Abstract
Cyphenothrin, one of the synthetic pyrethroids, developed as an alternative to organophosphorus and carbamate pesticides. It is used in veterinary medicine and household application against insects. Due to the contamination of the aquatic ecosystems, non-target aquatic organisms are affected. The current study aimed to evaluate the cyphenothrin effects on in vitro and in vivo models of freshwater mussels Unio delicatus Lea, 1863. While antioxidant enzyme (glutathione) was measured in both models, the total hemocyte counts were only detected in vivo models after exposure to cyphenothrin (1 and 10 μg/L) for 24-h and 48-h exposure times. A decrease in total hemocyte count occurred depending on the dose and duration (p < 0.001). In both in vitro and in vivo models of gill and digestive gland tissues, higher glutathione levels were obtained at a dose of 10 μg/L compared to the control groups in both exposure times (p < 0.001). The results of the study suggest that the antioxidant parameters could represent biomarkers to evaluate the effects of pollutants on in vitro and in vivo models of freshwater mussels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic activities cause pollution of aquatic ecosystem with pesticides. The pesticides in the aquatic ecosystems result in various effects on aquatic organisms (Timpano et al., 2022). The main types of pesticides found in the marine and freshwaters are herbicides, insecticides, and fungicides (Staley et al., 2015). Among insecticides, synthetic pyrethroids are widely used chemicals. They are derived from pyrethrin extracts from the flowers of Chrysanthemum cinerariaefolium (Ensley, 2018). Many pyrethroid varieties show high efficacy as insecticides for agricultural and domestic use. Due to their low toxicity to mammals and their short-term persistence in the environment, they are highly used in insecticide species (Feng et al., 2009; Lutnicka et al., 1999). Synthetic pyrethroids are classified into two types. While type I compounds that lack an alpha-cyano moiety include permethrin, resmethrin, and tetramethrin, type II compounds contain an alpha-cyano-3-phenoxybenzyl substituent including cypermethrin and cyphenothrin (Ensley, 2018).
Cyphenothrin (CAS No. 39515–40-7) is commonly used in veterinary medicine against ectoparasites and household application against flies, mosquitoes, and cockroaches due to its lower toxicity and effective insecticidal potential (Huang et al., 2020; Yücel & Özkul, 2016). However, this widespread use of cyphenothrin contaminates terrestrial and aquatic ecosystems, affecting non-target species as well (Huang et al., 2020; Zhan et al., 2020). Lethal concentration values (LC50) of cyphenothrin were investigated in studies with different aquatic species. The LC50 values are found for Daphnia magna as 0.92 μg/L and 0.43 μg/L for 48 h (USEPA/OPP, EFED, 2020), for Barbus macrops as 15 μg/L for 24 h, 12 μg/L for 48 h, and 10 μg/L for 72 h (Yameogo et al., 1991), for Chrysichthys longidorsalis as 630 μg/L for 24 h and 220 μg/L for 48 h (Yameogo et al., 1991), for Oncorhynchus mykiss as 0.34 μg/L and 0.37 μg/L for 96 h (USEPA/OPP, EFED, 2020), and for Lebistes reticulatus as 48.7 μg/L for 96 h (Erkmen et al., 2000). In addition, in a study conducted with common carp Cyprinus carpio exposed to cyphenothrin at a dose of 5 μg/L for 75 days, it was shown that histopathological findings were obtained in the gill, liver, kidney, and brain tissues (Yücel & Özkul, 2016).
Water pollutants have toxic effects on aquatic organisms and a wide range of biomarkers are used to investigate their effects on the cellular, biochemical, and physiological parameters (Wu & Wang, 2010). These methods measure the specific responses of organisms such as the alteration of total hemocyte counts to reflect the species’ health/immune system (Andreyeva et al., 2019) or the glutathione values to show for oxidative stress (Almeida et al., 2005).
