Abstract
99mTc–rufloxacin (99mTc–RUN) complex was prepared by reaction of different amounts of reduced sodium pertechnetate with different amount of Rufloxacin (RUN) antibiotic for the in vivo scintigraphic localization of the Staphylococcus aureus (S. aureus) infectious foci in Male Wister Rats (MWR) model. The 99mTc–RUN complex was radiochemically and biologically characterized in terms of radiochemical stability in saline, serum, in vitro binding with S. aureus and biodistribution in artificially infected with S. aureus MWR. The 99mTc–RUN complex showed stability more than 90% up to 240 min in normal saline with a maximum stability value of 98.10 ± 0.18% at 30 min after reconstitution. At 37 °C the complex showed in vitro permanence in serum up to 16 h with 13.90% side products during incubation. The 99mTc–RUN complex showed saturated in vitro binding with S. aureus at different intervals with a maximum uptake value of 71.50%. Infected to normal muscle, infected to inflamed and inflamed to normal muscles ratios were approximately 6.04, 4.31 and 1.40. Based on the stability of the complex in saline, serum, in vitro binding with S. aureus and biodistribution results, the 99mTc–RUN complex is recommended for in vivo scintigraphic localization of the S. aureus in vivo infectious foci in human.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Imaging of bacterial infection is still a serious concern of the medical community in spite of the innovation in the diagnostic facilities and expansion in the antibiotics. The role of the advanced diagnostic facilities like Computerized Tomography (CT) and Magnetic Resonance Imaging (MRI) has proven inadequate in the diagnosis of infection and its discrimination from inflammation in the early stages. The Nuclear Medicine Scintigraphy (NMS) has been recognized as a promising technique for the diagnosis of infection and its discrimination from inflammation [1, 2].
The available radiopharmaceuticals [3–15] and our recently reported ones [16–24] have supported the NMS and the promising diagnostic results encouraged us to look for more receptive and explicit agents for the localization of infectious foci.
Rufloxacin (RUN) Fig. 1a. 9-fluoro-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-{1,4}thiazino {2,3,4-ij} quinoline-6-carboxylic acid is a new quinoline broad spectrum antibiotic intended for the treatment of a wide range of infections caused by various pathogens [25, 26]. In the current investigation the radiolabeling of RUN with a gamma (γ-) emitter technetium-99m (99mTc) was investigated. The effect of various reacting species on the percent radiochemical purity yield, stability in normal saline at 30, 60, 90, 120 and 240 min, in vitro stability in serum at 37 °C up to 16 h, in vitro binding with Staphylococcus aureus (S. aureus) and biodistribution in infected (S. aureus) rats of the 99mTc–RUN complex was assessed.
Experimental
Materials
Rufloxacin (RUN) (Jinan Haohua Industry Co., Ltd Shandong, China), TLC (Merck, Germany) and the other chemicals and solvents of analytical grade (Sigma). RP-HPLC (Shimadzu, Japan) well counter and scalar count rate meter (Ludlum, USA) Dose calibrator (Capintech, USA) and Gamma camera GKS-1000 (GEADE Nuclearmedizine system, Germany).
Methods
Radiosynthesis of the 99mTc–rufloxacin complex
Rufloxacin (RUN), 0.5–5.0 mg (with 0.5 mg rise) was taken in ten sterilized syringes. After that sodium pertechnetate 0.5–5.0 mCi (with 0.5 mCi increase) were taken in ten different nitrogen gas filled vials. Thereafter, stannous chloride 25–250 μL (with 25 μL: μg/μL 0.01 N HCL) were added to the ten vials followed by injection of RUN through syringes. The pH of the reaction mixtures was set between 5.1 and 6.0 (with 0.1 unit augment). The reaction mixtures were incubated at room temperature followed by filtration through Millipore.
Characterization of the 99mTc–RUN complex
The 99mTc–RUN complex was radiocharacterized using the HPLC method reported earlier [22]. Briefly, 10 5 μL of the 99mTc–RUN complex was loaded on the C-18 column of the Shimadzu SCL-10 AVP system, equipped with SDP-10 AVP UV detector operating at 254 nm, Packard 500 TR series flow scintillation analyzer, binary pump and online degasser. Thereafter, the column was eluted with 1 mL/min of water:acetonitrile (1:9) mixture for 15 min followed by collection of the radio-fractions in separate vials. The radio-fractions were measured for radioactivity in well counter interface with scalar count rate meter (WCSR). The 99mTc–RUN complex was characterized at 30, 60, 90 and 120 min, subsequent to the reconstitution for determination of various radiospecies.
