Abstract
Radio-complexation of sitafloxacin dithiocarbamate (SFDE) with technetium-99m (99mTc) using [99mTc(OH2)3(CO)3]+ precursor was investigated and compared with the 99mTc labeled SFDE prepared through 99mTcN core. The 99mTc(CO)3–SFDE radiocomlpex was assessed in terms of radiochemical purity (RCP), eternalness in serum, in vitro binding with Staphylococcus aureus (S. aureus) and biodistribution in artificially Staphylococcus aureus infected rats (SAIR). The feasibility of the 99mTc(CO)3–SFDE radiocomplex as a suitable S. aureus infection radiotracer was evaluated in SAIR. The complex showed maximum RCP of 98.45 ± 0.21% in saline and was remained tagged more than 90% up to 4 h. The complex was found stable in serum and after 16 h only 17.95% de-tagged radio-fractions was observed. Similar saturated in vitro binding behaviour was observed for both the radiocomplexes (99mTc(CO)3–SFDE and 99mTcN–SFDE) with living S. aureus. Both the radiocomplexes showed almost similar in vivo biodistribution in SAIR. Significantly higher but similar infected to normal muscle ratio was observed for both the radiocomplexes in SAIR. The results of radiochemical purity (RCP), eternalness in serum, in vitro binding and in vivo biodistribution in SAIR posed the 99mTc(CO)3–SFDE radiocomplex as suitable S. aureus infection radiotracer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of techniques are available to diagnose in vivo suspected infection, like Nuclear Medicine Scintigraphy (NMS), Ultrasonography (US), Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) [1]. However selection of the right technique is always a big problem for the clinicians with special reference to the fever of unknown origin [2].
The MRI, CT and US have been found sensitive techniques in imaging infection but the specificity and after treatment monitoring failure limits their use [3, 4]. In the last two decades, the NMS tenders substantial expectation after the development of the promising specific infection radiotracers [5–12]. Recently, we have reported technetium-99m (99mTc) labeled antibiotics and evaluated their efficacy as radiotracers [13–18]. High RCP values in saline, stability in serum, in vitro bacterial uptake, promising biodistribution, high target to non-target ratio and specific imaging results support our effort for the development of infection radiotracers.
The role of (TcO)3+ core in the development of newer and better 99mTc radiopharmaceuticals intended for various imaging and therapy is very vital. The [99mTc(CO)3(H2O)3]+ organometallic water stable precursor have explored new ways for the development of promising diagnostic and therapeutic agents. Using the [99mTc(CO)3(H2O)3]+ as the staring material escorted, to form promising newer radiopharmaceuticals, have re-energized the research in the nuclear medicine technology [19–23].
In the current investigation the radiolabeling of sitafloxacin dithocarbamate (SFDE) (Fig. 1b) with 99mTc is reported using the [99mTc(OH2)3(CO)3]+ precursor. The RCP values, stability in serum, in vitro bacterial uptake and biodistribution were determined and compared with 99mTc labeled SFDE complex prepared through 99mTcN core.
Experimental
Materials
Sitafloxacin (STF) (Daiichi Sanko, Japan), succinic dihydrazide (SDH), propylenediamine tetra acetic acid (PDTA) (Aldrich, USA) and all the other chemicals and solvents of analytical grade (Sigma)., RP-HPLC (Shimadzu, Japan), well counter, scalar count rate meter (Ludlum, USA), Dose calibrator (Capintech USA) and the Gamma camera (GEADE Nuclearmedizine system, Germany).
Method
Sitafloxacin dithocarbamate (SFDE) preparation
Sitafloxacin dithocarbamate (GXND) was prepared using the reported method [18]. Briefly, Sitafloxacin (STF) (3.625 g) was mixed with 1.2 g of sodium hydroxide and dissolved in 11 mL of 40% tetrahydrofuran (THF) followed by continuous shaking in ice-bath for 10 min. Thereafter, 1 mL of carbon disulfide (CS2) was added followed by 8 h continuous shaking in an ice-bath. The final product (SFDE) was recovered and characterized.
