Abstract
Radiosynthesis of 99mTc-sitafloxacin (99mTc-STF) complex and its efficacy as a potential infection imaging agent was evaluated. Effect of sitafloxacin (STF) concentration, sodium pertechnetate (Na99mTcO4), stannous chloride dihydrate (SnCl2·2H2O), and pH on the % radiochemical purity yield (RCP) of 99mTc-STF complex was studied. A stable 99mTc-STF complex up to 120 min with maximum %RCP yield was observed by mixing 2 mg of STF with 3 mCi of Na99mTcO4 and 150 μL of SnCl2·2H2O (1 μg/μL in 0.01 N HCl) at a pH 5.5. Artificially infected rats with Staphylococcus aureus were used for studying the biodistribution behavior of the 99mTc-STF complex. After 30 min of the intravenous (I.V.) administration of the 99mTc-STF complex, 7.50 ± 0.10% was absorbed in the infected thigh of the rats and the uptake gradually increased to 18.50 ± 0.20% within 90 min. Rabbits with artificially induced infection were used for evaluating the scintigraphic accuracy. Higher uptake in the infected thigh was observed after 2 h of I.V. administration of the 99mTc-STF complex. Target to non-target organ ratio of the % absorbed dose incase of infected/normal muscle was 6.82 ± 0.40, 17.11 ± 0.60, and 23.13 ± 1.00% at 30, 60 and 90 min of administration. Stable and higher %RCP, higher uptake in the infected thigh, and spectral studies, recommend the 99mTc-STF for routine infection imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diagnosis of infection and its discrimination from inflammation at early stage is quite important for clinicians for its rapid treatment with appropriate drug to reduce the rate of morbidity and mortality [1]. Sophisticated technologies like computerized tomography (CT) and magnetic resonance imaging (MRI) are failed to localize the infectious foci in preliminary phase [2, 3].
67Gallium citrate and 111Indium labeled leucocytes were used for infection imaging but their high radiation dose and other disadvantages as discussed in literature and insignificant clinical results abandoned their use as infection imaging agent [4, 5]. Recently, technetium-99m (99mTc) labeled antibiotics like ciprofloxacin [6, 7], ciprofloxacin dithiocarbamate [8], cefoperazone [9], lomefloxacin, ofloxacin [10], cefuroxine [11], pefloxacin [12], kanamycin [13], sparafloxacin [14], and moxifloxacin [15], are proposed for infection localization because of their higher %RCP yields, better biodistribution behavior and easy preparation then the 111In and 99mTc labeled leukocytes [16].
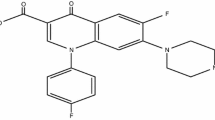
Sitafloxacin (STF), {(−)-7-[(7S)-7-amino-5-azaspiro[2.4]heptan-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluoro-1-cyclopropyl]-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid} is another new antimicrobial agent, having similar 4-fluroquinolone skeleton (Fig. 1) that inhibit the growth of various aerobic and anaerobic gram-positive (G+) and gram-negative (G−) bacteria [17]. The antimicrobial activity of STF, against G+ and G− bacteria, is found better than the available reported range [18].
The aim of the present investigation is to synthesize 99mTc-STF complex and to determine its stability and efficacy as a potential infection imaging agent in terms of percent radiochemical purity yield (%RCP), biodistribution and animal scintigraphy, using different concentration of the STF, Na99mTcO4, SnCl2·2H2O, at pH 5–6.
Experimental
Materials and methods
Sitafloxacin (STF) from Daiichi Sanko, Japan, analytical grade stannous chloride dihydrate, from Sigma, thin layer chromatography (TLC) aluminum strip and insulin syringes from Merck, were used in this investigation.
Equipment
Microwave oven from Panasonic, Matsushita Electric Industrial Co. model MN-S674 MF, single well counter interface with scalar count rate meter from Ludlum Measurements, Inc., USA, (Model 243 & 2000), and Gamma camera from GEADE Nuclearmedizine, Germany (model GKS-1000: SPECT system), were used.
Animal and pathogen
Rats, rabbit, and pathogen (Staphylococcus aureus) from Veterinary Research Institute, Peshawar, Pakistan.
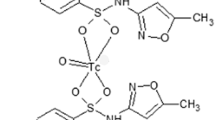
Preparation of 99mTc-STF complex
Five sets of 50, 100, 150 and 200 μL of SnCl2·2H2O (1 μg/μL in 0.01 N HCl) solutions were prepared in different vials at pH 5.0, 5.2, 5.5, 5.7 and 6. 1–5 mg of STF and 1–5 mCi of Na99mTcO4 were added through sterilized syringe and heated in a microwave oven up to 100 °C for 15 s. All the solutions were then filtered through 0.22 μm cellulose ester membrane and the products were analyzed for %RCP yield.