Mussels, filter-feeding organisms, are used as sentinels of aquatic toxicology studies and considered a non-target group to water pollutants toxicity (Wang et al., 2019). There are many studies conducted on marine and freshwater mussels that reported the alterations of cellular, biochemical, and physiological parameters of the organisms exposed to pollutants (Almeida et al., 2005; Verlecar et al., 2008; Wu & Wang, 2010; Wang et al., 2019; Stara et al., 2020; Katalay et al., 2022). One of the first physiological responses that points to environmental stresses in mussels is the change in the number of cells in the hemolymph; hemolymph is a tissue responsible for substance transport and the immune system in the organisms (Andreyeva et al., 2019). Abiotic or biotic-induced changes in the aquatic ecosystem may affect the normal metabolic activities of mussels. The oxidative stress mechanism is activated due to the production of reactive oxygen species (ROS) in organisms. The antioxidant defense system develops a defense system against this oxidative stress. These antioxidant systems, both enzymatic and non-enzymatic, are biomarkers used in environmental monitoring studies (Verlecar et al., 2008).
The use of mussels in scientific studies is increasing day by day both in the development of mussel farming areas and in basic research and analysis of pollutants. Studies with mussels are starting to leave their place to alternative models with the development of the 3R rule (refinement, reduction, and replacement) over time (Barrick et al., 2019). In vitro studies have been developed as an alternative to the investigation of toxic substances in in vivo animal experiments, providing rapid results and keeping the number and cost of experimental animals low. These models reveal the toxic effects of pollutants with great precision and reproducibility (Gómez-Mendikute et al., 2005). In vitro systems developed from different tissues of mussels are used to investigate the effects of aquatic pollutants (Yurdakök-Dikmen et al., 2018; Gómez-Mendikute et al., 2005; Arslan et al., 2021).
It is observed that studies comparing in vitro and in vivo applications are generally used in fields such as biomedical applications (Guzmán-Soto et al., 2021; Samadian et al., 2021), cancer research (Zhu et al., 2022), and pharmacological (Xiao et al., 2021) studies. The number of studies in the field of aquatic toxicology is quite low. Besides, the comparison of the effects of pollutants on the marine or freshwater mussels in vitro and in vivo models is limited (Panfoli et al., 2020; Barrick et al., 2019). Besides, when the studies of cyphenothrin on aquatic organisms are examined, no studies have been found on physiological or biochemical studies with freshwater mussels. The aim of this study is (1) to determine the first response of cyphenothrin on total hemocyte counts in vivo and (2) to investigate the effects of cyphenothrin on mussel gill and digestive gland cells in vivo and in vitro by using the glutathione parameter end-point.
2 Materials and Methods
2.1 Experiment Organisms
The model organism of this study is Unio delicatus Lea, 1863. Due to habituating a wide range area including river basins of the southwest to the east of Anatolia, the species is used for biomonitoring of the aquatic ecosystems to investigate the water quality and pollution (Lopes-Lima et al., 2021). Gölbaşı Lake located south Anatolia (Adıyaman, Turkey) has rich biodiversity including Unio delicatus (Alkan Uçkun, 2018). The freshwater mussels were obtained from local fishermen from Gölbaşı Lake and were brought to the laboratory in the aerated water. To adapt to laboratory conditions, they were placed in 15 L aquariums containing 10 L of dechlorinated tap water for 2 weeks. The water was changed every 2 days by siphoning. During the adaptation period, the mussels were fed by Cyanobacteria Spirulina sp.
2.2 Chemical Preparation
The stock concentration of cyphenothrin (purity 94%) was prepared via dimethylsulfoxide (DMSO) as 10 mg/L. Before the experiments, the sublethal concentrations of the insecticide were determined by the preliminary experiments as 1 and 10 μg/L. The stock concentration was diluted with water for in vivo experiments while it was diluted with cell culture medium (Leibovitz 15) for in vitro experiments.
2.3 In Vivo Exposure Experiments
For 24 h and 48 h exposure times, there were two controls (non-treated and solvent) and two cyphenothrin groups (1 and 10 μg/L CP) in the experiments (n = 80). Each groups contained 10 mussels which were selected randomly from stock aquariums. After each exposure time, ten mussels were sampled and taken their weight (34.35 ± 6.06 g) and length (5.05 ± 0.41 cm) parameters. With the help of 2.5 mL injection, the hemolymph tissues were taken from mussels. The total hemocyte counts (THCs) were evaluated according to Yavuzcan and Benli (2004). Then, the mussels were dissected, and their gill and digestive gland tissues were taken. The tissues were kept in − 80 °C until the biochemical analysis.
