Abstract
Effects of the pyrethroid lambda-cyhalothrin (LCH) were investigated in matrinxa Brycon amazonicus, a non-target freshwater teleost. The fish were submitted to a single-pulse exposure (10% of LC50; 96 h, 0.65 μg L−1), followed by 7 days of recovery in clean water. Hematologic parameters indicated impairments in oxygen transport, which were not recovered. Plasma [Na+], [Cl−], and protein were diminished, and only [Na+] remained low after recovery. Gill Na+/K+ATPase activity was increased and recovered to basal values. Brain acetylcholinesterase activity was not responsive to LCH. Liver ascorbic acid concentration was not altered, and reduced glutathione levels remained augmented even after recovery. LCH inhibited hepatic superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities, while glutathione-S-transferase (GST) and glucose-6-phosphate dehydrogenase (G6PDH) activities were steady. After recovery, SOD remained low, and GPx was augmented. Liver depicted lipid peroxidation, which was not observed after recovery. Hepatic morphology was affected by LCH and was not completely recovered. These responses, combined with the persistence of changes even after recovery span, clearly show the feasibility of these biomarkers in evaluating LCH toxic potential to non-target organisms, highlighting the importance of pyrethroids’ responsible use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biocides are chemical compounds largely employed in anthropogenic activities, which often results in high ecological impacts. These xenobiotics reach freshwater bodies, threatening both animal life and human health. Synthetic pyrethroids (SP) are pyrethrin-derived insecticides that can be classified as types I and II, depending on cyano-radical absence or presence, respectively (Haya 1989; Kumar et al. 2009). They interfere with voltage-dependent Na+ channels of nervous cells, preventing membrane repolarization and avoiding termination of nervous impulse transmission (Soderlund et al. 2002; Prusty et al. 2015). These biocides arose as an alternative to organochlorines and organophosphates, due to their high efficiency in agricultural and domestic pest control, and being less harmful to birds and mammals (Soderlund et al. 2002; Corcellas et al. 2015). In addition, SP are also used to control fish ectoparasites in a concentration range of 2.0–5.0 μg L−1 (SEPA. Scottish Environment Protection Agency 2008; Van Geest et al. 2014; Aznar-Alemany et al. 2017), which contributes to their presence in all environments. Notwithstanding the benefits, these xenobiotics are extremely toxic to fishes.
Exposure to SP can result in physiologic, metabolic, and morphological injuries to freshwater fish (Muranli and Güner 2011; Guardiola et al. 2014; Narra 2016). Blood alterations in fish are often related to SP exposure (Fawole and Yekeen 2014). In unraveling these biocide effects, some key enzymes are also widely used, such as acetylcholinesterase (AChE), which is responsible for terminating neurotransmission (Tilton et al. 2011), and Na+K+/ATPase (NKA), pivotal in fish osmolality balance maintenance (Begum 2011). Recent findings report that SP act as endocrine disruptors, and their metabolites exhibit great estrogenic activity (Wielogórska et al. 2015; Brander et al. 2016). Studies on bioaccumulation in aquatic organisms questioned SP safety, raising concerns on human consumption of contaminated fish (Corcellas et al. 2015; Tu et al. 2016; Muggelberg et al. 2017).
Detoxification mechanisms might contribute to the observed imbalances of antioxidant metabolism in teleosts exposed to SP (Marigoudar et al. 2013; Bacchetta et al. 2014; Abdelkhalek et al. 2015). Some organs are highly responsive to chemical exposure, such as gills, which are the first organs in contact with the surrounding water, and liver, which plays a vital role on metabolism and detoxification of chemical stressors. Hepatic responses are normally fast and efficient to cope with stressors, but SP can affect regulatory mechanisms and, besides the organ’s high plasticity, lead to structural injuries (Kan et al. 2012; Marigoudar et al. 2013).
Lambda-cyhalothrin (LCH) is a type II SP widely used worldwide (Fonseca-González et al. 2011; Muranli and Güner 2011; Kumar et al. 2014), and often observed in water bodies (Marino and Ronco 2005; Jabeen et al. 2015). Cypermethrin and LCH are reported in soy production areas of Argentina, Brazil, and Paraguay at concentrations able to cause acute toxicity in aquatic organisms (Hunt et al. 2016). Matrinxa Brycon amazonicus (Spix & Agassiz 1829) presents high commercial relevance in South American countries, since it is one of the main aquaculture species produced. Additionally, it is sensitive to xenobiotic exposures (Avilez et al. 2013; Moraes et al. 2018), meeting toxicology model needs.
In this context, the aim of this study was to evaluate LCH impacts on B. amazonicus, considering antioxidant metabolism, osmoregulation, and morphological changes. Such experimental design was able to test the responsiveness of these biomarkers to SP contamination. Since xenobiotics presence in water bodies is intermittent (Handy 1994), fish recovery was also accessed, enabling a wide frame on this pyrethroid’s impact on a freshwater species. The proposed investigations are critical in understanding LCH effects in non-target organisms, besides shedding light on the outcomes of water contamination by this unreasonable insecticide use.
Material and methods
Chemicals
The commercial formulation Trinca Caps® (DVA), containing 250 g L−1 of the active ingredient lambda-cyhalothrin (LCH) was used. Butylated hydroxytoluene, pyrogallol, NADP+, NADPH++H+, 1,1,3,3-tetramethoxypropane, and reduced glutathione were from Sigma-Aldrich; all other reagents were from Merck.
Fish maintenance and experimental design
Juvenile B. amazonicus were kindly donated by Polettini Fish Farm (Mogi-Mirim, Sao Paulo, Brazil). Fish were acclimated for 2 weeks at 2500-L tanks, filled with dechlorinated tap water, in a flow-through water system, under a 12–12-h photoperiod and fed daily with commercial pellets. Afterward, 48 fish (82.6 ± 18.6 g) were equally divided into 12 250-L tanks, under the same acclimation conditions, remaining undisturbed for 6 days. Feeding was discontinued 24 h before the beginning of the experimental span and animals were not fed until the end of the experiment. Six tanks were assigned as exposure and received 0.65 μg L−1 of LCH, which corresponds to 10% of the LC50;96 h (Moraes et al. 2013), ensuring a sub-lethal exposure. The remaining tanks, assigned as control, remained in xenobiotic-free water. After the pesticide was added, the water flow was interrupted and the whole system (exposed and control tanks) was kept static for 96 h. Water parameters were monitored daily and remained stable over the experiment: pH (7.3–7.5); T °C (27.3 ± 0.8); pO2 mg L−1 (6.0–7.0); [NH3-NH4+] μmol L−1 (0.38–0.42); hardness as mg L−1 HCO3−/CO32− (19–20); alkalinity as CaCO3 mg L−1 (22–24).
