the 99mTcN–GXND complex for in vivo investigation of MDRSA and PRSC infection in human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection identification and its discrimination from inflammation is a challenging dilemma in remedial practices. The implications of in time diagnosis on the appropriate management of infectious foci’s are very significant [1, 2].
In the last two decades, great advances in infection imaging have been achieved [3–14]. To facilitate the clinicians and to treat the patients appropriately in case of indecisive clinical history, we have recently reported a number of technetium-99m (99mTc) labeled antibiotics as potential in vivo infection radiotracers [15–20]. Based on our results of high radiochemical purity (RCP) yield, stability in serum, saturated in vitro binding with bacteria, better biodistribution in artificially infected animals, high target to non target ratio and scintigraphy that give clinically useful images of infectious foci. These findings encourage us to investigate new antibiotics for infection imaging with greater promise.
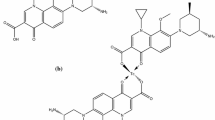
The major reasons for relentless infections are gram positive (G+) bacteria which over the decade gradually extended resistance to the existing drugs thus stimulated the investigation for a better antibiotic. Garenoxacin (GXN) is a des-fluoro(6)-quinolones (1-cyclopropyl-8-(difluoromethoxy)-7-[(1R)-1-methyl-2,3-dihydro-1H-isoindol-5-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid) (Fig. 1a) that emerged as an effective antibiotic against multiresistant Staphylococcus aureus (MDRSA) and penicillin-resistant Streptococci (PRSC), posing its suitability as promising agent against both the pathogen [21, 22].
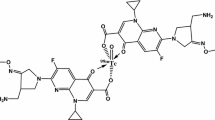
In continuation to our ongoing investigation, now we report the garenoxacin dithiocarbamate (GXND) as the tetradentate chelator for the radiosynthesis of the 99mTcN–GXND complex (Fig. 1c). In the present study, viability of the 99mTcN–GXND complex to trace soft tissue infections in animal models was assessed. The suitability of the complex was further examined in terms of in vitro permanence in saline and serum, in vitro binding with MDRSA and PRSC, biodistribution in AFRT.
Experimental
Materials
Garenoxacin from (Bristol-Myers Squibb, Syracuse, UK), TLC (Merck), succinic dihydrazide (SDH), propylenediamine tetra acetic acid (PDTA) and all the other chemicals and solvents of analytical grade (Sigma). RP-HPLC (Shimadzu, Japan), well counter, scalar count rate meter (Ludlum, USA), Dose calibrator (Capintech, USA), and Gamma camera GKS-1000 (GEADE Nuclearmedizine system, Germany).
Method
Preparation of GXND
Garenoxacin dithiocarbamate was synthesized by mixing 3.625 g of GXN with 1.2 g NaOH followed by the addition of 11 mL of 40% tetrahydrofuran in a sterilized flask. After stirring for 10 min in ice-bath 1 mL carbon disulfide was added to the reaction mixture followed by 8 h continuous shaking in an ice-bath. The solvent was removed under low pressure and the product was recovered.
Synthesis of 99mTcN–GXND complex
Sodium pertechnetate (Na99mTcO4 −) freshly eluted, 0.5 mL (1–2 mCi) was added to 0.05 mg SnCl2·2H2O, 5.0 mg PDTA, 5.0 mg SDH. The mixture was incubated at room temperature for 10 min and thereafter 2 mg of GXND was added.
Characterization of 99mTcN–GXND complex
99mTcN–GXND complex was characterized by RP-HPLC chromatography using Shimadzu SCL-10 AVP system equipped with SDP-10 AVP UV detector operating at 254 nm, Packard 500 TR series flow scintillation analyzer, binary pump and online degasser. 10 μL of the labeled complex was injected into C-18 column (4.6 × 150 mm2). The flow rate of 1 mL/min was applied for 15 min using water (W) and methanol (M) as mobile phase. For 0–2 min (100% W), 2–5 min (100–75% W), 5–7 min (75–66% W), 7–10 min (34–100% M), 10–12 min (100% M) and 12–15 min (100% M–100% W). Single well counter interface with scalar count rate meter (SWCSR) was employed for measuring the radiochemical activity in each fraction collected in a sterile vial.
Serum stability
In vitro stability of the 99mTcN–GXND complex was evaluated in serum by incubating 0.2 mL of the preparation with 1.8 mL serum at 37 °C. Aliquots, at different intervals post incubation were obtained and for thin layer chromatography executed on polyamide strip. The strip was developed in saline and CH2Cl2:CH3OH (9:1) (v/v) for the determination of various components of the complex. The developed strips were measured for activity using SWCSR.