Preparation of the 99mTc(CO)3–SFDE complex
Na99mTcO4 −, 0.5 mL (1–2 mCi) freshly eluted was injected to the kit (Isolink) and incubated for 15 min followed by addition of 0.1 mol/L HCL. Thereafter 2 mg of SFDE in water was added and incubated for 15 min.
Partition coefficient of the 99mTc(CO)3–SFDE complex
The 99mTc(CO)3–SFDE complex was mixed with octanol and phosphate buffer in equal volume and vortexed at room temperature for 2 min. Thereafter, the mixture was centrifuged at 5000 rpm/min for 10 min. Aliquot, 0.1 mL was obtained at different intervals and counted for activity using well counter interface with scalar count rate meter. The partition coefficient was determined using the following formula.
The same process was repeated for the 99mTcN–SFDE and 99mTc–STF radiocomplexes for comparison.
RP-HPLC Characterization of the 99mTc(CO)3–SFDE radiocomplex
RP-HPLC chromatography was used to characterized the 99mTc(CO)3–SFDE radiocomplex using SCL-10 AVP shimadzu system fitted with SDP-10 AVP UV detector operating at 254 nm, Packard 500 TR series flow scintillation analyzer, binary pump, and online degasser. The 99mTc(CO)3–SFDE radiocomplex, 10 μL was introduced to the main unit of the SCL-10 AVP shimadzu system through the C-18 column (4.6 × 150 mm). Water:ethanol was used as a mobile phase, with a flow rate of 1 mL/min for 15 min (0–3 min (100%: W), 3–5 min (100–75% W), 5–8 min (75–66% W), 8–10 (34–100 M), 10–13 (100% M) and 13–15 (100% M to 100% W)). The fractions collected at 1–15 min were counted for activity using well counter interface with scalar count rate meter. The RCP values of the 99mTc(CO)3–SFDE radiocomplex was compared with the 99mTcN–SFDE radiocomlpex.
Serum stability
The in vitro immovability of the 99mTc(CO)3–SFDE radiocomplex in serum was investigated using TLC. The radiocomplex, 0.2 mL was incubated at 37 °C with 1.8 mL serum. Thereafter, aliquots obtained at 2, 4, 6, 8, 10, 12 14 and 16 h of incubation were spotted on the polyamide strip and developed in saline and CH2Cl2:CH3OH (9:1) (v/v). The developed polyamide strips were counted for activity using well counter interface with scalar count rate meter. The in vitro serum stability profile of the 99mTc(CO)3–SFDE was compared with the 99mTcN–SFDE radiocomplex.
In vitro binding with pathogens
In vitro binding of S. aureus was assessed by using the reported method [24]. Briefly, 99mTc(CO)3–SFDE radiocomplex, 10 MBq was transferred to a flask holding 0.1 mL sodium phosphate buffer (Na-PB). Subsequently, 0.8 mL (50%, v/v) 0.01 M acetic acid containing approximately 1 × 108 colony forming units (CFU) of S. aureus was added. Thereafter, at 4 °C the preparations were incubated for 1 h and the pH 5 was adjusted. The mixture was centrifuged for 10 min (2000 rpm) followed by removal of the supernatant. The pellets were resuspended in Na-BP (2 mL) and re-centrifuged for 10 min with the same spin speed. The activity uptake in pellets was estimated using well counter interface with scalar count rate meter. The in vitro bacterial binding of the 99mTc(CO)3–SFDE was compared with the 99mTcN–SFDE radiocomplex.
