Abstract
The acute effect of carbofuran, a carbamate insecticide, was studied on adenosine triphosphatase enzymes in gill, kidney, liver and muscle tissues of a food fish, Clarias batrachus. Glycogen and glycogen phosphorylase were investigated in gill and kidney only. Thirty-six fish were exposed to sublethal concentration (7.6 mg/L) for 6 days. After 6 days, 18 fish were released into freshwater in order to study the recovery response. Eighteen fish were kept in clean water as control. Tissues were isolated from control, exposed and recovery fish at the end of 1, 3 and 6 days and used for the assay of enzymes. Total ATPase was inhibited in kidney and muscle tissues throughout the exposure period, whereas branchial and hepatic tissues showed initial induction followed by inhibition. Na+-K+ ATPase activity was induced in gill till day 3, whereas in other tissues inhibition was throughout the exposure period. Mg+2 ATPase activity was inhibited in all tissues except liver. When the fish were released into freshwater, liver recovered almost to control values and other tissues showed organ-specific response. Glycogen content of gill increased initially followed by decrease, and in kidney initial decrease was noted. The recovery response was more in kidney than in gill. Induction in the activity of glycogen phosphorylases was observed in kidney, whereas gill tissue showed mixed response. Recovery was not observed in phosphorylases. Thus, the results of the present study demonstrated the acute effect of carbofuran on a food fish and organ-specific recovery response to insecticidal treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On the global basis, more than two thousand million kilograms of conventional pesticides with agriculture, forest and disease control applications are used annually. These synthetic poisons, once released into the environment, can affect other living beings and cause unpredictable effects. About 70% of the pesticides used in agricultural fields reach adjoining water bodies through rain or irrigation (Ridgway et al. 1978) or by direct use in the water bodies for control of aquatic weeds (Li 1975). Many of the pesticides and their metabolites, which contaminate aquatic ecosystems, have yet to be identified, and their impact on aquatic life is to be determined. Therefore, the exposure, fate and effects of pesticides in the aquatic ecosystems have been extensively studied by environmental toxicologists. The carbamate insecticide, carbofuran is a broad-spectrum pesticide, commonly used in agriculture throughout the world. It is frequently used because of its relatively short life in the environment and fast action on the target pest. The combination of high solubility and low sorption causes carbofuran to be mobile in groundwater, and its toxic degradation products (3 hydroxy-carbofuran) are also soluble in water (Munster et al. 1996). Carbofuran is being used extensively in rice fields to control rice pests, and contamination of water bodies adjacent to rice fields by this carbamate, mainly through run-off, is quite possible (Moreno et al. 2008). Carbofuran elicits its primary effects by virtue of reversible inhibition of acetylcholinesterase activity at nerve terminals (Fukuto 1990). Reports on action of carbofuran on brain and muscle acetylcholinesterase enzyme of gold fish, Carassius auratus (Bretaud et al. 2000) and in Gambusia yucatana (Osten et al. 2005) are available. Carbofuran, in addition to inhibition of AChE activity, affects many nonspecific sites. Moreno et al. (2008) reported the long-term effects of carbofuran on hepatic cytochrome and UDPGT in tench (Tinca tinca L.). Toxicity of carbofuran on nucleotide hydrolysis in Zebra fish (Danio rerio) brain membrane was reported by Senger et al. (2005). Reports are available on the sublethal effect of carbofuran on biochemical parameters of the fish, Clarias batrachus (Begum 2001, 2004, 2008) and on ovarian maturity and recovery response in Cyprinus carpio (Chandra et al. 2004). Fish are extremely sensitive to many waterborne pollutants, and the gills respond to most of the stressors being potentially an excellent indicator of environmental quality and/or stress level in the intact animal (Wendelaar Bonga 1997). The gill epithelium provides an extensive surface of contact with the environment to facilitate ion transport, gaseous exchange and exchange of aquatic toxicants and hazardous agents (Perry 1997). Liver is the primary site involved in the metabolism of various drugs and chemicals including carbofuran (Pineiro-Carrero and Pineiro 2004) and is most widely studied tissue for the mechanisms of organ toxicity. The kidney plays a central role in excreting physiological compounds and metabolites formed in it and the other tissues, in particular in the liver. Muscle tissue is the main consumable part of the fish. An attempt has been made to investigate the organ-specific response of carbofuran in physiologically important tissues viz; gill, liver, muscle and kidneys of a fish, Clarias batrachus, special attention was given to the recovery response since such information is not known. ATPases are the membrane-bound enzymes responsible for the transport of ions through biological membranes and thus regulates cellular volume, osmotic pressure and membrane permeability (Sancho et al. 2003). ATPases are considered as sensitive biomarkers used for assessment of the membrane fragility of organs (Stagg et al. 1992). Hence, adenosine triphosphatase (Na+-K+, Mg+2 and total ATPase in gill, liver, muscle and kidney tissues), along with glycogen and glycogen phosphorylase enzymes (a and ab in gill and kidneys only) as biomarkers of carbofuran toxicity, was selected for the present study. The parameters were studied at the end of 1, 3 and 6 days of exposure as well as recovery period.