Binding with MDRSA and PRSC
MDRSA and PRSC in vitro up take was assessed by adopting the reported method with slight modification [23]. Briefly, 10 MBq of 99mTcN–GXND was transferred to a sterilized test tube containing 0.1 mL sodium phosphate buffer (Na-PB) followed by addition of 0.8 mL (50%, v/v) 0.01 M acetic acid containing approximately 1 × 108 colony forming units (CFU) of MDRSA and PRSC. The mixture was incubated at 4 °C for 1 h and the pH was adjusted to pH 5 followed by centrifugation with 2,000 rpm for 10 min. Thereafter, the supernatant was removed and the pellets were resuspended in 2 mL of Na-PB and repeated the centrifugation with the same spin speed. Finally, the pellets were counted for activity using SWCSR.
Biodistribution
Twenty healthy (Sprague–Dawley male rat: weight range, 180–220 g) were selected and arranged in four groups (G-I, G-II, G-III, and G-IV) of five rats each. Intramuscularly (IM) sterile turpentine oil 0.2 mL was injected to the left thigh of all the selected rats. G-I was IM (right thigh) infected with living MDRSA (1 × 108 cfu) and G-II with heat killed. Similarly, G-III and G-IV were IM infected with living and heat killed PRSC (1 × 108 cfu), respectively. After, 24 h, 0.5 mL (37 MBq) of the 99mTcN–GXND were injected intravenously to all the animals (G-I to G-IV). Thereafter, the animals were killed (in accordance with the rules and regulation stipulated in the manual of the Nuclear Medicine Research Laboratory Part-I and II). Percent absorbed dose per gram (each) in blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed muscle and normal muscle was determined using SWCSR.
Result and discussion
Radiochemistry and HPLC characterization of 99mTcN–GXND
The tetradentate GXND (two sulfur atoms, a carboxyl and hydroxyl group) under substitution reaction with an intermediate [99mTcN]2+core yield 99mTcN–GXND complex as given in Fig. 1c. By similarity with the structure of bis(diethyldithiocarbamato) nitride technetium-99m complex [24]. We propose a similar structure for our complex (Fig. 1c), with a square pyramidal geometry having a TcN:Ligand ratio of 1:1.
Figure 2 showed two peaks, one at 4.4 and the second one at 12.6 min. The radio-peaks at 4.4 represents [99mTc-N]2+ intermediate and of 12.6 min the 99mTcN–GXND complex.
The radiochemical purity observed at different intervals are shown in Fig. 3. It was observed that the complex showed high and longer stability in saline. The maximum RCP value observed was 98.00 ± 0.22% at 1 min and remained more than 90% stable up to 240 min after reconstitution for 99mTcN–GXND.
Stability of 99mTcN–GXND in serum
At 37 °C the complex showed good stability in serum as shown in Fig. 4. The complex was found stable in serum up to 4 h however it went down to 78% after 16 h.
In vitro binding with MDRSA and PRSC
The in vitro binding affinity of the complex with MDRSA and PRSC is shown in Fig. 5. The complex showed saturated in vitro binding with living MDRSA and PRSC respectively.
Biodistribution MDRSA and PRSC infected rats
The percent up take of the 99mTcN–GXND in various organs of the artificially MDRSA and PRSC infected rats (AFRT) are given in Tables 1 and 2. It was observed that activity in blood of the rat infected with MDRSA and PRSC was high but it went down with the passage of time. No significant difference in the activity up take with regards to blood was noted in all animals either infected by MDRSA or PRSC (alive or heat killed). The uptake of 99mTcN–GXND to G-I to G-IV, infected with MDRSA and PRSC gave almost similar profile with a seven fold up take in the target organ as compared to the inflamed and normal muscle. Initially, the higher uptake in liver, spleen, and blood was observed which significantly declined with time. A reciprocal behavior was noted in kidney, where the activity went up with time. The appearance of activity in urinary system and disappearance from the circulatory system confirmed the normal route of excretion. The appearance of activity almost seven fold in the infected muscle further confirmed the feasibility of the radiotracer as specific and potential radiopharmaceutical for the diagnosis of infection caused by MDRSA and PRSC.
Conclusion
Garenoxacin dithiocarbamate radiolabeling with 99mTc through [99mTc-N]2+ core was investigated and its feasibility as a potential MDRSA and PRSC infection radiotracer was evaluated in AFRT. Based on the high RCP, stability in serum, better in vitro binding, biodistribution behavior and target to non-target (infected to inflamed muscle) ratio we recommend the 99mTcN–GXND for in vivo localization of MDRSA and PRSC infection in human.