After 96 h, three exposure and three control tanks from the 12 experimental ones were randomly chosen. Four fish from each of them were sampled (n = 12, per condition, exposure and control), blood was withdrawn, and liver, brain, and gills were excised as detailed below. The water of the six remaining tanks was renewed, and kept in continuous water flow for 7 days. This period corresponded to the recovery span. After recovery, blood and tissues were sampled as explained below.
Blood and tissue sampling
At the end of experimental spans (96 h of exposure and 7 days of recovery), fish were anesthetized into eugenol solution (Inoue et al. 2003) and blood was drawn from the caudal vein. A blood aliquot was centrifuged for 3 min at 13,400×g for plasma obtainment. Fish were then killed by spinal cord section for liver, brain, and gills excision. These organs were immediately rinsed with cold saline solution (NaCl 0.9%). Liver and brain were flash frozen in liquid nitrogen, while gills were immersed in SEI buffer (sucrose-EDTA-imidazole, pH 7.4) before freezing. Tissues and plasma were stored in − 80 °C until analyses. Liver slices (3 mm) were fixed in buffered 2.5% glutaraldehyde solution for histology.
Blood and plasma parameters
Hematocrit (Hct%) was determined in heparinized microcapillaries after centrifugation for 3 min at 13,400×g. Red blood cells (RBC, cells mm−3) were counted in a Neubauer chamber. Total hemoglobin concentration (Hb, g dL−1) was determined according to Drabkin (1948). Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCHb), and mean corpuscular hemoglobin concentration (MCHC) were calculated from hematimetric data (Wintrobe 1934).
The ions Na+, K+, and Cl− were determined in plasma after dilution in distilled water (1:100 v/v). A flame photometer was used to estimate Na+ and K+ concentrations, against standard solutions of 140 mEq of Na+ and 5.0 mEq of K+. Chloride anion was quantified at 480 nm using 0.9% mercury thiocyanate diluted in ethanol and 0.4 M nitric acid at 3:10 v/v (APHA 1980), against NaCl standard solution. Plasma protein concentrations were determined with Bradford reagent against a standard solution of bovine serum albumin (BSA) (Kruger 1994).
Cell-free extracts
Liver was homogenized in 5% TCA (trichloroacetic acid) at 1:50 (tissue: TCA) for 1 min, in a Turrax® T10-Ika homogenizer at 1000 rpm under ice-bath, and centrifuged at 13,400×g for 3 min at 4 °C. Supernatants were used for ascorbic acid quantification. Tissue extracts for GSH determinations followed similar procedure using liver pieces at 1:20 (tissue: 0.2 M PO42− buffer pH 7.0).
Cell homogenates
Hepatic tissues were homogenized as described above in 0.1 M PO42− buffer pH 7.0 with 0.25 M sucrose at 1:1 tissue:buffer. Homogenates were centrifuged for 10 min, at 15,000×g at 4 °C. The supernatant was used as a crude antioxidant enzyme source. Brain samples were homogenized in 0.1 M PO42− buffer pH 7.0 in anhydrous glycerine (v/v) at 1:1 tissue:buffer and centrifuged at 13,400×g for 3 min at 4 °C. The supernatant was used as a crude AChE-enzyme source. Gill filaments were thawed, homogenized in SEI (sucrose-EDTA-imidazole) buffer pH 7.4, as reported above and centrifuged at 10,000×g for 5 min at 4 °C. Supernatant (0.7 mg protein) was used as a crude NKA-enzyme source.
Hepatic antioxidant biomarkers
Ascorbic acid was quantified with 2,6-dichlorophenolindophenol in meta-phosphoric/sulfuric acid medium and dinitrophenyl hydrazine at 524 nm, against ascorbic acid standard solution (Carr et al. 1983). Reduced glutathione (GSH) concentration was determined through the interaction of GSH with 5,5′-dithiobis-2-nitrobenzoic acid in 0.1 M PO42− buffer pH 8.0, resulting in 2-nitro-5-thiobenzoate, which was read at 412 nm (Beutler 1984).
SOD was assayed by pyrogallol self-oxidation, which is inhibited in the presence of SOD (Beutler 1984, modified). Absorbance readings were performed at 420 nm, considering that 1.0 UI inhibits 50% the pyrogallol self-oxidation. CAT activity was assayed by reading H2O2 decay at 230 nm (Beutler 1984). One unit of CAT was defined as the amount of enzyme required in 1.0 μmol of H2O2 min−1 oxidation, and the molar absorptivity used was (H2O2)ελ230 = 0.071 mM cm−1. GPx was assayed through NADPH+H+ extinction, read at 340 nm with glutathione reductase as an auxiliary enzyme (Beutler 1984). Molar absorptivity used was (NADPH+H+/NAP)ελ340 = 6.20 mM cm−1. GST assay was based in GSH conjugation to 1-chloro-2,4 dinitrobenzene (CDNB), catalyzed by GST, and read at 340 nm (Habig and Jakoby 1981). Molar absorptivity used was (CDNB)ελ340 = 9.6 mM cm−1. G6PDH was assessed through NADP supply to antioxidant enzymes, which was performed reading NADP+ extinction, at 340 nm (Beutler 1984). Molar absorptivity used was (NADPH+H+/NADP)ελ340 = 6.3 mM cm−1.
TBARS were determined as Draper and Hadley (1990). Liver aliquots were homogenized in 5% (w/v) TCA with 0.5 g L−1 of butylated hydroxytoluene (BHT). The homogenates were boiled for 30 min, cooled in ice, and centrifuged at 1000×g for 10 min. Thiobarbituric acid (TBA) was mixed to the supernatants (1:1 v/v) and mixture was boiled again for 30 min. The samples were read at 532 nm against a standard curve of tetramethoxypropane.