Materials and methods
The air-breathing food fish, Clarias batrachus (Linnaeus) (30–32 g and 22–24 cm), were purchased from a local fish market in Hyderabad (India) and brought to the laboratory. They were acclimatized for 15 days at 26 ± 2°C with 12 h: 12 h light and dark period before using for experiments. They were kept in glass aquaria and fed ad libitum with chopped boiled beef liver and egg albumin, and the food was with held from 24 h prior to the experiment. The procurement and acclimation of fish and the experimental conditions were according to OECD guidelines (1992). The commercial-grade carbofuran (2,3-dihydro 2,2 dimethyl-7-benzofuranyl-methyl carbamate) containing 3% granules of active ingredients of carbofuran was purchased from local agrochemical shop. The stock solution of carbofuran was prepared in 100% ethanol, and the 96 h LC50 value was determined by the method of Finney (1964). The physicochemical characteristics of the laboratory water were in the following range: pH 7.3–7.5, dissolved oxygen 7.5–8 mg/L, temperature 27–29°C, hardness 98–100 mg/L as CaCO3 and alkalinity 85–90 mg/L as CaCO3. A group of 36 fish was exposed to 7.66 mg/L commercial carbofuran in 36 litre of laboratory water in glass aquaria (60 × 30 × 30 cm) for 6 days; this served as exposed group. The exposed concentration is one-third the value of the 96 h LC50 value for commercial carbofuran (23 mg/L). After 6 days of exposure period, 18 fish were transferred into carbofuran-free water and held for 6 days to study the recovery response. The recovery response was noted at the end of 1, 3 and 6 days. A third group of 18 fish were kept in toxicant-free water as controls. The test conditions were semi-static; the water was replenished daily in all the groups, and carbofuran was added only in the exposed group in order to maintain the concentration constant for 6 days. The fish were fed during the experiment. Fish from exposed, recovery and control were killed by severe blow to the head after 1, 3 and 6 days in each condition. The tissues examined were gill, kidney, liver and muscle; these were dissected out and frozen at 4°C and used with in an hour for the estimation of adenosine triphosphatase, glycogen and glycogen phosphorylase enzymes. Six individual observations of each enzyme or glycogen were made, and mean and standard error were calculated. Student’s t-test was used to compare the differences between control and exposure group and also between control and recovery group.
The enzyme ATPase (Adenosine triphosphatase, ATPase phosphorylase EC 3.6.1.3) was assayed by the method of Kaplay (1978), and glycogen by the method of Kemp and Kitsvan Heijinger (1954). Glycogen phosphorylase enzyme (a and ab) (1, 4 glucon orthophosphate glucosyltransferase, EC 2.4.1.1) was estimated by the method of Cori et al. (1955). Proteins were estimated by the method of Lowry et al. (1951). For the assay of ATPase activity, 5% (W/V) homogenates of gill, kidney, liver and muscle were prepared in 0.25 M ice-cold sucrose solution containing 1 mM EDTA and 30 mM histidine (pH 7.4) in an ice-jacketed homogenizer with a motor-driven pestle. The homogenates were centrifuged in the cold centrifuge machine at 3000×g for 20 min to remove nuclei and cell debris. Clear cell-free extracts were used for the assay. The reaction mixture in a volume of 0.5 ml contained 0.1 ml homogenate, 3 mM ATP, 3 mM MgCl2, 140 mM NaCl, 14 mM KCL, 20 mM tris HCL buffer (pH 7.4) and 0.1 mM ouabain (to the tubes of Mg+2 ATPase only). The reaction mixture was incubated at 37°C for 15 min. After 15 min, the reaction was stopped by adding 1 ml of 10% TCA, and the liberated inorganic phosphate (Pi) was measured by the colorimetric method of Taussay and Shorr (1953). ATPase activity in the tubes without ouabain was taken as total ATPase (Na+-K+, Mg+2), Mg+2 ATPase activity was measured in the presence of 0.1 mM ouabain, a specific inhibitor of Na+-K+ ATPase (Matsumura and Patil 1969), and Na+-K+ ATPase activity was obtained by subtracting the Mg+2 ATPase from total ATPase. The results were expressed as micromole of Pi formed/mg protein/h. A detailed protocol for the assay of glycogen phosphorylase was given elsewhere (Begum 2004).