Biodistribution
Twelve male Sprague–Dawley rats (weight, 180–220 g) were randomly selected and divided into two groups (A and B) in each of the group six rats were injected with 0.2 mL sterile turpentine oil intramuscularly (I.M.) to the left thigh. Approximately, 1 × 108 CFU of living S. aureus in 0.2 mL normal saline was injected to the left thigh of the rats of the group A and a similar amount of heat killed S. aureus to the group B rats. After 24 h, 0.5 mCi (0.5 mL) of the 99mTc(CO)3–SFDE was administered to all the rats, separately and then exterminated in accordance with the Nuclear Medicine Research Laboratory University of Peshawar rules and regulations. The absorbed dose (%) per gram (each) in blood, liver, spleen, stomach, intestine, kidney, infected muscle, inflamed and normal muscle was calculated using well counter interface with scalar count rate meter. The biodistribution profile of the 99mTc(CO)3–SFDE radiocomplex was compared with the 99mTcN–SFDE radiocomplex.
Result and discussion
Chemistry of the 99mTc(CO)3–SFDE radiocomplex
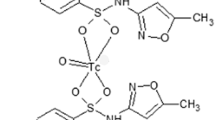
Sitafloxacin (Fig. 1a) was converted to its dithiocarbamate (Fig. 1b) which was radiolabeled with 99mTc using [99mTc(OH2)3(CO)3]+ precursor giving the proposed structure (Fig. 1c) having a tetrahedral bipyramidal geometry and stoichiometry of Lig:Tc(CO)3 as 1:2 [25].
The two sulfur atoms of the bidentate SFDE under substitution reaction with an intermediate [99mTcN]2+core yield 99mTcN–SFDE radiocomplex while in the fac-[99mTc(CO)3(H2O)3]+ precursor readily displaced H2O to give the 99mTc(CO)3–SFDE radiocomplex.
Participation coefficient of the 99mTc(CO)3–SFDE radiocomplex
The value of participation coefficient determined for the 99mTc(CO)3–SFDE, 99mTc–STF and 99mTcN–SFDE radiocomplexes were 0.47 ± 0.02, −1.06 ± 0.05 and 1.12 ± 0.01, respectively. The participation coefficient values of the 99mTc(CO)3–SFDE was decreased than 99mTcN–SFDE and increased then 99mTc–STF radiocomplex. The values of the participation coefficient for the 99mTc(CO)3–SFDE and 99mTcN–SFDE radiocomplexes suggest that both are lipophilic and the 99mTc–STF are hydrophilic.
HPLC characterization of the 99mTc(CO)3–SFDE radiocomplex
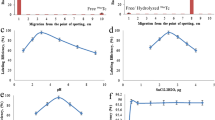
The HPLC radio-chromatogram of the 99mTc(CO)3–SFDE radiocomplex (Fig. 2) demonstrated two peaks with the retention times (RT) of 2.1 and 8.2 min. The peak at 8.2 min represents the yield of 99mTc(CO)3–SFDE radiocomplex. However, the RT observed for the SFDE radiocomplex prepared through [99mTcN] 2+ core using the same parameters were 3.2 and 10.5 min. The activity peak observed at 10.5 min represents the 99mTcN–SFDE radiocomplex.
The 99mTc(CO)3–SFDE radiocomplex showed stable radiochemical profile in saline at different intervals after reconstitution as shown in Fig. 3. The maximum RCP value observed for the 99mTc(CO)3–SFDE radiocomplex was 98.45 ± 0.21% at 30 min after reconstitution and showed more than 90% stability up to 4 h. No significant change was observed between the RCP values of the 99mTc(CO)3–SFDE and 99mTcN–SFDE radiocomplexes.
In vitro stability of the 99mTc(CO)3–SFDE radiocomplex in serum
A comparative in vitro stability profile of the 99mTc(CO)3–SFDE and 99mTcN–SFDE radiocomplexes is shown in Fig. 4. Similar stable in vitro profile in serum at 37 °C was observed for the 99mTc(CO)3–SFDE radiocomplex up to 4 h and insignificantly the stability went down to 80.50 ± 0.29% up to 16 h after reconstitution.
In vitro pathogenic uptake
The 99mTc(CO)3–SFDE radiocomplex showed saturated in vitro binding with living S. aureus like 99mTcN–SFDE radiocomplex. A comparative in vitro binding affinity of the 99mTc(CO)3–SFDE and 99mTcN–SFDE radiocomplex is given in Fig. 5.