References
Basu S, Chryssikos T, Moghadam-Kia S, Zhuang H, Torigian DA, Alvai A (2009) Positron emission tomography as a diagnostic tool in infection: present role and future possibilities. Semin Nucl Med 39:36
Gallagher H, Ramsay SC, Barnes J, Maggs J, Cassidy N, Ketheesan N (2006) Neutrophil labeling with [99mTc]-technetium stannous colloid is complement receptor 3-mediated and increases the neutrophil priming response to lipopolysaccharide. Nucl Med Biol 33:433
Lahiri S, Sarkar S (2007) Studies on 66, 67 Ga- and 199 Tl-poly(N-vinylpyrrolidone) complexes. Appl Radiat Isot 65:309
Zhang J, Wang X, Tian C (2006) Synthesis of a bis-(N-butyl-dithiocarbamto)-nitrido 99mTc complex: a potential new brain imaging agent. J Radioanal Nucl Chem 273:15
Zhang J, Wang X (2000) Synthesis of 99mTcN(IPDTC)2 and its biodistribution in mice. J Radioanal Nucl Chem 249:573
Hong Z, Ningyi J, Lin Z (2009) Experimental studies on imaging of infected site with 99mTc-labeled ciprofloxacin in mice. Chin Med J 122:1907
Oh SJ, Ryu J, Shin JW, Yoon EJ, Ha H, Cheon JH, Lee HK (2002) Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Appl Radiat Isot 57:193
Zhang J, Guo H, Zhang S, Lin Y, Wang X (2008) Synthesis and biodistribution of a novel 99mTcN complex of ciprofloxacin dithiocarbamate as a potential agent for infection imaging. Bioorg Med Chem Lett 18:51
Motaleb MA (2007) Preparation of 99mTc-cefoperazone complex, a novel agent for detecting sites of infection. J Radioanal Nucl Chem 272:167
Motaleb MA (2007) Preparation and biodistribution of 99mTc-lomefloxacin and 99mTc-olfloxacin complex. J Radioanal Nucl Chem 272:95
EL-Gany EA, EL-Kolaly MT, Amine AM, EL-Sayed AS, Abdel-Gelil F (2005) Synthesis of 99mTc-pefloxacin:a new targeting agent for infectious foci. J Radioanal Nucl Chem 266:131
Roohi S, Mushtaq A, Jehangir M, Ashfaq MS (2006) Synthesis, quality control and biodistribution of 99mTc-kanamycin. J Radioanal Nucl Chem 267:561
Motaleb MA (2009) Preparation, quality control and stability of 99mTc-sparafloxacin complex, a novel agent for detecting sites of infection. J Label Compd Radiopharm 52:415
Chattopadhyay S, Das SS, Chandra S, De K, Mishra M, Sarkar BR, Sinha S, Ganguly S (2010) Synthesis and evaluation of 99mTc-moxifloxacin, a potential infection specific imaging agent. Appl Radiat Isot 68:314
Qaiser SS, Khan AU, Khan MR (2010) Synthesis, biodistribution and evaluation of 99mTc-Sitafloxacin kit: a novel infection imaging agent. J Radioanal Nucl Chem 284:189
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis of 99mTc-nitrifuratonin a novel radiotracer for in vivo imaging of Escherichia coli infection. J Radioanal Nucl Chem (in press)
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biodistribution of 99mTc-rifampicin: a novel radiotracer for in vivo infection imaging. Appl Radiat Isot 68:2255
Shah SQ, Khan AU, Khan MR (2010) 99mTc-Novobiocin: a novel radiotracer for infection imaging. Radiochim Acta (in press)
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis, biodistribution and scintigraphy of the 99mTc-Teicoplanin complex in artificially infected animal models. J Label Compd Radiopharm (in press)
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biological evaluation of 99mTcN-sitafloxacin dithiocarbamate as a potential radiotracer for Staphylococcus aureus infection. J Radioanal Nucl Chem (in press)
Ohsaki Y, Morita K, Takeda H, Kishino S, Okumura S, Fujiuchi S (2010) Pharmacokinetics of garenoxacin in elderly patients with respiratory tract infections. Int J Antimicrob Agents 35:603
Goto K, Yabe K, Suzuki T, Jindo T, Sanbuissho A (2010) Chondrotoxicity and toxicokinetics of novel quinolone antibacterial agents DC-159a and DX-619 in juvenile rats. Toxicology 276:122
Welling MM, Paulusma-Annema A, Batler HS, Pauwels EKJ, Nibbering PH (2000) Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med 27:292
Baldas J, Bonnyman J, Poer PM, Williams GA, Mackay MF (1981) Synthesis And Structure of bis(diethyldithiocarbamato)nitridotechnetium(V)—a technetium-nitrogen triple bond. J Chem Soc Dalton Trans 9:1798
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S.Q., Khan, A.U. & Khan, M.R. Radiosynthesis and biodistribution of 99mTcN–Garenoxacin dithiocarbamate complex a potential infection imaging agent. J Radioanal Nucl Chem 288, 59–64 (2011). https://doi.org/10.1007/s10967-010-0871-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0871-3