Results
During the exposure to a sublethal concentration of carbofuran, observations were made to detect the external signs of poisoning on the exposed fish. No mortality and visible symptoms by toxic reaction were observed in the fish exposed to 1/3 of LC50 concentration.
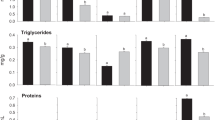
The results on the effect of carbofuran and recovery response on the adenosine triphosphatase, glycogen and glycogen phosphorylase activity in gill, kidney, liver and muscle tissues of C. batrachus are shown in Figs. 1, 2 and 3. Gill total ATPase activity induced slightly at the end of day 1 followed by significant inhibition till the end of the recovery period (Fig. 1). Total ATPase activity was decreased in kidney during exposure period, and there was a recovery when the fish were released into fresh water (Fig. 2). Liver tissue exhibited a pattern similar to that in gill, with a slight induction in total ATPase after 1 day of exposure followed by inhibition. Total ATPase activity was inhibited in muscle of C. batrachus during carbofuran exposure, and recovery was observed after 3 and 6 days of released into toxicant-free water (Fig. 3). Recovery in total ATPase activity was more in liver followed by kidney, gill and muscle. Gill and kidney Mg+2 ATPase activity was inhibited throughout the exposure period. In liver, Mg+2 ATPase activities was elevated on day 1 followed by inhibition after 3 and 6 days of exposure, and recovery was observed during recovery period (Fig. 3). There was a consistent and significant inhibition of Mg+2 ATPase in muscle tissue on all days of exposure as well as early recovery period, after which there was recovery response. The maximum impact of carbofuran on Mg+2 ATPase activity was observed in kidney followed by muscle, gill and liver. Of all the tissues investigated, liver Mg+2 ATPase recovered to maximum (difference between control and recovery group is −0.9%) followed by gill, kidney and muscle tissue. Na+-K+ ATPase activity in gill tissue showed varying response during exposure and recovery period. Na+-K+ ATPase activity was inhibited in kidney during treatment period and continues to inhibit in the early period of recovery. There was a consisted inhibition in the activity of Na+-K+ ATPase in liver tissue followed by substantial recovery. Na+-K+ adenosine triphosphatase enzyme activity was declined in muscle tissue on all days of exposure as well as early in the recovery period, after which it rebounded. The recovery response in Na+-K+ ATPase activity was in the order of liver > muscle > kidney > gill.
Glycogen content enhanced significantly in gill up to 3 days of exposure period followed by decrease (Fig. 1), recovery was observed after 1 day of transfer of fish into carbofuran-free water. Kidney glycogen content was declined up to 3 days of carbofuran treatment followed by enhancement. When the fish were transferred to clean water, the difference between the glycogen content in kidney of control and recovery fish is +2.5%. Glycogen phosphorylase a and ab activities in gill tissue showed varying responses during exposure period. When the fish were released into toxicant free water, there was a slight recovery in glycogen phosphorylase a and not much in ab activities. In kidneys, glycogen phosphorylase a activity was enhanced and reached to maximum at the end of treatment period (Fig. 2). The recovery response was very little in kidneys when compared to gill tissue. Kidney glycogen phosphorylase ab activity was induced by carbofuran treatment, and the induction was persisted after transfer of fish into carbofuran-free water.