Acetylcholinesterase (AChE) and Na+K+/ATPase (NKA) assays
Brain AChE was assayed in 100 mM PO42− buffer pH 7.0 with acetylthiocholine as substrate and 5,5-dithiobis-2-nitrobenzoic acid as a reagent (Ellman et al. 1961). AChE activity was recorded at 412 nm (ελ412 = 16,950 M−1 cm−1). Gill NKA activity was determined with ouabain as inhibitor through optical detection of phosphate, read at 620 nm (Quabius et al. 1997).
Protein
Protein was determined with Bradford reagent against a standard solution of BSA (Kruger 1994).
Histological analysis
After fixation, samples were dehydrated in ethanol and embedded in Historesin® (Leica, Heidelberg, Germany). Liver sections of 2 μm were stained with toluidine blue and basic fuchsin. Ten random microscopic fields of each section were observed with an optic microscope (Olympus BX51). Images were captured with a digital camera and analyzed with the Motic Images Plus 2.0 software. Histopathological index (HI) was calculated as Camargo and Martinez (2007), modified from Poleksic and Mitrovic-Tutundzic (1994). It is based on location, type, and severity of tissue lesions, which are classified into four groups: parenchymal lesions; cytoplasmic and nuclear modifications; blood vessel changes and necrosis. After the environmental stressor is withdrawn, the damages are classified into three progressive stages considering the organ recovery: stage I (SI), the lesions are not severe, do not affect organ normal function, and are reversible; stage II (SII), moderate to severe lesions, can affect the organ function, might be reversible when the environment is improved; stage III (SIII), very severe lesions, great impairment of organ function, irreversible even with environment amelioration. Estimative of HI is based on the sum of tissue lesions times the stage index, accordingly HI = 100ΣSI + 101ΣSII + 102ΣSIII (Poleksic and Mitrovic-Tutundzic 1994), in which ΣSI, ΣSII, and ΣSIII are the sum of alterations in each stage and 100, 101, and 102 are the calculated factors, based on lesion severities. The HI values are grouped into the following categories: 0–10 = structurally normal organ; 11–20 = slight to moderate organ injuries; 21–50 = moderate to severe organ injuries; 51–100 = severe organ injuries; and > 100 = irreparably injured organ.
Statistical analysis
The normality of data was tested by Kolmogorov-Smirnov test. Then, the parametric test t of Student or the non-parametric test of Mann-Whitney was used, accordingly. The statistical significances of differences among control × exposure and control of recovery × recovery are reported accepting a confidence interval of 95% (p < 0.05). The used software was GraphPad Prism, 5.0.
Results
After LCH exposure, there was an increase of Hct (10%), Hb (31%), RBC (18%), MCHb (26%), and MCHC (17%). Hematocrit and Hb remained high after recovery, at 9.5% and 24%, respectively. Concerning plasma ionic balance, Na+ and Cl− concentrations diminished due to LCH exposure, in 15% and 19%, respectively. No changes were observed in plasma K+ concentrations (4.57 ± 0.6 mEq mL−1). After recovery, only Na+ was held low at 5%. Plasma protein was 13% lower in LCH-exposed fish, while no changes occurred after recovery. The activity of gills NKA was raised in 43% after sub-lethal exposure, while no differences were observed in recovered fish. Table 1 brings blood and osmoregulatory outcomes.
Brain AChE activity was unchanged at nearly 0.22 mU min−1 mg protein−1 in both experimental spans.
Hepatic ascorbic acid concentration remained steady at both evaluations, being not responsive to LCH. On the other hand, GSH augmented 41% after sub-lethal exposure, and 29% after recovery. The antioxidant enzymatic activities of SOD, CAT, and GPx presented decreases of 37%, 35%, and 32%, respectively, while GST and G6PDH remained stable after LCH exposure. After recovery, SOD activity diminished 19% and GPx activity increased 141%. Enzyme activity of CAT was recovered to control values. In spite of G6PDH activity being not directly linked to ROS scavenging and no significant changes were observed in the experiment, its role is discussed moving forward. Lipid peroxidation (LPO) occurred in the liver of B. amazonicus after LCH exposure, as shown by a 22% increment of TBARS in exposed animals. There was no hepatic TBARS generation after recovery. Table 2 presents the aforementioned data.
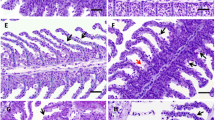
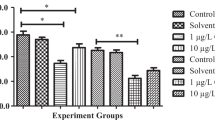
Liver of exposed fish presented blood vessel hyperplasia, cellular deformation, hepatic cell cord disorganization, nuclear and cytoplasmic vacuolization, eosinophilic aggregates, and lipid accumulation. The HI of exposed groups was indicative of moderate to severe organ injuries (HI control 11.0 ± 1.2 and HI exposed 45.8 ± 2.5; Fig. 1). Recovered fish presented hepatic cellular and nuclear hypertrophy, cellular deformation, cell cord disorganization, cytoplasmic and nuclear vacuolization, eosinophilic aggregates, and cytoplasmic and nuclear degeneration. The recovery span was not enough for hepatic recovery, as can be inferred from the HI values (HI control of recovery 15.3 ± 1.5 and HI recovery 44.7 ± 3.1; Fig. 1). Table 3 brings the frequency of alterations, and Fig. 2 exhibits the histopathological observations.
Liver histopathological index (HI) of Brycon amazonicus exposed to 0.65 μg L−1 of lambda-cyhalotrhin and recovered. C, control; E, exposed; CR, control of recovery; R, recovery. Asterisk indicates differences between control and exposed groups and between control of recovery and recovery groups (p < 0.05). Data are presented as mean ± standard error (n = 12)
Photomicrographs of Brycon amazonicus liver sections. C, control. Tubular arrangement of hepatocytes indicated by (H). CR, control of recovery. Sinusoids indicated by (S). E, exposed. Simple arrow: nuclear deformation. Double arrow: cellular deformation. R, recovery. Triple arrow: eosinophil aggregates. Toluidine blue and basic fuchsin. Scale bar, 20 μm
Discussion
Toxic effects caused by synthetic pyrethroids in non-target organisms are not always lethal, and yet they can cause serious sub-lethal effects, leading to impairments in individual and population fitness and survival. The LCH concentration here reported is environmentally relevant, already found at water bodies and recommended for fish parasite treatment (Marino and Ronco 2005; Hunt et al. 2016; United Kingdom 2017). B. amazonicus exposed to LCH presented hematologic parameter alterations, imbalances in antioxidant metabolism, and changes in liver structure; a physiological frame that was not recovered even after 1 week under clean water.