Determination of RCP yield
TLC was used to check the %RCP yield of the 99mTc-STF complex. TLC strip was used as stationary and acetone as the mobile phase to separate the tagged 99mTc-STF and reduced hydrolyzed technetium (99mTcO2) from the free technetium (99mTcO −)4 . In this system, the tagged 99mTc-STF and 99mTcO2 stayed at the origin (Rf 2–3), while the 99mTcO4 − component moved with the solvent front (Rf 7–9). To separate 99mTc-STF from 99mTcO2, the TLC strip was impregnated with human serum albumin (HSA), using ethanol:ammonia:water (2:1:5) as the mobile phase. In this system 99mTc-STF moved with the solvent front (Rf 7–9) leaving 99mTcO2 at the bottom (Rf 2–3). The developed TLC strip was divided into two equal parts (Rf 5) and each part was then counted for activity, using single well interface with scalar count rate meter.
Animal model and biodistribution
Male rats (weight range, 20–25 g) were sedated with intraperitonial injection of 0.1 mL in saline containing 1 mg fluanisone and 0.03 mg fentanyl citrate. 0.05 mL broth containing 2 × 108 organisms of Staphylococcus aureus was injected into the right thigh for inducing artificial infection and after 24 h, 0.1 mL of the sterile turpentine oil was injected in the left thigh of the rat. 37 MBq of the 99mTc-STF was injected and after 30 min, 1 and 2 h, the animals were sacrificed. Blood sample was directly obtained from the heart through needle aspiration and samples of the infected, inflamed, normal muscle, liver, spleen, lungs and kidney were weighed and % injected dose absorbed per gram was calculated using single well counter interface with scalar count rate meter.
Animal scintigraphy
Broth, 0.1 mL containing 2 × 108 organisms of Staphylococcus aureus injected into the right thigh of a pathogen free rabbit before 12 h of scintigraphy. 0.1 mL of the sterile turpentine oil was I.M. injected in left thigh and 74 MBq of the 99mTc-STF was I.V. injected to the same rabbit. Whole body images were obtained using Gamma camera at 30, 60, and 90 min.
Results and discussion
%RCP yield and effect of stannous chloride dihydrate
The %RCP yield was determined at 15 s, 30, 60, 90, 120 and 240 min after labeling and the results are shown in Table. 1. A stable 99mTc-STF complex up to 120 min with maximum %RCP yield (98.96 ± 0.10 %) was observed by mixing 2 mg of STF with 3 mCi of Na99mTcO4 and 150 μL of SnCl2·2H2O (1 μg/μL in 0.01 N HCl) at a pH 5.5. Decrease in %RCP yield and tagging time was observed at the lower and the higher concentration of the stannous chloride than 150 μL as shown in Fig. 2.
Effect of pH, concentration of STF and sodium pertechnetate
The effect of pH on %RCP values was studied from pH 5 to 6 and the results are shown in Fig. 3. Maximum %RCP yield was observed at pH 5.5 and minimum at pH 6. The effect of concentration of STF on 99mTc-STF complex was checked between 1 to 5 mg and the results are shown in Fig. 4. Higher RCP yield was observed using 2 mg of STF. Sodium pertechnetate amount was characterized over a range of 5 mCi. It was observed that the labeling shows maximum RCP yield at 3 mCi and the values gradually decreases as the amount of sodium pertechnetate, either increases or decreases from 3 mCi, as shown in Fig. 5.
Biodistribution in various organs of rat
Biodistribution behavior of the labeled preparation was investigated in artificially infected rats after 30, 60 and 90 min of I.V. administration of the 99mTc-STF complex and the results are shown in Table 2. The % adsorbed dose in infected muscle was 7.50 ± 0.10, 16.25 ± 0.15 and 18.50 ± 0.20%. In artificially inflamed and normal muscles a decrease in the % absorbed dose was observed and the value of % absorbed dose goes down from 1.25 ± 0.15 to 0.90 ± 0.10% and 1.00 ± 0.25 to 0.80 ± 0.20%. Target to non-target organ ratio of the % absorbed dose incase of infected/normal muscle was 6.82 ± 0.40, 17.11 ± 0.60, and 23.13 ± 1.00% at 30, 60 and 90 min of administration. The same trailing pattern was observed for liver and the % absorbed activity was gradually decreased from 20.50 ± 0.50 to 5.50 ± 0.00%. In kidney the % absorbed activity was 10.50 ± 0.50 and gradually the uptake goes higher up to 27.25 ± 0.22% within 90 min. This increases in the uptake values for kidney suggested that drug is biodegradable and its route of excretion is kidney. The values of the activity in blood gradually deceased from 22.60 ± 0.25 to 2.50 ± 0.20%. This decrease in the % absorbed dose suggested that the labeled drug shows normal biodistribution of tagged STF.