2.4 In Vitro Exposure Experiments
Three mussels, mean length of 4.98 ± 0.07 cm and mean weight of 33.09 ± 1.57 g, were chosen randomly from stock aquariums. The primary cell culture from gill and digestive gland tissues was made according to Yurdakök-Dikmen et al. (2018). To eliminate the contamination sources, the mussels were kept in absolute ethanol 1 min and the shells were flamed for 3 s. Then, the mussels were dissected with sterile scissors and pens under sterile conditions. The gill and digestive gland tissues were taken into sterile Petri dishes. After cutting into 3–5 mm pieces, the tissues were kept with trypsin solution (Capricorn Scientific, Germany) containing penicillin–streptomycin (Capricorn Scientific, Germany), amphotericin B (Capricorn Scientific, Germany), and fetal bovine serum (Capricorn Scientific, Germany) for 4 h. The cell culture medium Leibovitz 15 (Sigma Aldrich, USA) was added and the Petri dishes were incubated for 24 h. Then, the solution of Petri dishes was pipetted and filtered with 200 μm cell strainers. The filtered solutions containing cells were centrifugated for 10 min at 400 rpm and the supernatant was removed. The cell pellets were ready to exposure experiments. For the cyphenothrin exposure, the cells were placed into 24-well plate at the density of 105 cells/well. The cells were incubated for 24 h to attach to the well surface. The cell medium was removed and cyphenothrin concentrations (1 and 10 μg/L CP) were applied three replicates on each plate. The exposure durations were 24 h and 48 h. In each plate, there were two control groups: non-treated cells and solvent control group (DMSO).
2.5 Glutathione Assay
Glutathione analysis was performed according to the Ellman (1959). For this, cell and tissue samples were homogenized with metaphosphoric acid and then centrifuged at 3500 rpm + 4 °C for 10 min. Samples whose supernatants were taken were mixed with Ellman reagent and measured spectrophotometrically at wavelengths of 410 (A1) and 420 (A2) nm. After the determination of cell and tissue protein amounts by the Bradford (1976), glutathione values were obtained as (A1-A2)/protein.
2.6 Statistical Information
All the parameters in this study were distributed normally according to the Kolmogorov–Smirnov normality test. Data were expressed as mean ± standard deviation in the graphs. Differences were tested by one-way ANOVA test. The values P < 0.05 were considered statistically significant. Statistical analysis was done using GraphPad Prism 5 program.
3 Results
In this study, in vitro and in vivo systems of freshwater mussels were exposed to cyphenothrin concentrations of 1 and 10 μg/L for 24 h and 48 h. At the end of the exposure times, the total hemocyte count was examined in in vivo systems, while the glutathione parameter was examined in in vitro and in vivo systems.
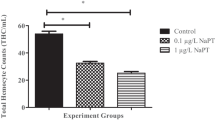
As in vivo study with freshwater mussels, the total hemocyte counts were evaluated for determination of the physiological effects of cyphenothrin. The changes of the total hemocyte counts of mussels exposed to two different cyphenothrin concentrations for 24-h and 48-h exposure periods is shown in Fig. 1. At the end of the 24-h exposure, the total hemocyte count was higher in the control groups. In 1 and 10 μg/L dose groups, 1.66 and 1.22 times less THCs were detected compared to the control group, respectively (p = 0.018). Similarly, after 48 h of exposure, the total hemocyte count was higher in the control groups. While 1.45 times less THCs were detected in the 1 μg/L dose group, 0.5 times less THCs were found in the 10 μg/L dose group compared to the control group (p = 0.002). When 24-h and 48-h exposure conditions were compared, high THC values were observed in the control, solvent control, and dose groups during the 24-h exposure period compared to 48-h exposure groups. Thus, there is a significant difference between the two periods (p < 0.001). The trend of change in the total hemocyte counts in U. delicatus was found to be non-linear exposed to cyphenothrin concentrations.