The energetic cost to metabolize xenobiotics is a critical point in detoxification processes (Hammer 1995; Geeraerts and Belpaire 2010) and the energy demanded comes preferentially from aerobic metabolism. For this reason, cell strategies aiming to achieve uptake and transport of oxygen are usually enhanced after poisoning by xenobiotics. It is well known that one of the major routes of toxicant entry into the fish body is through the gills, a relevant interface between blood and water. Lipophilic compounds, such as SP, rapidly permeate this barrier for their chemical affinity with cell membranes. This mechanism is reported as mainly responsible for the body burden of these xenobiotics (Randall et al. 1998). Gills are the main organs for gas exchange in fish, and accumulation of lipophilic compounds might interfere with the permeability of cell membranes, impairing transports executed by these structures (Payne et al. 1978). Moreover, gill morphological changes reported in fish exposed to SP, e.g., cell hyperplasia, augment the water-blood barrier, hindering gas exchange (Yildirim et al. 2006; Cunha et al. 2018; Moraes et al. 2018). Along these lines, LCH presence in water can impair oxygen uptake, which would lead to the compensatory blood changes observed in B. amazonicus (augmented Hb, Htc, and RBC). These responses are also seen in fish submitted to environmental hypoxia (Moraes et al. 2002; Gaulke et al. 2014).
Stress related to LCH intoxication might also explain the blood outcomes. Stress for SP exposure is reported to increase blood cortisol in fish (Saravanan et al. 2009; Firat et al. 2011), a potential reason for RBC enhancement. The recovery period of 7 days was not enough to eliminate the stress caused in B. amazonicus by LCH. This fact is central considering the health status of fish that inhabit water bodies close to agricultural lands, as this pesticide is heavily used in crop farms.
In freshwater fish, ionic and hydric balances are held by glomerular filtration rates and urine flow, associated with active reabsorption of ions by branchial epithelium (Evans et al. 2005). Ion transfer through gills is mediated by NKA activity in chloride cells. NKA is a target for SP toxicity, as seen upregulated NKA encoding genes in liver of Atlantic salmon Salmo salar L. exposed to deltamethrin, which are downregulated in gills (Olsvik et al. 2014). Additionally, structural changes of ATPases are supposed to be partially responsible for the neurotoxic action of these xenobiotics (Kakko et al. 2003; Riar et al. 2013). Hemodilution presented by B. amazonicus exposed to LCH might happen due to an inhibition of active ion uptake by NKA, resulting in a decrease of plasma concentrations of anions and cations (Wendelaar Bonga and Lock 1992). Nevertheless, the rise of NKA activity in fish exposed to LCH might also be a result of high levels of blood catecholamines, due to intoxication stress. Increases of NKA would work as a compensatory adaptation to cope with low plasma ion concentration, although such compensatory response was not enough to keep normal patterns of blood Na+ and Cl−.
Increases in enzyme expression, as observed in NKA, can worsen the metabolic syndrome associated with xenobiotic exposure. Proteins can be the main energy source in detoxification processes, leading to hypoproteinemia, as reported in Clarias batrachus L. exposed to SP (Kumar et al. 2011). Moreover, osmoregulation disturbances inferred from low concentrations of Na+, Cl−, and protein can also be attributed to kidney and/or intestine dysfunction, particularly considering SP nephrotoxicity (Haya 1989; Mohapatra et al. 2012). In addition, impairment of oxygen uptake caused by LCH, just discussed above, would cause blood acidification aggravated by increases of metabolic rate from detoxification processes. A decline in Na+ and Cl− concentrations, as observed in B. amazonicus, would keep the blood pH into acidic ranges, since adjustments to recover physiological values are primarily dependent on bicarbonate concentrations.
The decrease of plasma Na+ was the only ionic alteration observed after 7 days of recovery; even the NKA activity returned to basal levels. B. amazonicus was able to re-establish normal conditions after LCH poisoning considering these physiological parameters. Our findings agree with those observed in C. batrachus exposed to cypermethrin, in which NKA activity was restored after 10 days of recovery (Begum 2009). The osmoregulatory imbalance observed in B. amazonicus is an early biomarker of xenobiotic injuries, enabling diagnosis before morphological changes are noticed.
Acetylcholinesterase activity is not the main target of SP toxicity, nevertheless some authors suggest that its use as a biomarker should not be limited to organophosphorous and carbamates, instead it might be extended to other pollutants (Guilhermino et al. 1998). Brain AChE in some fish is reported as an SP biomarker (Toumi et al. 2015; Singh et al. 2018); however, some studies do not observe altered AChE activity due to SP intoxication (Wheelock et al. 2005; Hernández-Moreno et al. 2010; Ensibi et al. 2014). Notwithstanding its role as an important biomarker widely used in fish, AChE activity changes due to SP exposure seem highly inconsistent. We hypothesize that other esterases might be affected by SP intoxication, since B. amazonicus brain AChE activity is unresponsive to this neurotoxicant action. Other esterases might interact with pyrethroids in this fish species, and should be considered in future studies.
Reactive oxygen species (ROS) are continually produced as a natural consequence of aerobic life, but their overproduction brings on severe consequences. B. amazonicus exposed to LCH depicted characteristics of hepatic oxidative stress. Xenobiotics are able to produce high levels of oxyradicals through metabolic reactions of detoxification (Kelly et al. 1998); moreover, SP are known for inhibiting mitochondrial complex I (Gassner et al. 1997), which would contribute to sharp rises in cellular ROS. A plausible hypothesis for the observed metabolic frame is that oxidative stress and all of the injuries associated to it (LPO, enzyme inhibition, DNA injuries) were already set in 96 h, indicating that B. amazonicus responses to LCH insults were insufficient.
The SOD-CAT joint activities are the organism’s first approach in dealing with ROS, being pivotal for the proper functioning of the antioxidant enzymatic system (Doria et al. 2018). This pair inhibition, after 96 h of LCH exposure, might have contributed for the development of LPO chain (e.g., TBARS increase) and consequent cellular injuries. The presence of superoxide radicals that were not vanished by the fish defenses would exacerbate Harber-Weiss reaction, boosting the damages caused by hydroxyl radicals, including enzyme inactivation (e.g., SOD, CAT, and GPx inhibitions) and LPO. Additionally, high hepatic levels of O2•- radicals were probably responsible for CAT and GPx inhibition (Odajima and Yamazaki 1972; Kono and Fridovich 1982). Regarding SOD activity, it could have been inhibited both by hydroxyl radicals and superoxide radicals, which is its own substrate (Bagnyukova et al. 2006; Modesto and Martinez 2010). Moreover, some studies link excessive H2O2 levels arisen from a lack of CAT activity to SOD inhibition (Kono and Fridovich 1983).