Radiochemical stability in serum
The in vitro radiochemical stability of the 99mTc–RUN complex was assessed in serum for 16 h at 37 °C. The 99mTc–RUN complex, 0.2 mL was incubated with 1.8 mL of serum at 37 °C. Thereafter, 1 μL aliquots of the incubated mixture immediately after incubation and at 2, 4, 6, 8, 10, 12, 14 and 16 h was taken and spotted on TLC strips. The strips were then developed in CH2Cl2:CH3OH (9:1) (v/v) and thereafter divided into two equal parts for measurement of the radiochemical stability using WCSR.
In vitro binding with S. aureus
The binding of 99mTc–RUN complex with S. aureus was assessed using the reported method [27]. Briefly, to a sterilized test tube having 0.1 mL of sodium phosphate buffer (Na-PB) was added 0.2 mL of the freshly prepared 99mTc–RUN complex. Subsequently 0.01 M (0.8 mL) acetic acid (50% v/v) including 1 × 108 colony forming units (CFU) of the S. aureus was added to the test tube followed by incubation for 1 h at 4 °C (pH 5). Thereafter, the mixture was centrifuged at 2,000 rpm for 10 min followed by removal of the supernatant. The pellets were resuspended in 4 mL Na-PB and repeated the incubation and centrifugation process again as described above. For percent uptake calculation the bacterial pellets were counted for activity using WCSR.
Biodistribution in S. aureus infected MWR
Biologically the percent absorption of the 99mTc–RUN complex in blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle was studied in artificially infected (S. aureus) MWR (weight, 140–180 g). Ten MWR in good physical condition and health were chosen and segregated into two groups of five each (A and B). Then 0.2 mL of sterile turpentine oil was infused intramuscularly (I.M.) (left thigh) to the healthy rats of group A and B for inducing artificial inflammation. Subsequently, 0.2 mL of living S. aureus in normal saline was I.M. infused (right thigh) to the group A and heat killed S. aureus to the group B (MWR) for induction of artificial infection. After 20 h, 0.5 mCi of the 99mTc–RUN complex was intravenously injected to the group A and B (MWR) followed by execution in accordance with the permitted regulations of the Nuclear Medicine Research Laboratory (NMRL), University of Peshawar. The percent uptake of the 99mTc–RUN complex per gram of the blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle was measured WCSR.
Results and discussion
Chemistry and characterization of the 99mTc–RUN complex
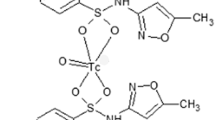
The role of acidic stannous chloride dihydrate (25–250 μL with 25 μL rise) (μg/μL 0.01 N HCL) was to reduce the 99mTc radioactive metal (required for complexation with ligand) and to suppress the formation of radio-colloids. The reduced 99mTc radioactive metal easily reacts with the two sulfur atoms, a carboxyl and hydroxyl groups of the bidentate rufloxacin (RUN) (Fig. 1a under substitution reaction gave in high yield the 99mTc–RUN complex as shown in Fig. 1b.
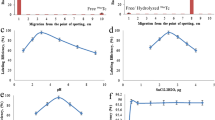
High radiochemical yield (98.10 ± 0.18%) of the 99mTc–RUN complex was observed by mixing 2.5 mCi of sodium pertechnetate with 125 μg/μL stannous chloride (μg/μL 0.01 N HCL) and 2 mg of RUN at pH 5.5. The radiochemical yield of the complex decreased to 91.00 ± 0.16 from 98.10 ± 0.18% within 240 min. The radiochemical yield went down in either condition whether we increase or decrease the amount of the reacting species from 2.5 mCi of the sodium pertechnetate, 125 μg/μL stannous chloride (μg/μL 0.01 N HCL), 2 mg of RUN and pH 5.5. The radiochemical yield of the 99mTc–RUN complex determined at 30, 60, 90, 120 and 240 min after reconstitution is given in Fig. 2.
The HPLC radiochromatogram of the 99mTc–RUN complex is given in Fig. 3. Two distinctly variable radiopeaks at 3.8 and 11.2 min of retention was observed. The radiopeak at 3.8 min of retention characterize the free pertechnetate and that at 11.2 min the succumb of the 99mTc–RUN complex.