Discussion
The results of the present study showed significant alterations in the activities of enzymes and time-bound organ-specific response to carbofuran stress. Carbofuran caused slight induction in liver and gill total ATPases at the end of day 1; thereafter, there was inhibition. Total ATPases in kidney and muscle tissues were inhibited on all sampling days. Branchial ATPases are intimately involved in osmoregulation, acid–base regulation and respiration of fish (Parvez et al. 2006). The slight induction in gill ATPase after day 1 could be due to the increase rate of respiration when the fish were under carbofuran stress. ATPases are a group of enzymes that play an important role in intracellular functions and are considered to be a sensitive indicator of toxicity (Yadwad et al. 1990). Reddy and Philip (1994) have reported that ATPases are intimately associated with synaptic transmission at the neuromuscular junction. Inhibition in the muscle ATPases in the present study could be due to imbalance in the level of synaptic transmission at neuromuscular junction under carbofuran stress as pointed out by Reddy and Philip (1994). When C. batrachus were released into clean water, the return in total ATPase activity only in liver tissue indicates its tissue-specific response to carbofuran intoxication.
Mg+2 ATPase is associated with the synthesis of ATP through oxidative phosphorylation in mitochondria (Begum and Vijayaraghavan 1994). The enzyme mediates an ATP-driven uptake of various extracellular solutes against an electrochemical gradient. Mg+2 ATPase activity in the hepatic tissue was induced significantly after day 1; thereafter, there was inhibition. The induction might be due to stimulation of anaerobic metabolism at the expense of aerobic processes and also to an enhanced transport of ions across the membranes to overcome the stress of carbofuran, facilitating the exchange of nutrients. Mg+2 ATPase is responsible for transepithelial regulation of Mg+ ions, which are essential to the integrity of the cellular membrane, intracellular cements and the stabilization of branchial permeability (Parvez et al. 2006). In the present study, the Mg+2 ATPase activity showed progressive inhibition in gill, kidney and muscle. The inhibition of Mg+2 ion-dependent ATPase (Mg+2 ATPase) in C. batrachus probably might have caused a blockage in the transport of ions across the membrane and reduced synthesis of ATP production. The decreased Mg+2 ATPase activities could also be attributed to the damage in mitochondrial membranes, which may interfere with the conversion of oxidative energy to phosphate bond energy. It is evident from the results that the carbofuran had a profound impact on the enzymes involved in energy synthesis and utilization processes and can in turn affect on the general metabolism of the exposed fish. When the fish were released into freshwater to investigate the recovery response, liver was the first organ to exhibit complete recovery as it is the primary organ for metabolism and excretion of carbofuran. Liver was followed by gill, kidney and muscle tissues.
Na+-Ka+ ATPase is found in the basolateral membrane of gill epithelial cells and is involved in the active electrolyte transport across the gills. The induction in the activity of Na+-Ka+ ATPase in gill tissue at the end of 1 and 3 days of carbofuran exposure could be due to enhance active transport of electrolyte across the branchial epithelial cells under the impact of carbofuran toxicity. Na+-Ka+ ATPase enzyme in the branchial tissue of carbofuran-exposed fish exhibited organ-specific response. Organ-specific responses are related to their anatomical location determining the exposure route, distribution and bioaccumulation of pollutants, as well as their defensive capacity (Ahmad et al. 2006). Environmental pollutants usually affect the Na+-Ka+ ATPase by decreasing its activity (Haya et al. 1983). Na+-Ka+ ATPase is an important component of active transport system, and it is a biochemical expression of active transport of Na+-Ka+ in the cells (Skou 1975). The inhibition in Na+-Ka+ ATPase activity in kidney, liver and muscle throughout the exposure is similar to decrease in Na+-Ka+ activity in fish tissues exposed to pesticide, reported by Begum (2009) and Sancho et al. (2003). Inhibition in the activity of Na+-Ka+ ATPase activity revealed that the transport of vital ions and nutrients that are essential to maintain the physiological requirements of the cells and organs is reduced. In freshwater fishes, Na+-Ka+ ATPase enzyme is involved in the regulation of blood and tissue osmolality (Sancho et al. 2003). The disturbed osmolality could have created an osmotic gradient in the cells, and the cells might tend to increase the water content as reported by Heath (1984). The inhibition in Na+-Ka+ ATPase observed during carbofuran intoxication could be due to the perturbation in regulation of blood and tissue osmolality as suggested by Sancho et al. (2003). When the fish C. batrachus were transferred into freshwater, the Na+-Ka+ ATPase in gill and kidney tissue were still inhibited significantly when compared to control fish. However, liver showed recovery near to the control value, and the difference between control and recovered value is 3.08%.