Glutathione peroxidase (GPx) reduces H2O2, preventing the expansion of chain reactions that cause lipid peroxidation (Di Giulio et al. 1989). This enzymatic defense inhibition after 96 h of exposure leads to an augmented Fenton reaction due to a surplus of H2O2, which induced higher HO− levels, contributing for LPO development. When installed, LPO causes loss of membrane fluidity, increased permeability, possible release of cell and organelle content, and inactivation of membrane enzymes and protein receptors (Kappus 1985; Gutteridge 1995). Although being a potent antioxidant, GSH by itself is not able to protect the tissue from lipid peroxidation, even when in higher levels as observed in B. amazonicus.
After 7 days of recovery, B. amazonicus antioxidant metabolism was not completely restored, but it seemed less affected by oxyradicals than after exposure, presenting a delayed adaptive response. SOD activity remained lower than control, but noticeably higher than that after exposure (inhibition of 37% and 19%, respectively). This increase on SOD activity would provide substrate (H2O2) for CAT and GPx activities. Indeed, CAT activity was retaken towards control levels, while GPx was sharply increased. Both enzymes might have taken part on the blocking of LPO chain development, since TBARS were no longer detected, besides preventing further enzyme inhibition. Reduced glutathione contents were still high, accounting for the rises in GPx and tissue protection against ROS deleterious effects.
Glutathione-S-transferase is fundamental in a particular type of detoxification mechanism, in which GSH is chemically conjugated to the xenobiotic molecule. Electrophilic metabolite conjugation to GSH is one of the major drug detoxification pathways known (Halliwell 2006; Di Giulio and Meyer 2008). Conjugation of GSH to toxicant molecules is a way of transforming them, usually reducing their deleterious effects. The unaltered activity of GST observed after LCH exposure and recovery was probably due to the type of mechanisms elected to cope with this xenobiotic. Conjugation to GSH should not be relevant to detoxify LCH in B. amazonicus, at least over the present experimental span. Contrastingly, GST hepatic activity is stimulated by LCH in Nile tilapia Oreochromis niloticus L. (Piner and Üner 2012). On the other hand, CAT and SOD activities and GSH and TBARS levels in Prussian carp Carassius auratus gibelio (Bloch 1782), submitted to deltamethrin, are similar to those observed in B. amazonicus (Dinu et al. 2010).
A couple of pentose’s pathway enzymes, such as dehydrogenases, are critical in maintaining the cell redox potential. The activity of G6PDH is key in NADPH+H+ production, which is involved in anabolic redox reactions. Unaltered G6PDH activity all over the experiment indicates that LCH did not affect NADPH+H+ supply to the metabolism of B. amazonicus. In Oncorhynchus mykiss, G6PDH is inhibited by cypermethrin and deltamethrin (Sentürk et al. 2009). Considering the oxidative stress, NADPH+H+ is oxidized to NADP+ by glutathione reductase to re-establish GSH levels. The steady activity of G6PDH in B. amazonicus should contribute in maintaining the observed elevated levels of that peptide. The upkeep of hepatic ascorbic acid levels could also be explained by G6PDH regular activity maintenance, since NADPH+H+ acts in the regeneration of vitamins C and E oxidized forms.
Liver is a crucial organ in detoxification processes. The histopathologic observations in B. amazonicus evince LCH toxic effects, and have been reported in other freshwater fish exposed to SP (Muthuviveganandavel et al. 2013). However, the morphological frame cannot be interpreted as a specific response to LCH intoxication; instead, it can be attributed to xenobiotics in general. One of the most frequent hepatic alterations observed after exposure to xenobiotics is an increase of vascularization. This physiologic response leads to raises in blood flux and increases of catabolite excretion from LCH metabolism, which occurs mainly through bile (Bradbury and Coats 1989; Kolo et al. 2010). Eosinophil aggregates are typical of inflammatory reactions from several etiologies such as parasitism, allergies, infections, and injuries (Ferrero-Milani et al. 2007). In particular, two possibilities arise to explain eosinophil aggregates: allergic processes, local or generalized, for the poisoning effects; or local lesions caused directly by LCH. Local lesions seem the most plausible hypothesis and might be due to an exacerbated ROS production, owing to LCH action on cell mitochondria. The influence of SP in the electron transport chain, already discussed, could increase ROS generation, and consequently enhance local cellular lesions.
There was a clear cytoplasmic vacuolization in hepatocytes of B. amazonicus linked to LCH intoxication. This was likely associated to inhibition of protein synthesis, energy depletion for detoxification processes, and disaggregation of microtubules, as also proposed by Hinton and Laurén (1990). In this regard, inhibition and/or depletion of antioxidant enzymes were just discussed above, and likewise, other proteins are also liable of suffering the same kind of injuries. Besides being reversible, cell and cytoplasmic degeneration are considered severe injuries, since the metabolic active volume is diminished (Maduenho and Martinez 2008). Hepatic modifications observed in B. amazonicus were enough to alter normal functions of the liver, although being considered moderate to severe. Disorganization of typical hepatocytes’ cords suggests that the organelle distribution was altered, affecting the organ functions as just proposed (Akaishi et al. 2004; Marinho et al. 2014). Considering the range of HI estimated to B. amazonicus in the present degree of poisoning, and the return of GPx and GSH to normal levels, it is possible to assume that the liver injury level can be reverted if LCH action is withdrawn in time. Nevertheless, the recovery span was not enough to allow complete re-establishment of hepatic normal morphology.
Shifts in hematologic parameters and osmoregulatory issues, combined with imbalances in hepatic antioxidant metabolism and morphological changes, were observed in B. amazonicus after LCH exposure, and homeostasis was not completely restored after recovery. These are critical alterations, since they could potentially impair the species’ regular life cycle. Considering the risks offered by SP contamination, reliable biomarkers are of great interest in detecting and evaluating these xenobiotics’ effects on non-target species. The reported metabolic frame emphasizes the relevance of the responsible use of this synthetic pyrethroid, along with adequate legislation to its use and discharge, especially considering the impairments caused in this non-target species exposed to low LCH doses.