The proposed structure of 99mTc–RUN complex (Fig. 1b) will have a square planner pyramidal geometry and 99mTcO:RUN ratio of 1:2 with the bidentate ligand.
Effects of the amount of RUN, sodium pertechnetate, reducing agent and pH on the percent radiochemical purity yield are given in Fig. 4. The 99mTc–RUN complex prepared by mixing amount other than 2.5 mCi of the sodium pertechnetate, 125 μg/μL stannous chloride (μg/μL 0.01 N HCL), 2 mg of RUN and pH 5.5, ultimately lowering the radiochemical purity yield.
Radiochemical stability in serum
The radiochemical permanence of the 99mTc–RUN complex in serum at 37 °C determined at different interval of incubation is shown in Fig. 5. A stable behaviour was noted with an unwanted 13.90% free radioactivity at 16 h after incubation. However, 90% stability was seen up to 4 h.
In vitro binding with S. aureus
The complex shows saturated in vitro binding with the 99mTc–RUN complex at 30, 60, 90 and 120 min. The in vitro binding of the 99mTc–RUN complex with S. aureus determined at 30, 60, 90 and 120 min is shown in Fig. 6. The maximum and saturated binding of 65.75–76.75% was observed between 30 to 120 min.
Biodistribution in S. aureus infected MWR
The in vivo absorption (%) of the 99mTc–RUN complex in blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle of MWR measured at 30, 60, 90 and 120 min of the I.V. administration is given in Table 1. The absorption (%) of the complex per gram of the blood was initially high and decreased to 4.50 ± 0.30 from 22.15 ± 0.32% within 120 min in group A (MWR). Almost similar profile was noted in MWR of group B. Similar trailing patron was seen in liver, spleen, stomach and intestine where the uptake gradually decreased from 18.85 ± 0.36 to 5.70 ± 0.34%, 10.10 ± 0.30 to 4.35 ± 0.32% and 8.50 ± 0.30 to 4.35 ± 0.36% respectively. However, in kidney the activity was gradually increased from 9.25 ± 0.34 to 23.50 ± 0.36% in 120 min. In infected muscle high activity was seen as compared to the inflamed and normal muscle. Figure 7 gives the activity uptake ratios of infected to normal muscle, infected to inflamed muscle and inflamed to normal muscle.
Conclusion
A novel 99mTc–rufloxacin complex was prepared for the in vivo scintigraphic localization of the S. aureus infectious foci in male Wister rats’ model. The permanence of the complex in saline, serum, in vitro binding with S. aureus and biodistribution results we recommend the 99mTc–rufloxacin complex for the in vivo scintigraphic localization of the S. aureus infectious foci in human.
References
Gallagher H, Ramsay SC, Barnes J, Maggs J, Cassidy N, Ketheesan N (2006) Neutrophil labeling with [99mTc]-technetium stannous colloid is complement receptor 3-mediated and increases the neutrophil priming response to lipopolysaccharide. Nucl Med Biol 33:433
Stumpe KDM, Dazzi H, Schaffner A, Schulthess GK (2000) Infection imaging using whole-body FDG-PET. Eur J Nucl Med 27:822
Chattopadhyay S, Das SS, Chandra S, De K, Mishra M, Sarkar BR, Sinha S, Ganguly S (2010) Synthesis and evaluation of 99mTc–moxifloxacin, a potential infection specific imaging agent. Appl Radiat Isot 68:314
Motaleb MA (2007) Preparation of 99mTc–cefoperazone complex, a novel agent for detecting sites of infection. J Radioanal Nucl Chem 272:167
Motaleb MA (2007) Preparation and biodistribution of 99mTc–lomefloxacin and 99mTc–olfloxacin complex. J Radioanal Nucl Chem 272:95
Zhang J, Guo H, Zhang S, Lin Y, Wang X (2008) Synthesis and biodistribution of a novel 99mTcN complex of ciprofloxacin dithiocarbamate as a potential agent for infection imaging. Bioorg Med Chem Lett 18:51
Roohi S, Mushtaq A, Jehangir M, Ashfaq MS (2006) Synthesis, quality control and biodistribution of 99mTc–kanamycin. J Radioanal Nucl Chem 267:561
Oh SJ, Ryu J, Shin JW, Yoon EJ, Ha H, Cheon JH, Lee HK (2002) Synthesis of 99mTc–ciprofloxacin by different methods and its biodistribution. Appl Radiat Isot 57:193