In the present study, glycogen content in gill tissue enhanced till day 3 followed by slight decrease at the end of day 6. A steady increase in glycogen may be associated with enhanced glycogenesis with active turnover of glycogen under the stress of carbofuran. The glycogen content of the kidney responds quite opposite to gill by exhibiting a decrease up to 3 days followed by significant increase after 6 days. This shows the tissue-specific response of the fish Clarias batrachus to carbofuran toxicity. The increased glycogen content in gill recovers to a greater extent, whereas kidney glycogen was near to control after cessation of carbofuran toxicity. An elevation in the activity of glycogen phosphorylase a and ab in kidney on all days of carbofuran treatment was observed. The increase in the phosphorylase a form might be due to the rapid conversion of inactive b form into active a form, thereby quantitatively elevating phosphorylase a to compact the stress of carbofuran insecticide. Author has reported induction in phosphorylase a in liver and muscle tissues of same fish exposed to carbofuran (Begum 2004). The exact mechanism involved in the induction of glycogen phosphorylase ab in gill and muscle is not clear, and further studies are required to elucidate the importance of increased phosphorylase ab in the carbofuran-induced fish. Release of 6-day carbofuran-intoxicated fish into freshwater exhibited no recovery response in both phosphorylases.
Conclusion
Acute exposure of the fish Clarias batrachus to carbofuran caused significant perturbation in adenosine triphosphatase enzymes viz; Mg+2, Na+-K+ and total ATPase in physiological important tissues such as gill, kidney, liver and muscle. Glycogen and glycogen phosphorylase (a and ab) enzymes are also affected in gill and kidney tissues. Some tissues exhibited similar response for some parameters, and some organ-specific responses are also noted in the present study. When C. batrachus were released into freshwater to study recovery pattern, Mg+2 ATPase recovered almost to control level followed by total and Na+-K+ ATPase in hepatic tissue. Glycogen recovery was more in kidney than in gill; however, no recovery response was observed in the glycogen phosphorylase enzymes. The results of the present study furnish information about the adverse effects of a carbamate insecticide on food fish and how the fish respond after cessation of exposure, a way to treat the polluted fish that are cultured near the areas of insecticidal treatment.
References
Ahmad I, Maria VL, Oliveira M, Pacheco M, Santos MA (2006) Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to β-naphtho flavone. Mutat Res 608:16–28
Begum G (2001) Carbofuran toxicity on total lipids and free fatty acids in air breathing fish during exposure and cessation of exposure—in vivo. Environ Monit Asses 70:233–239
Begum G (2004) Carbofuran insecticide induced biochemical alterations in liver and muscle tissues of the fish Clarias batrachus (linn) and recovery response. Aquat Toxicol 66:83–92
Begum G (2008) Assessment of biochemical markers of carbofuran toxicity and recovery response in tissues of the freshwater teleost, Clarias batrachus (linn). Bull Environ Contam Toxicol 81:480–484
Begum G (2009) Enzyme as biomarkers of cypermethrin toxicity: response of Clarias batrachus tissue ATPase and glycogen phosphorylase as a function of exposure and recovery at sublethal level. Toxicol Mech Method 19:29–39
Begum G, Vijayaraghavan S (1994) In vivo inhibition of branchial Na+-K+, Mg+2 ATPase of Clarias batrachus exposed to sub-lethal concentration of dimethoate. Pollut Res 13:213–216
Bretaud S, Toutant JP, Sanglio P (2000) Effects of carbofuran, diuron, and nicosulfuraon on acetylcholinesterase activity in gold fish (Carassius auratus). Ecotoxicol Environ Saf 47:117–124
Chandra S, Ram RN, Singh IJ (2004) First ovarian maturity and recovery response in common carp after exposure to carbofuran. J Environ Biol 25(3):239–249
Cori GT, Illingworth B, Keller PJ (1955) Muscle phosphorylase. In: Colowick SP, Kalpan O (eds) Methods in enzymology, vol 1. Academic Press, New York, pp 200–205