References
Abdelkhalek NKM, Ghazy EW, Abdel-Daim MM (2015) Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res 22:3023–3031
Akaishi FM, Silva de Assis HC, Jakobi SCG, Eiras-Stofella DR, St-Jean SD, Courtenay SC, Lima EF, Wagener ALR, Scofield AL, Oliveira Ribeiro CA (2004) Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to water soluble fraction (WSF) of crude oil. Arch Environ Contam Toxicol 46:244–253
APHA (1980) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington
Avilez IM, Aguiar LH, Hori TS, Moraes G (2013) Metabolic responses of matrinxã, Brycon amazonicus (Spix & Agassiz, 1829), exposed to environmental nitrite. Aquaculture 44:596–603
Aznar-Alemany O, Eljarrat E, Barceló D (2017) Effect of pyrethroid treatment against sea lice in salmon farming regarding consumers’ health. Food Chem Toxicol 105:347–354
Bacchetta C, Rossi A, Ale A, Campana M, Parma MJ, Cazenave J (2014) Combined toxicological effects of pesticides: a fish multi-biomarker approach. Ecol Indic 36:532–538
Bagnyukova TV, Chahrak OI, Luschak VI (2006) Coordinated response of goldfish antioxidant defenses to environmental stress. Aquat Toxicol 78:325–331
Begum G (2009) Enzymes as biomarkers of cypermethrin toxicity: response of Clarias batrachus tissues ATPase and glycogen phosphorylase as a function of exposure and recovery at sublethal level. Toxicol Mech Method 19:29–39
Begum G (2011) Organ-specific ATPase and phosphorylase enzyme activities in a food fish exposed to a carbamate insecticide and recovery response. Fish Physiol Biochem 37:61–69
Beutler E (1984) Red cell metabolism: a manual of biochemical methods, 3rd edn. Grune & Straton, Michigan 187p
Bradbury SP, Coats JR (1989) Toxicokinetics and toxicodynamics of pyrethroid insecticides in fish. Environ Toxicol Chem 8:373–380
Brander SM, Gabler MK, Fowler NL, Connon RE, Schlenk D (2016) Pyrethroid pesticides as endocrine disruptors: molecular mechanisms in vertebrates with focus on fishes. Environ Sci Tech 50:8977–8992
Camargo MMP, Martinez CBR (2007) Histopathology of gills, kidney and liver of a neotropical fish caged in an urban stream. Neotrop Ichthyol 5:327–336
Carr RS, Bally MB, Thomas P, Neff JM (1983) Comparision of methods for determination of ascorbic acid in animal tissues. Anal Chem 55:1229–1232
Corcellas C, Eljarrat E, Barceló D (2015) First report of pyrethroid bioaccumulation in wild river fish: a case study in Iberian river basins (Spain). Environ Int 75:110–116
Cunha FS et al (2018) Deltamethrin-induced nuclear erythrocyte alteration and damage to the gills and liver of Colossoma macropomum. Environ Sci Pollut Res Int 25:15102–12110
Di Giulio RT, Meyer JN (2008) Reactive oxygen species and oxidative stress. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press, Florida, pp 273–326
Di Giulio RT, Washburn PC, Wenning RJ (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123
Dinu D, Marinescu D, Munteanu MC, Staicu AC, Costache M, Dinischiotu A (2010) Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and intestine. Arch Environ Con Tox 58:757–764
Doria HB, Ferreira MB, Rodrigues SD, Lo SM, Domingues CE, Nakao LS, de Campos SX, Ribeiro CAO, Randi MAF (2018) Time does matter! Acute copper exposure abolishes rhythmicity of clock gene in Danio rerio. Ecotoxicol Environ Saf 155:26–36
Drabkin DL (1948) The standardization of haemoglobin measurement. Am J Med Sci 215:110–111
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Method Enzymol 186:421–431
Ellman GL, Courtney KD, Andres JR, Featherstone RM (1961) A new rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ensibi C, Hernández-Moreno D, Santiyán MPM, Yahya MND, Rodriguez FS, Pérez-López M (2014) Effects of carbofuran and deltamethrin on acetylcholinesterase activity in brain and muscle of the common carp. Environ Toxicol 29:386–393
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fawole OO, Yekeen TA (2014) Alterations in haematological and biochemical parameters of African catfish Clarias gariepinus exposed to sublethal concentrations of deltamethrin. Zool Ecol 24:355–360
Ferrero-Milani L, Nielsen OH, Andersen PS, Girardin SE (2007) Chronic inflammation: the importance of NOD2 and NALP3 in interleukin-1B generation. Clin Exp Immunol 147:227–235
Firat O, Cogun HY, Yuzereroglu TA, Gok G, Firat O, Kargin F, Kotemen Y (2011) A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 37:657–666
Fonseca-González I, Quinones ML, Lenhart A, Brogdon WG (2011) Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag Sci 67:430–437
Gassner B, Wüthrich A, Scholtysik G, Solioz M (1997) The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J Pharmacol Exp Ther 281:855–860
Gaulke GL, Denis CE, Wahl SH, Suski CD (2014) Acclimation to a low oxygen environment alters the haematology of largemouth bass (Micropterus salmoides). Fish Physiol Biochem 40:129–140
Geeraerts C, Belpaire C (2010) The effects of contaminants in European eel: a review. Ecotoxicology 19:239–266
Guardiola FA, Gónzalez-Párraga P, Meseguer J, Cuesta A, Esteban MA (2014) Modulatory effects of deltamethrin-exposure on the immune status, metabolism and oxidative stress in gilthead sea bream (Sparus aurata L.). Fish Shellfish Immun 36:120–129
Guilhermino L, Barros P, Silva MC, Soares AMVM (1998) Should the use of inhibition of cholinesterases as a specific biomarker for organophosphate and carbamate pesticides be questioned? Biomarkers 3:157–163
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 1819–1828
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione-S-transferases. Methods Enzymol 77:398–405
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hammer C (1995) Fatigue and exercise tests in fish. Comp Biochem Physiol 1:1–20