The changes in glutathione level, which is one of the oxidative stress parameters, were investigated in the gill and digestive gland tissues of freshwater mussels exposed to cyphenothrin (Fig. 2). It was observed that significantly higher values were obtained in the gill tissues in the groups at the dose of 10 μg/L at 24-h and 48-h exposure compared to the control group (p < 0.001). Glutathione levels at the dose of 10 μg/L increased 1.9 and 1.5 times in the gill tissue compared to the control group during 24 h and 48 h of exposure. Unlike gills, glutathione level at a dose of 10 μg/L decreased 1.6 times but increased 1.4 times in the digestive gland tissue compared to the control group in 24 h and 48 h of exposure, respectively (p < 0.001).
Similar results have been obtained in in vitro studies of freshwater mussels as well as in vivo experiments (Fig. 3). Glutathione levels in the gill cell culture were increased by 1.34 times at 24-h exposure and 1.61 times at 48 h of exposure in the 10 μg/L dose group compared to the control group (p < 0.001). Like gill cell cultures, in the digestive gland cell culture, a 0.79-fold increase in 24-h exposure and 1.52-fold increase in 48 h were observed compared to the control group cells (p < 0.001).
4 Discussion
Mussels, important species diversity among aquatic organisms, absorb aquatic pollutants through filtering the water and lead to bioaccumulation in the food chain. Due to these properties, they help to reduce the pollutant load in those systems by transferring them to aquatic systems where the pollutant load is known to increase. Thus, they are used to reflect a load of pollutants, especially in field studies, as well as to investigate the effects of pollutants at different biological levels in laboratory studies (Doyotte et al., 1997; Stara et al., 2020). Therefore, they are considered model organisms to biomonitoring the aquatic system health status to provide accurate biological endpoints such as physiological, cellular, and biochemical (Faggio et al., 2018).
Due to the increase in pollution in aquatic ecosystems, the immune system of mussels weakens and the potential for disease increases. Against this phenomenon, the immune system, which consists of cellular and humoral components, comes into the situation. The hemocyte cells in the hemolymph tissue, which contains the immune system elements, reveal the physiological and immunological status of the mussels (de la Ballina et al., 2022). There are studies in which the total hemocyte counts decrease or increase in marine and freshwater mussels exposed to different pollutants (Arslan, 2022; Gürkan, 2022; Li et al., 2022). The THC results of this study showed a rapid decrease in the lowest concentration of cyphenothrin in both exposure times. These results indicate that hemocyte circulation decreased in the hemolymph tissues of organisms exposed to chemical (Gürkan, 2022). Moreover, the THCs at low dose of cyphenothrin are less than that at high dose can be explained by the slow response of the organism’s metabolism. Ray et al. (2013) obtained similar results to the current study in aquatic invertebrates exposed to cypermethrin and fenvalerate.
Glutathione, one of the compounds with a thiol group in the cell, is metabolically active in the organism against exposure to endogenous and exogenous chemicals and is one of the cell defense mechanisms (Canesi et al., 1999). Glutathione, which is among the antioxidant mechanisms, is used as a biomarker for contaminant-mediated in many aquatic organisms (Almeida et al., 2005; Regoli, 1998). In the current study, both models of freshwater mussels marked alterations in the glutathione levels of digestive glands and gills.
One of the tissues used in the screening of health indicators of mussels is the digestive gland tissue. The digestive gland is involved in processes such as secretion, enzyme production, and digestion. In addition, lysosomes in cells take part in the detoxification process of toxic substances (Faggio et al., 2018). Due to the importance of xenobiotics in the detoxification process in the organism, digestive gland tissues have been studied as in vivo or in vitro models in many studies (Wilhelm Filho et al., 2001; Canesi et al., 2007; Yurdakök-Dikmen et al., 2018; Gürkan, 2022). In this study, the effect of the insecticide on the glutathione parameter was investigated in the digestive gland tissue both in vitro and in vivo. In both models, the glutathione levels were higher in the high-dose group than in the control groups during the exposure times. Obtaining similar results in both models proves that digestive gland primary cell cultures can be used as an alternative to in vivo experiments. In addition, these results support the use of digestive gland cell cultures made with marine and freshwater organisms as a usable model for investigating oxidative stress parameters caused by pollutants (Birmelin et al., 1999; Parolini et al., 2011; Balbi et al., 2017).