Finney DJ (1964) Probit analysis. Cambridge University Press, Cambridge, p 20
Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87:245–254
Haya K, Waiwood BA, Johnston DW (1983) Adenylate energy change and ATPase activity of Lobster (Homarus americanus) during sublethal exposure to zinc. Aquat Toxicol 3:115–126
Heath AG (1984) Changes in tissue adenylates and water content of bluegill, Leomis macrochirus, exposed to copper. J Fish Biol 24:299–309
Kaplay SS (1978) Erythrocyte membrane Na+-K+ activated ATPase in protein–caloric malnutrition. Am J Clin Nutr 31:579–584
Kemp A, Kitsvan Heijinger AJM (1954) A colorimetric micro method for the determination of glucose and glycogen. Biochem J 56:646–648
Li M (1975) Pollution in nation’s estuaries: origination from the agricultural use of pesticides. In: Estuarine pollution control and assessment. Proceedings of a conference, office of water planning and standards, Washington, DC, pp 451–466
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-Phenol reagent. J Biol Chem 193:265–275
Matsumura F, Patil KC (1969) Adenosine triphosphate sensitive to DDT in synapses of rat brain. Science 166:121–122
Moreno DH, Rodríguez FS, Santiyán M, López PM (2008) Hepatic monooxygenase (CYP1A and CYP3A) and UDPGT enzymatic activities as biomarkers for long-term carbofuran exposure in tench (Tinca tinca L.). J Environ Sci Health Part B 43:395–404
Munster CL, Skaggs RW, Pemmireddy VR (1996) Effect of water table management on the fate of the pesticide aldicarb. ASAE 39:55–66
Organisation of Economic Co-operation and Development (1992) Guidelines for the testing of chemicals, 203: fish, acute toxicity test. OECD, Paris
Osten RJ, Arana OA, Guilhermino L, Soares AMVM (2005) In vivo evaluation of three biomarkers in the mosquito fish (Gambusia yaucatana) exposed to pesticides. Chemosphere 58:627–636
Parvez S, Sayeed I, Raisuddin S (2006) Decreased gill ATPase activities in the freshwater fish Channa punctata (Bloch) exposed to a diluted paper mill effluent. Ecotoxicol Environ Saf 65:2–66
Perry SF (1997) The chloride cell: structure and function in the gills of freshwater fishes. Ann Rev Physiol 59:325–347
Pineiro-Carrero VM, Pineiro EO (2004) Liver. Pediatrics 113(4):1097–1106
Reddy PM, Philip GH (1994) In vivo inhibition of AChE and ATPase activities in the tissues of freshwater fish, Cyprinus carpio exposed to technical grade cypermethrin. Bull Environ Contam Toxicol 52:619–626
Ridgway RL, Finney JC, McGregor NJ (1978) Pesticide use in agriculture. Environ Health Perspect 27:103–112
Sancho E, Fernandez-Vega C, Ferrando MD, Andreu-Moliner E (2003) Eel ATPase activity as biomarker of thiobencarb exposure. Ecotoxicol Environ Saf 56:434–441
Senger RM, Rico PE, Arizi BM, Rosemberg BD, Dias DR, Bogo RM, Bonan DC (2005) Carbofuran and malathion inhibit nucleotide hydrolysis in zebrafish (Danio rerio) brain membranes. Toxicology 212(2–3):107–115
Skou JC (1975) The Na+-Ka+ activated enzyme system and its relationship to transport sodium and potassium. Rev Biophys 7:401–434
Stagg RM, Rusin J, Brown F (1992) Na+-K+ ATPase activity in the gills of the flounder (Platichthys flesus) in a relation to mercury contamination in the Firth of Forth. Mar Environ Res 33:255–266
Taussay HH, Shorr E (1953) A micro colorimetric method for the determination of inorganic phosphate. J Biol Chem 202:675–685
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Yadwad VB, Kallapur VL, Basalingappa S (1990) Inhibition of gill Na+-K+ ATPase activity in dragon fly larva Pantala flavesens, by endosulfan. Bull Environ Contam Toxicol 44:585–589
Acknowledgments
The author is grateful to the Council of Scientific and Industrial Research for the award of fellowship during Research Associateship. She also thanks Professor Shantha Vijayaraghavan (Rtd) for constant guidance and help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Begum, G. Organ-specific ATPase and phosphorylase enzyme activities in a food fish exposed to a carbamate insecticide and recovery response. Fish Physiol Biochem 37, 61–69 (2011). https://doi.org/10.1007/s10695-010-9417-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-010-9417-4