Handy RD (1994) Intermittent exposure to aquatic pollutants: assessment, toxicity and sublethal responses in fish and invertebrates. Comp Biochem Physiol 107C(2):171–184
Haya K (1989) Toxicity of pyrethroid insecticide to fish. Environ Toxicol Chem 8:381–391
Hernández-Moreno D, Soler F, Míguez MP, Pérez-López M (2010) Brain acetylcholinesterase, malondialdehyde and reduced glutathione as biomarkers of continuous exposure of tench, Tinca tinca, to carbofuran or deltamethrin. Sci Total Environ 408:4976–4983
Hinton DE, Laurén DJ (1990) Integrative histopathological effects of environmental stressors in fishes. Am Fish S S 8:51–66
Hunt L, Bonetto C, Resh VH, Buss DF, Fanelli S, Marrochi N, Lydy MJ (2016) Insecticide concentrations in stream sediments of soy production regions of South America. Sci Total Environ 547:114–124
Inoue LAKA, Neto CS, Moraes G (2003) Clove oil as an anesthetic for juveniles of matrinxa Brycon cephalus (Gunther, 1896). Cienc Rural 33:943–947
Jabeen F, Chaudhry AS, Manzoor S, Shaheen T (2015) Examining pyrethroids, carbamates, and neonicotinoids in fish, water and sediments from the Indus River for potential health risks. Environ Monit Assess 187:1–11
Kakko I, Toimela T, Tahti H (2003) The synaptosomal membrane bound ATPase as a target for the neurotoxic effects of pyrethroids, permethrin, and cypermethrin. Chemosphere 51:475–480
Kan Y, Cengiz EI, Ugurlu P, Yanar M (2012) The protective role of vitamin E on gill and liver tissue histopathology and micronucleus frequencies in peripheral erythrocytes of Oreochromis niloticus exposed to deltamethrin. Environ Toxicol Pharmacol 34:170–179
Kappus H (1985) Lipid peroxidation: mechanisms, analysis, enzymology and biological relevance. In: Oxidative Stress (Sies H, ed). London: Academic Press, 273–310
Kelly SA, Havrilla CM, Brady TC, Abramo KH, Levin ED (1998) Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ Health Perspect 106:375–384
Kolo RJ, Lamai SL, Ojutiku RO (2010) Subacute toxicity of Karate to Sarotherodon galileus (Linneu, 1758). J Water Chem Technol 32:107–112
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Kono Y, Fridovich I (1983) Inhibition and reactivation of Mn-catalase: implications for valence changes at the active site manganese. J Biol Chem 258:13646–13648
Kruger NJ (1994) The Bradford method for protein quantification. Walker JM (ed) Methods in Molecular Biology, Basic Protein and Peptide Protocols vol 32. Humana Press Inc, Totowa, 15–21
Kumar A, Rai DK, Sharma B, Pandey RS (2009) λ-Cyhalothrin and cypermethrin induced in-vivo alterations in the activity of acetylcholinesterase in a freshwater fish, Channa punctatus (Bloch). Pestic Biochem Physiol 93:96–99
Kumar A, Sharma B, Pandey RS (2011) Cypermethrin induced alterations in nitrogen metabolism in freshwater fishes. Chemosphere 83:492–501
Kumar A, Sharma B, Pandey RS (2014) λ-Cyhalothrin and cypermethrin induce stress in the freshwater muddy fish, Clarias batrachus. Toxicol Environ Chem 96:136–149
Maduenho LP, Martinez CBR (2008) Acute effects of diflubenzuron on the freshwater fish Prochilodus lineatus. Comp Biochem Physiol C 148:265–272
Marigoudar SR, Ahmed RZ, David M (2013) Ultrastructural responses and oxidative stress induced by cypermethrin in the liver of Labeo rohita. Chem Ecol 29:296–308
Marinho JFU, Correia JE, Marcato ACC, Pedro-Escher J, Fontanetti CS (2014) Sugarcane vinasse in water bodies: impact assessed by liver histopathology in tilapia. Ecotoxicol Environ Saf 110:239–245
Marino D, Ronco A (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826
Modesto KA, Martinez CBR (2010) Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78(3):294–299
Mohapatra S, Chakraborty T, Prusty AK, Kumar K, Prasad KP, Mohanta KN (2012) Fenvalerate-induced stress mitigation by dietary supplementation of the multispecies probiotic mixture in a tropical freshwater fish, Labeo rohita (Hamilton). Pestic Biochem Physiol 104:28–37
Moraes G, Avilez IM, Altran AE, Barbosa CC (2002) Biochemical and haematological responses of the banded knife fish Gymnotus carapo (Linnaeus, 1758) exposed to environmental hypoxia. Braz J Biol 62:633–640
Moraes FD, Venturini FP, Cortella LRX, Rossi PA, Moraes G (2013) Acute toxicity of pyrethroid-based insecticides in the neotropical freshwater fish Brycon amazonicus. Ecotoxicol Environ Contam 8:59–64
Moraes FD, Venturini FP, Rossi PA, Avilez IM, Souza NES, Moraes G (2018) Assessment of biomarkers in the neotropical fish Brycon amazonicus exposed to cypermethrin-based insecticide. Ecotoxicology 27:188–197
Muggelberg LL, Hartz KEH, Nutile SA, Harwood AD, Heim JR, Derby AP, Weston DP, Lydy MJ (2017) Do pyrethroid-resistant Hyalella azteca have greater bioaccumulation potential compared to non-resistant populations? Implications for bioaccumulation in fish. Environ Pollut 220A:375–382
Muranli FD, Güner U (2011) Induction of micronuclei and nuclear abnormalities in erythrocytes of mosquito fish (Gambusia affinis) following exposure to the pyrethroid insecticide lambda-cyhalothrin. Mut Res 94:104–108
Muthuviveganandavel V, Hwang I, Anita V, Malarani PS, Selvam C, Hemalatha M, Pandurangan M (2013) Synthetic pyrethroid effect on blood plasma biomarker enzymes and histological changes in Catla catla. Int J Exp Pathol 94:104–108
Narra MR (2016) Single and cartel effect of pesticides on the biochemical and haematological status of Clarias batrachus: a long-term monitoring. Chemosphere 144:966–974
Odajima T, Yamazaki I (1972) Myeloperoxidase of the leukocyte of normal blood. III. The reaction of ferric myeloperoxidase with superoxide anion. Biochim Biophys Acta 284:355–359