Gill tissue is responsible for the respiration of the mussels (Wu et al., 2022) and is the first organ contact the xenobiotics in the aquatic environment (Günal et al., 2021; Nimet et al., 2020). In this study, in which the oxidative parameter of the gills was evaluated in vitro and in vivo systems, it was observed that the glutathione level increased at high doses compared to the control groups, while a decrease occurred at low doses. Like digestive gland cell cultures, primary gill cell cultures are also useful tool for the evaluating the toxic effects of pollutants (Arslan et al., 2021; Parolini et al., 2011; Yurdakök-Dikmen et al., 2018).
In conclusion, cyphenothrin is toxic freshwater mussels. Among physiological parameters, the lowest THCs values were found in the lower concentrations of cyphenothrin in both exposure times, but the higher concentrations were also decreased compared to control groups. This is due to the difference in metabolism in the recovery process of the organism against the dose of insecticide. On the other hand, the glutathione levels of the digestive gland and gill tissues were increased in the higher concentration of cyphenothrin in both exposure times. Similar results were obtained in both in vitro and in vivo tissue comparisons. Therefore, the current study demonstrated that Unio delicatus primary gill and digestive gland cell cultures should be absolutely considered in screening ecotoxicological evaluations to assess the potential hazard of aquatic pollutants. Hence, the 3R rule can be adopted by turning to in vitro systems when carrying out the studies on aquatic pollutants.
Data Availability
The research has no associate data.
References
AlkanUçkun, A. (2018). Investigation of toxic metal contamination in water and sediments of Gölbaşı Lake (Adıyaman). Adıyaman University Journal of Science, 8(2), 129–140.
Almeida, E. A., Bainy, A. C. D., Dafre, A. L., Gomes, O. F., Medeiros, M. H., & Di Mascio, P. (2005). Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. Journal of Experimental Marine Biology and Ecology, 318(1), 21–30. https://doi.org/10.1016/j.jembe.2004.12.007
Andreyeva, A. Y., Efremova, E. S., & Kukhareva, T. A. (2019). Morphological and functional characterization of hemocytes in cultivated mussel (Mytilus galloprovincialis) and effect of hypoxia on hemocyte parameters. Fish & Shellfish Immunology, 89, 361–367. https://doi.org/10.1016/j.fsi.2019.04.017
Arslan, P., Yurdakok-Dikmen, B., Ozeren, S. C., Kuzukıran, O., & Filazi, A. (2021). In vitro effects of erythromycin and florfenicol on primary cell lines of Unio crassus and Cyprinus carpio. Environmental Science and Pollution Research, 28, 48408–48416. https://doi.org/10.1007/s11356-021-14139-3
Arslan, P. (2022). Determinations of the effects of cyfluthrin on the hemocytes parameters of freshwater mussel (Unio delicatus). Ege Journal of Fisheries and Aquatic Sciences, 39(1), 39–45.
Balbi, T., Ciacci, C., Grasselli, E., Smerilli, A., Voci, A., & Canesi, L. (2017). Utilization of Mytilus digestive gland cells for the in vitro screening of potential metabolic disruptors in aquatic invertebrates. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 191, 26–35. https://doi.org/10.1016/j.cbpc.2016.08.009
Barrick, A., Manier, N., Lonchambon, P., Flahaut, E., Jrad, N., Mouneyrac, C., & Châtel, A. (2019). Investigating a transcriptomic approach on marine mussel hemocytes exposed to carbon nanofibers: An in vitro/in vivo comparison. Aquatic Toxicology, 207, 19–28. https://doi.org/10.1016/j.aquatox.2018.11.020
Birmelin, C., Pipe, R. K., Goldfarb, P. S., & Livingstone, D. R. (1999). Primary cell-culture of the digestive gland of the marine mussel Mytilus edulis: A time-course study of antioxidant-and biotransformation-enzyme activity and ultrastructural changes. Marine Biology, 135(1), 65–75.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Canesi, L., Borghi, C., Fabbri, R., Ciacci, C., Lorusso, L. C., Gall, G., & Vergani, L. (2007). Effects of 17β-estradiol on mussel digestive gland. General and Comparative Endocrinology, 153(1–3), 40–46. https://doi.org/10.1016/j.ygcen.2007.02.005
Canesi, L., Viarengo, A., Leonzio, C., Filippelli, M., & Gallo, G. (1999). Heavy metals and glutathione metabolism in mussel tissues. Aquatic Toxicology, 46(1), 67–76. https://doi.org/10.1016/S0166-445X(98)00116-7
de la Ballina, N. R., Francesco, M., Asunción, C., & Antonio, V. (2022). Bivalve haemocyte subpopulations: A review. Frontiers in Immunology, 13.https://doi.org/10.3389/fimmu.2022.826255.