Animal scintigraphy
The efficacy of the 99mTc-STF was finally evaluated using rabbits, having artificially induced infection and inflammation in contra-lateral thighs. Whole body static images of the infected rabbits were obtained at 30, 60 and 90 min after I.V. administration of the 99mTc-STF. The images are shown in Fig. 6a–c. Initially the activity was higher in the heart, liver and spleen of the rabbit as shown in Fig. 6a, and uniform distribution in the infected, inflamed, normal muscles and other organs were observed. Higher uptake in the infected muscle than inflamed and normal muscle was observed after 60 min as shown in Fig. 6b. However the scan after 60 min of the I.V. administration was blurred giving insignificant diagnostic information. The uptake in the infected muscle significantly increases after 90 min giving clear visibility of the infected region of the rabbit than the normal and inflamed portions as shown in Fig. 6c.
Conclusion
The 99mTc-STF complex showed stability up to 98.96 ± 0.10% and remained >90% tagged up to 2 h using 2 mg of STF, 3 mCi Na99mTcO4, 150 μL SnCl2·2H2O at pH 5.5. The labeled complex under similar conditions showed maximum uptake in the infected muscle from 7.50 ± 0.10, 16.25 ± 0.15 to 18.50 ± 0.20 within 2 h after I.V. administration to the rats. Target to non-target organ ratio of the absorbed dose incase of infected/normal muscle was 6.82 ± 0.40, 17.11 ± 0.60, and 23.13 ± 1.00 at 30, 60 and 90 min of administration. The scintigraphic accuracy of the 99mTc-STF complex is significantly high, clearly showing the uptake in the infected region. The 99mTc-STF complex is recommended for imaging the infectious foci at early stage.
References
Rusckowski M, Gupta S, Liu G, Dou S, Hnatowich DJ (2004) J Nucl Med 45:1201
Welling MM, Lupetti A, Balter HS, Lanzzeri S, Souto B, Rey AM, Savio EO, Paulusma-Annema A, Pauwels EKJ, Nibbering. PH (2001) J Nucl Med 42:788
Sarda L, Cremieux A, Lebellec Y, Meulemans A, Lebtahi R, Hayem G, Genin R, Delahaye N, Huten D, Guludec DL (2003) J Nucl Med 44:920
Yamamoto N, Fujita J, Shinzato T, Higa F, Tateyama M, Tohyama M, Nakasone I, Yamane N (2006) Int J Antimicrob Agents 27:171
Fujikawa K, Chiba M, Tanaka M, Sato K (2005) Antimicrob Agent Chemother 49:3040
Chattopadhyay S, Das SS, Chandra S, De K, Mishra M, Sarkar BR, Sinha S, Ganguly S (2010) Appl Radiat Isot 68:314
Motaleb MA (2009) J Label Compd Radiopharm 52:415
Zhang J, Guo H, Zhang S, Lin Y, Wang X (2008) Bioorg Med Chem Lett 18:5168
Hong Z, Ning-yi J, Lin Z (2009) Chin Med J 16:1907
Roohi S, Mustaq A, Jehangir M, Malik SA (2006) J Radioanal Nucl Chem 267:561
Lumbrecht FY, Yilmaz O, Unak P, Seytigolu B, Durkan K, Baskan H (2008) J Radioanal Nucl Chem 277:491
Motaleb MA (2007) J Radioanal Nucl Chem 272:167
El-Ghany EA, Amine AM, El-Sayed AS, El-Kolaly MT, Abdel-Gelil F (2005) J Radioanal Nucl Chem 266:125
Amin AM, Gouda AA, El-Sheikh R, Seddik U, Hussien H (2009) J Radioanal Nucl Chem 280:589
Motaleb MA (2007) J Radioanal Nucl Chem 272:95
Oh SJ, Ryu J, Shin JW, Yoon EJ, Ha HJ, Cheon JH, Lee HK (2002) Appl Radiat Isot 57:193
Suzuki T, Kitaoka H, Miwa Y, Taga T (2000) Anal Sci 16:343
Nakamura S, Yanagihara K, Araki N, Morinaga Y, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, Kamihira S, Kohno S (2009) Int J Antimicrob Agents 34:210
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qaiser, S.S., Khan, A.U. & Khan, M.R. Synthesis, biodistribution and evaluation of 99mTc-sitafloxacin kit: a novel infection imaging agent. J Radioanal Nucl Chem 284, 189–193 (2010). https://doi.org/10.1007/s10967-010-0470-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0470-3