Biodistribution
The in vivo biodistribution of the 99mTc(CO)3–SFDE radiocomplex in rats of group A and B is given in Table 1. It was observed that the amount of the 99mTc(CO)3–SFDE radiocomplex in blood of the group A and B rats went down with time. The radiocomplex showed almost similar blood activity profile in all the infected rats irrespective of the live or heat killed pathogen. The uptake of the 99mTc(CO)3–SFDE radiocomplex in group A showed significantly higher amount in the infected muscle as compared to the inflamed and normal muscle. In case of group B rats almost similar uptake was observed in infected, inflamed and normal muscle. The uptake of the radiocomplex in case of group A (live S. aureus infected rats) was approximately 6 time higher in the infected muscle as compared with the inflamed and normal while in the heat killed (group B) no significant difference was observed. The activity significantly decreased in liver and spleen and increased in kidney with time in infected rats of group A and B. The appearance of activity of the 99mTc(CO)3–SFDE radiocomplex in urinary system and disappearance from circulatory system confirmed the normal route of excretion.
The infected to normal muscle uptake ratio of the 99mTc(CO)3–SFDE and 99mTcN–SFDE radiocomplexes in SAIR are given in Figure 6. Both the radiocomplexes showed almost similar uptake patron. Significantly, higher up take was observed in infected muscle as compared to inflamed and normal muscle.
Conclusion
In the current investigation the feasibility of the sitafloxacin dithiocarbamate (SFDE) radiolabeling with 99mTc using [99mTc(OH2)3(CO) 3]+ precursor and biological evaluation in artificially infected Staphylococcus aureus rats was assessed to verify the achievability of the 99mTc(CO)3–SFDE radiocomplex as a potential Staphylococcus aureus in vivo infection radiotracer. The high radiochemical stability in saline, supercilious in vitro serum immovability, targeted biodistribution profile and desired target to non-target (infected to inflamed muscle) ratio we recommend the 99mTc(CO)3–SFDE radiocomplex as a radiotracer for in vivo localization of Staphylococcus aureus infection in human.
References
Bruggen W, Bleeker-Rovers CP, Boerman OC, Gotthardt M, Oyen WJG (2010) PET and SPECT in osteomyelitis and prosthetic bone and joint infections: a systematic review. Semin Nucl Med 40:3
Basu S, Chryssikos T, Moghadam-Kia S, Zhuang H, Torigian DA, Alvai A (2009) Positron Emission Tomography as a diagnostic tool in infection: Present role and future possibilities. Semin Nucl Med 39:36
Gallagher H, Ramsay SC, Barnes J, Maggs J, Cassidy N, Ketheesan N (2006) Neutrophil labeling with [99mTc]-technetium stannous colloid is complement receptor 3-mediated and increases the neutrophil priming response to lipopolysaccharide. Nucl Med Biol 33:433
Lahiri S, Sarkar S (2007) Studies on 66, 67Ga- and 199Tl-poly(N-vinylpyrrolidone) complexes. Appl Radiat Isot 65:309
Motaleb MA (2007) Preparation of 99mTc-cefoperazone complex, a novel agent for detecting sites of infection. J Radioanal Nucl Chem 272:167
Motaleb MA (2007) Preparation and biodistribution of 99mTc-lomefloxacin and 99mTc-olfloxacin complex. J Radioanal Nucl Chem 272:95
Oh SJ, Ryu J, Shin JW, Yoon EJ, Ha H, Cheon JH, Lee HK (2002) Synthesis of 99mTc-ciprofloxacin by different methods and its biodistribution. Appl Radiat Isot 57:193
Zhang J, Guo H, Zhang S, Lin Y, Wang X (2008) Synthesis and biodistribution of a novel 99mTcN complex of ciprofloxacin dithiocarbamate as a potential agent for infection imaging. Bioorg Med Chem Lett 18:51