Doyotte, A., Cossu, C., Jacquin, M. C., Babut, M., & Vasseur, P. (1997). Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquatic Toxicology, 39(2), 93–110. https://doi.org/10.1016/S0166-445X(97)00024-6
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Ensley, S. M. (2018). Pyrethrins and pyrethroids. In Veterinary Toxicology Basic and Clinical Principles (pp. 515–520). Academic Press. https://doi.org/10.1016/B978-0-12-811410-0.00039-8
Erkmen, B., Caliskan, M., & Yerli, S. V. (2000). Histopathological effects of cyphenothrin on the gills of Lebistes reticulatus. Veterinary and Human Toxicology, 42(1), 5–7.
Faggio, C., Tsarpali, V., & Dailianis, S. (2018). Mussel digestive gland as a model tissue for assessing xenobiotics: An overview. Science of the Total Environment, 636, 220–229. https://doi.org/10.1016/j.scitotenv.2018.04.264
Feng, D., Ke, C., Li, S., Lu, C., & Guo, F. (2009). Pyrethroids as promising marine antifoulants: Laboratory and field studies. Marine Biotechnology, 11(2), 153–160. https://doi.org/10.1007/s10126-008-9130-9
Gómez-Mendikute, A., Elizondo, M., Venier, P., & Cajaraville, M. P. (2005). Characterization of mussel gill cells in vivo and in vitro. Cell and Tissue Research, 321(1), 131–140. https://doi.org/10.1007/s00441-005-1093-9
Guzmán-Soto, I., McTiernan, C., Gonzalez-Gomez, M., Ross, A., Gupta, K., Suuronen, E. J., Mah, T. F., Griffith, M., & Alarcon, E. I. (2021). Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. Iscience, 24(5), 102443. https://doi.org/10.1016/j.isci.2021.102443
Günal, A. Ç., Tunca, S. K., Arslan, P., Gül, G., & SepiciDinçel, A. (2021). How does sublethal permethrin effect non-target aquatic organisms? Environmental Science and Pollution Research, 28, 52405–52417. https://doi.org/10.1007/s11356-021-14475-4
Gürkan, S. E. (2022). Impact of nickel oxide nanoparticles (NiO) on oxidative stress biomarkers and hemocyte counts of Mytilus galloprovincialis. Biological Trace Element Research. https://doi.org/10.1007/s12011-022-03189-4
Huang, Y., Lin, Z., Zhang, W., Pang, S., Bhatt, P., Rene, E. R., Kumar, A. J., & Chen, S. (2020). New insights into the microbial degradation of D-cyphenothrin in contaminated water/soil environments. Microorganisms, 8, 473. https://doi.org/10.3390/microorganisms8040473
Katalay, S., Guner, A., Dagdeviren, M., Yigitturk, G., Yavasoglu, A., Gunal, A. C., Karabay Yavasoglu, N. U., & Oltulu, F., (2022). Oxidative stress-induced apoptotic changes after acute exposure to antifouling agent zinc pyrithione (ZnPT) in Mytilus galloprovincialis Lamark (Mediterranean mussels) tissues. Chemistry and Ecology, 1-18.https://doi.org/10.1080/02757540.2022.2047951.