Olsvik PA, Omsrud R, Lunestad BT, Steine N, Fredriksen BN (2014) Transcriptional responses in Atlantic salmon (Salmo salar) exposed to deltamethrin, alone or in combination with azamethiphos. Comp Biochem Physiol C 162:23–33
Payne JF, Kiceniuk JW, Squires WR, Fletcher GL (1978) Pathological changes in a marine fish after a six month exposure to petroleum. J Fish Res Board Can 35:665–667
Piner P, Üner N (2012) Oxidative and apoptotic effects of lambda-cyhalothrin modulated by piperonyl butoxide in the liver of Oreochromis niloticus. Environ Toxicol Pharmacol 33:414–420
Poleksic V, Mitrovic-Tutundzic V (1994) Fish gills as a monitor of sublethal and chronic effects of pollution. In: Muller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Fishing New Books, Oxford, pp 339–352
Prusty AK, Meena DK, Mohapatra S, Panikkar P, Das P, Gupta SK, Behera BK (2015) Synthetic pyrethroids (type II) and freshwater fish culture: perils and mitigation. Int Aquat Res 7:163–191
Quabius ES, Balm PH, Wendelaar Bonga S (1997) Interrenal stress responsiveness of tilapia (Oreochromis mossambicus) in impaired by dietary exposure to PCB 126. Gen Comp Endocrinol 108:472–482
Randall DJ, Connell DW, Yang R, Wu SS (1998) Concentrations of persistent lipophilic compounds in fish are determined by exchange across the gills, not through the food chain. Chemosphere 37:1263–1270
Riar N, Crago J, Jiang W, Maryoung LA, Gan J, Schlenk D (2013) Effects of salinity acclimation on the endocrine disruption and acute toxicity of bifenthrin in freshwater and euryhaline strains of Oncorhynchus mykiss. Environ Toxicol Chem 32:2779–2785
Saravanan R, Revathi K, Murthy PB (2009) Lambda-cyhalothrin induced alterations in Clarias batrachus. J Environ Biol 30:265–270
Sentürk M, Ceyhun SB, Erdogan O, Küfrevioglu ÖI (2009) In vitro and in vivo effects of some pesticides in glucose-6-phosphate dehydrogenase enzyme activity from rainbow trout (Onchorhynchus mykiss) erythrocytes. Pestic Biochem Physiol 95:95–99
SEPA. Scottish Environment Protection Agency (2008) Attachment XIV. Guidance note on the licensing of discharges of AMX (Deltamethrin) at marine cage fish. https://www.sepa.org.uk/media/114914/fish-farm-manual-attachment-14.pdf (last accessed 28 Dec 2017)
Singh S, Tiwari RK, Pandey RS (2018) Evaluation of acute toxicity of triazophos and deltamethrin and the inhibitory effect on AChE activity in Channa punctatus. Toxicol Rep 5:85–89
Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171:3–59
Tilton FA, Bammler TK, Gallagher EP (2011) Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp Biochem Physiol C 153:9–16
Toumi H, Boumaiza M, Millet M, Radetski CM, Felten V, Férard JF (2015) Is acetylcholinesterase a biomarker of susceptibility in Daphnia magna (Crustacea, Cladocera) after deltamethrin exposure? Chemosphere 120:351–356
Tu W, Xu C, Lu B, Lin C, Wu Y, Liu W (2016) Acute exposure to synthetic pyrethroids causes bioconcentration and disruption of the hypothalamus–pituitary–thyroid axis in zebrafish embryos. Sci Total Environ 542:876–885
United Kingdom (2017) Summary of product characteristcs – AlphaMax®. Available at: https://www.google.com.br/url?sa=t&rct=j&q=&esrc=s&source=web&cd=5&ved=0ahUKEwjq1ovs5vLXAhVJHpAKHQf4DbcQFghIMAQ&url=http%3A%2F%2Fwww.vmd.defra.gov.uk%2Fproductinformationdatabase%2FSPC_Documents%2FSPC_229187.DOC&usg=AOvVaw2xfI1sTEpk5pYxv5jtgWIP. (last accessed 20 December 2017)
Van Geest JL, Burridge LE, Kidd KA (2014) Toxicity of two pyrethroid-based anti-sea lice pesticides, AlphaMax® and Excis®, to a marine amphipod in aqueous and sediment exposures. Aquaculture 434:233–240
Wendelaar Bonga SE, Lock RAC (1992) Toxicants and osmoregulation in fish. Neth J Zool 42:478–493
Wheelock CE, Eder KJ, Werner I, Huang H, Jones PD, Brammell BF, Elskus AA, Hammock BD (2005) Individual variability in esterase activity and CYP1A levels in Chinook salmon (Oncorhynchus tshawytscha) exposed to fenvalerate and chlorpyrifos. Aquat Toxicol 74:172–192
Wielogórska E, Elliott CT, Danaher M, Connolly L (2015) Endocrine disruptor activity of multiple environmental food chain contaminants. Toxicol in Vitro 29:211–220
Wintrobe MM (1934) Variations in size and haemoglobin content of erythrocytes in the blood of various vertebrates. Fol Haematol 51:32–49
Yildirim MZ, Benlı AÇK, Selvı M, Özkul A, Erkoç F, Koçak O (2006) Acute toxicity, behavioural changes, and histopathological effects of deltamethrin on tissues (gills, liver, brain, spleen, kidney, muscle, skin) of Nile Tilapia (Oreochromis niloticus L.) fingerlings. Environ Toxicol 21(6):614–620
Acknowledgements
The authors are thankful to the technician Mr. da Silva ADA; to all colleagues of the Laboratory of Adaptive Biochemistry for the logistical support; to Polettini Fish Farm for providing the fish; and to DVA Agro do Brasil for kindly granting LCH (Trinca Caps®). We also thank two anonymous reviewers, for their considerations substantially improved the manuscript.
Funding
The present research was supported by Sao Paulo Research Foundation (FAPESP, proc. no 2010/17007-0), which also provided a PhD scholarship to Venturini FP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were approved by the Ethics Committee for Animal Research of the Federal University of Sao Carlos, under the license number CEUA 056/2011.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Venturini, F.P., de Moraes, F.D., Rossi, P.A. et al. A multi-biomarker approach to lambda-cyhalothrin effects on the freshwater teleost matrinxa Brycon amazonicus: single-pulse exposure and recovery. Fish Physiol Biochem 45, 341–353 (2019). https://doi.org/10.1007/s10695-018-0566-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0566-1