EL-Gany EA, EL-Kolaly MT, Amine AM, EL-Sayed AS, Abdel-Gelil F (2005) Synthesis of 99mTc-pefloxacin: a new targeting agent for infectious foci. J Radioanal Nucl Chem 266:131
Roohi S, Mushtaq A, Jehangir M, Ashfaq MS (2006) Synthesis, quality control and biodistribution of 99mTc-Kanamycin. J Radioanal Nucl Chem 267:561
Motaleb MA (2009) Preparation, quality control and stability of 99mTc-sparafloxacin complex, a novel agent for detecting sites of infection. J Label Compd Radiopharm 52:415
Chattopadhyay S, Das SS, Chandra S, De K, Mishra M, Sarkar BR, Sinha S, Ganguly S (2010) Synthesis and evaluation of 99mTc-moxifloxacin, a potential infection specific imaging agent. Appl Radiat Isotopes 68:314
Qaiser SS, Khan AU, Khan MR (2010) Synthesis, biodistribution and evaluation of 99mTc-Sitafloxacin kit: a novel infection imaging agent. J Radioanal Nucl Chem 284:189
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis of 99mTc-nitrifuratonin a novel radiotracer for in vivo imaging of Escherichia coli infection. J Radioanal Nucl Chem. Online published on 19 August 2010
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biodistribution of 99mTc-rifampicin: a novel radiotracer for in vivo infection imaging. Appl Radiat Isot 68:2255
Shah SQ, Khan AU, Khan MR (2010) 99mTc-Novobiocin: a novel radiotracer for infection imaging. Radiochim Acta (in press)
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis, biodistribution and scintigraphy of the 99mTc-Teicoplanin complex in artificially infected animal models. J Labelled Compd Radiopharm (in press)
Shah SQ, Khan AU, Khan MR (2010) Radiosynthesis and biological evaluation of 99mTc-sitafloxacin dithiocarbamate as potential radiotracer for Staphylococcus aureus infection. J Radioanal Nucl Chem. Online published on 19 September 2010
Xia J, Wang Y, Yu J, Li S, Tang L, Zheng M, Liu X, Li G, Cheng D, Liang S, Yin D (2008) Synthesis, in vitro and in vivo behavior of 188Re(I)-tricarbonyl complexes for the future functionalization of biomolecules. J Radioanal Nucl Chem 275:325
Zhang J, Wang X, Jin C (2007) Synthesis and biodistribution of the 99mTc(CO)3-DEDT complex as a potential new radiopharmaceutical for brain imaging. J Radioanal Nucl Chem 272:91
Djokic DD, Jankovic DL, Stamenkovic LL, Pirmettis I (2004) Chemical and biological evaluation of 99mTc (CO)3 and 99mTc complexes of some IDA derivatives. J Radioanal Nucl Chem 260:471
Xia J, Long S, Yu J, Wang Y, Cao Z (2009) Pyridyl derivatives provide new pathways for labeling protein with fac-[188Re(CO)3(H2O)3]+. J Radioanal Nucl Chem 281:493
Zhang JB, Wang XB, Jin C (2006) Synthesis of 99mTc(CO)3-NOET via [99mTc(OH2)3(CO)3]+ precursor and comparative biological studies with 99mTcN-NOET. J Radioanal Nucl Chem 269:227
Welling MM, Paulusma-Annema A, Batler HS, Pauwels EKJ, Nibbering PH (2000) Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med 27:292
Baldas J, Bonnyman J, Poer PM, Williams GA, Mackay MF (1981) Synthesis and structure of bis(diethyldithiocarbamato)nitridotechnetium(V)—a technetium-nitrogen triple bond. J Chem Soc Dalton Trans 9:1798
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, S.Q., Khan, A.U. & Khan, M.R. Radiosynthesis and biological evolution of 99mTc(CO)3–sitafloxacin dithiocarbamate complex: a promising Staphylococcus aureus infection radiotracer. J Radioanal Nucl Chem 288, 131–136 (2011). https://doi.org/10.1007/s10967-010-0880-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0880-2