Li, F., Liu, Z., Yao, L., Jiang, Y., Qu, M., Yu, Y., Gong, X., Tan, Z., & Li, Z. (2022). Immunotoxicity of perfluorooctanoic acid to the marine bivalve species Ruditapes philippinarum. Environmental Toxicology and Chemistry, 41(2), 426–436. https://doi.org/10.1002/etc.5263
Lopes-Lima, M., Gürlek, M. E., Kebapçı, Ü., Şereflişan, H., Yanık, T., Mirzajan, A., Neubert, E., Prie, V., Teixeira, A., Gomes-dos-Santos, A., Barros-Garcia, D., Bolotov, I. N., Kondakov, A. V., Vikhrev, I. V., Tomilova, A. A., Özcan, T., Altun, A., Gonçalves, D. V., Bogan, A. E., & Froufe, E. (2021). Diversity, biogeography, evolutionary relationships, and conservation of Eastern Mediterranean freshwater mussels (Bivalvia: Unionidae). Molecular Phylogenetics and Evolytion, 163, 107261. https://doi.org/10.1016/j.ympev.2021.107261
Lutnicka, H., Bogacka, T., & Wolska, L. (1999). Degradation of pyrethroids in an aquatic ecosystem model. Water Research, 33, 3441–3446. https://doi.org/10.1016/S0043-1354(99)00054-8
Nimet, J., Neves, M. P., Viana, N. P., de Arruda Amorim, J. P., & Luciana, R. (2020). Histopathological alterations in gills of a fish (Astyanax bifasciatus) in neotropical streams: Negative effects of riparian forest reduction and presence of pesticides. Environment Monitoring and Assessment, 192, 58. https://doi.org/10.1007/s10661-019-8030-y
Panfoli, I., Burlando, B., & Viarengo, A. (2020). Effects of heavy metals on phospholipase C in gill and digestive gland of the marine mussel Mytilus galloprovincialis Lam. Comparative Biochemistry and Physiology Part b: Biochemistry & Molecular Biology, 127(3), 391–397. https://doi.org/10.1016/s0305-0491(00)00272-8
Parolini, M., Quinn, B., Binelli, A., & Provini, A. (2011). Cytotoxicity assessment of four pharmaceutical compounds on the zebra mussel (Dreissena polymorpha) haemocytes, gill and digestive gland primary cell cultures. Chemosphere, 84(1), 91–100.
Ray, M., Bhunia, A. S., Bhunia, N. S., & Ray, S. (2013). Density shift, morphological damage, lysosomal fragility and apoptosis of hemocytes of Indian molluscs exposed to pyrethroid pesticides. Fish & Shellfish Immunology, 35(2), 499–512. https://doi.org/10.1016/j.fsi.2013.05.008
Regoli, F. (1998). Trace metals and antioxidant enzymes in gills and digestive gland of the Mediterranean Mussel Mytilus galloprovincialis. Archives Environmental Contamination and Toxicology, 34, 48–63. https://doi.org/10.1007/s002449900285
Samadian, H., Khastar, H., Ehterami, A., & Salehi, M. (2021). Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Scientific Reports, 11(1), 1–11. https://doi.org/10.1038/s41598-021-93367-6
Staley, Z. R., Harwood, V. J., & Rohr, J. R. (2015). A synthesis of the effects of pesticides on microbial persistence in aquatic ecosystems. Critical Reviews of Toxicology, 45(10), 813–836. https://doi.org/10.3109/10408444.2015.1065471
Stara, A., Pagano, M., Capillo, G., Fabrello, J., Sandova, M., Vazzana, I., Zuskova, E., Velisek, J., Matozzo, V., & Faggio, C. (2020). Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. Science of the Total Environment, 700, 134914. https://doi.org/10.1016/j.scitotenv.2019.134914
Timpano, A. J., Jones, J. W., Beaty, B., Hull, M., Soucek, D. J., & Zipper, C. E. (2022). Combined effects of copper, nickel, and zinc on growth of a freshwater mussel (Villosa iris) in an environmentally relevant context. Aquatic Toxicology, 242, 106038. https://doi.org/10.1016/j.aquatox.2021.106038
USEPA/OPP, EFED. (2000). Pesticide Ecotoxicity Database, as cited in the ECOTOX database. Available from, as of July 31, 2018: https://cfpub.epa.gov/ecotox/.