El-Gany EA, El-Kolaly MT, Amine AM, El-Sayed AS, Abdel-Gelil F (2005) Synthesis of 99mTc–pefloxacin: a new targeting agent for infectious foci. J Radioanal Nucl Chem 266:131
Motaleb MA (2009) Preparation, quality control and stability of 99mTc–sparafloxacin complex, a novel agent for detecting sites of infection. J Label Compd Radiopharm 52:415
Xia J, Wang Y, Yu J, Li S, Tang L, Zheng M, Liu X, Li G, Cheng D, Liang S, Yin D (2008) Synthesis, in vitro and in vivo behavior of 188Re(I)–tricarbonyl complexes for the future functionalization of biomolecules. J Radioanal Nucl Chem 275:325
Zhang J, Wang X, Jin C (2007) Synthesis and biodistribution of the 99mTc(CO)3–DEDT complex as a potential new radiopharmaceutical for brain imaging. J Radioanal Nucl Chem 272:91
Djokic DD, Jankovic DL, Stamenkovic LL, Pirmettis I (2004) Chemical and biological evaluation of 99mTc (CO)3 and 99mTc complexes of some IDA derivatives. J Radioanal Nucl Chem 260:471
Xia J, Long S, Yu J, Wang Y, Cao Z (2009) Pyridyl derivatives provide new pathways for labeling protein with fac-[188Re(CO)3(H2O)3]+. J Radioanal Nucl Chem 281:493
Zhang JB, Wang XB, Jin C (2006) Synthesis of 99mTc(CO)3-NOET via [99mTc(OH2)3(CO)3]+ precursor and comparative biological studies with 99mTcN-NOET. J Radioanal Nucl Chem 269:227
Qaiser SS, Khan AU, Khan MR (2010) Synthesis, biodistribution and evaluation of 99mTc–sitafloxacin kit: a novel infection imaging agent. J Radioanal Nucl Chem 284:189
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis of 99mTc–nitrifuratonin a novel radiotracer for in vivo imaging of Escherichia coli infection. J Radioanal Nucl Chem. doi:10.1007/s10967-010-0697-z
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biodistribution of 99mTc–rifampicin: a novel radiotracer for in vivo infection imaging. Appl Radiat Isot 68:2255
Shah SQ, Khan AU, Khan MR (2010) 99mTc–novobiocin: a novel radiotracer for infection imaging. Radiochim Acta (in press)
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis, biodistribution and scintigraphy of the 99mTc–teicoplanin complex in artificially infected animal models. J Label Compd Radiopharm. doi:10.1002/jlcr.1834
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biological evaluation of 99mTcN–sitafloxacin dithiocarbamate as a potential radiotracer for Staphylococcus aureus infection. J Radioanal Nucl Chem. doi:10.1007/s10967-010-0833-9
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biodistribution of 99mTcN–garenoxacin dithiocarbamate complex a potential infection imaging agent. J Radioanal Nucl Chem. doi:10.1007/s10967-010-0871-3
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biological evolution of 99mTc(CO)3–sitafloxacin dithiocarbamate complex: a promising Staphylococcus aureus infection radiotracer. J Radioanal Nucl Chem. doi:10.1007/s10967-010-0880-2
Shah SQ, Khan AU, Khan MR (2010) 99mTc(CO)3–Garenoxacin dithiocarbamate synthesis and biological evolution in rats infected with multiresistant Staphylococcus aureus and penicillin-resistant Streptococci. J Radioanal Nucl Chem. doi:10.1007/s10967-010-0892-y
Imbimbo BP, Klietmann W, Broccali GP, Cesana M, Aarons L (1997) Population pharmacokinetics of rufloxacin in patients with acute exacerbations of chronic bronchitis. Eur J Pharm Sci 5:37
Wang X, Zhao H, Nie L, Jin L, Zhang Z (2001) Europium sensitized chemiluminescence determination of rufloxacin. Anal Chim Acta 445:169
Welling MM, Paulusma-Annema A, Batler HS, Pauwels EKJ, Nibbering PH (2000) Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med 27:292
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S.Q., Khan, M.R. Radiocharacterization of the 99mTc–rufloxacin complex and biological evaluation in Staphylococcus aureus infected rat model. J Radioanal Nucl Chem 288, 373–378 (2011). https://doi.org/10.1007/s10967-010-0923-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0923-8