Verlecar, X. N., Jena, K. B., & Chainy, G. B. N. (2008). Seasonal variation of oxidative biomarkers in gills and digestive gland of green-lipped mussel Perna viridis from Arabian Sea. Estuarine, Coastal and Shelf Science, 76(4), 745–752.
Xiao, S., Yu, H., Xie, Y., Guo, Y., Fan, J., & Yao, W. (2021). The anti-inflammatory potential of Cinnamomum camphora (L.) J. Presl essential oil in vitro and in vivo. Journal of Ethnopharmacology, 267, 113516. https://doi.org/10.1016/j.jep.2020.113516.
Wang, T., Huang, X., Jiang, X., Hu, M., Huang, W., & Wang, Y. (2019). Differential in vivo hemocyte responses to nano titanium dioxide in mussels: Effects of particle size. Aquatic Toxicology, 212, 28–36. https://doi.org/10.1016/j.aquatox.2019.04.012
Wilhelm Filho, D., Tribess, T., Gaspari, C., Claudio, F. D., Torres, M. A., & Magalhaes, A. R. M. (2001). Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture, 203(1–2), 149–158. https://doi.org/10.1016/S0044-8486(01)00599-3
Wu, H., & Wang, W. X. (2010). NMR-based metabolomic studies on the toxicological effects of cadmium and copper on green mussels Perna viridis. Aquatic Toxicology, 100(4), 339–345. https://doi.org/10.1016/j.aquatox.2010.08.005
Wu, W., Geist, J., Beggel, S., Schmitz, C., Milz, S., & Sternecker, K. (2022). Immunohistochemical detection of various proteoglycans in the extracellular matrix of zebra mussels. Fishes, 7(2), 74. https://doi.org/10.3390/fishes7020074
Yameogo, L., Tapsoba, J. M., & Calamari, D. (1991). Laboratory toxicity of potential blackfly larvicides on some African fish species in the Onchocerciasis Control Programme area. Ecotoxicology and Environmental Safety, 21(3), 248–256. https://doi.org/10.1016/0147-6513(91)90063-u
Yavuzcan, H. Y., & Benli, A. Ç. K. (2004). Nitrite toxicity to crayfish, Astacus leptodactylus, the effects of sublethal nitrite exposure on hemolymph nitrite, total hemocyte counts, and hemolymph glucose. Ecotoxicology and Environmental Safety, 59(3), 370–375. https://doi.org/10.1016/j.ecoenv.2003.07.007
Yücel, G., & Özkul, İA. (2016). Pathomorphological evaluation of toxic effect of cypermethrin and cyphenothrin in common carp. Ankara University Journal of Veterinary Medicine Faculty, 63(4), 401–406.
Yurdakök-Dikmen, B., Arslan, P., Kuzukıran, Ö., Filazi, A., & Erkoç, F., (2018). Unio sp. primary cell culture potential in ecotoxicology research. Toxin Reviews, 37(1), 75–81. https://doi.org/10.1080/15569543.2017.1331360
Zhan, H., Huang, Y., Lin, Z., Bhatt, P., & Chen, S. (2020). New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environmental Research, 18, 109138. https://doi.org/10.1016/j.envres.2020.109138
Zhu, K., Deng, C., Du, P., Liu, T., Piao, J., Piao, Y., Yang, M., & Chen, L. (2022). G6PC indicated poor prognosis in cervical cancer and promoted cervical carcinogenesis in vitro and in vivo. Reproductive Biology and Endocrinology, 20, 50(2022). https://doi.org/10.1186/s12958-022-00921-6.
Acknowledgements
Special thanks to Prof. Dr. A. Çağlan Günal for providing cyphenothrin, and to Prof. Dr. Aylin Sepici-Dinçel for providing the Ellman’s reagent.
Author information
Authors and Affiliations
Contributions
The author designed the study, performed the experiments, evaluated the results, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arslan, P. How Does Cyphenothrin Affect the Freshwater Mussel as In Vitro and In Vivo Models?. Water Air Soil Pollut 233, 386 (2022). https://doi.org/10.1007/s11270-022-05860-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05860-x