Abstract
This 3 × 3 factorial study assessed pyrethroids, carbamates and neonicotenoids groups of pesticides in replicated samples of three fish species from low (S1, reference), medium (S2) and heavy (S3) polluted sites receiving agricultural run-offs around the Indus River. Water and sediment samples from the same sites were also analysed for these pesticides by using high-performance liquid chromatography. Out of nine investigated pesticides, only three pesticides (deltamethrin, carbofuran and cypermethrin) were detected in fish and sediment samples. Deltamethrin in Cyprinus carpio ranged from 0.490 to 0.839 μg/g, mostly exceeding 0.5 μg/g as the maximum residual limit suggested by FAO-WHO, whereas it ranged from 0.214 to 0.318 μg/g in the sampled sediments. The carbofuran concentrations were 0.0425–0.066 and 0.613–0.946 μg/g in Labeo rohita and Channa marulius muscles respectively and 0.069–0.081 μg/g in the corresponding sediment samples. These values were either higher or lower than the maximum limit (0.1 μg/g) as suggested by FAO-WHO. Conversely, the cypermethrin concentration ranged from 0.141 to 0.174 in Ch. marulius and 0.183–0.197 μg/g in sediments which were both below the FAO-WHO maximum limit of 2 μg/g. No pesticide residues were detected in water from these sampling sites. Most selected physicochemical variables were within the acceptable range of World Health Organization for the water quality for aquatic life. The detected pesticide contents were mostly higher in fish muscles from heavily polluted sites. This is worrying because these pesticides may pose health risks for the fish and people of the study area. However, a preliminary risk assessment indicated that the calculated daily intake of detected pesticides by people consuming fish from the Indus River was low and did not present an immediate risk to the fish-consuming people. This study may be used as a benchmark to determine the safety of fish meat in order to develop intervention strategies to maintain the water quality and to protect the health of fish and fish-consuming people.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution of aquatic systems is a very serious global problem. Various deleterious effects on aquatic organisms can occur when agricultural, industrial and commercial chemicals discharge into the aquatic environment. Fish are also known to accumulate these pollutants directly from the polluted water and indirectly from the food chain (Mohamed 2009; Chaudhry and Jabeen 2011). While pesticides are needed to control weeds and pests in agricultural farming, the residual pesticides can emigrate from treated fields to the air and water and thus can adversely affect the relevant organisms (Estevez et al. 2008). This is particularly true for aquatic environments where if pesticides are absorbed by the aquatic organisms such as fish, it can cause damage to the fish health and the eating quality of its meat for human beings (Anampiu 2011). These pesticides have the ability to survive for long periods of time in the environment after application, as these are not easily biodegradable (Richterova and Svobodova 2012).
Recently, organophosphates are replaced by pyrethroid pesticides such as bifenthrin, cypermethrin, cyfluthrin, deltamethrin and lambda cyhalothrin (Amweg et al. 2006). These are commonly used in landscape maintenance, structural pest control and residential gardens and homes. These pyrethroids are more toxic to mammals and birds at low than the high temperature. In fact, these chemicals are over 100 times more toxic for fish due to not only their high sensitivity to toxic agents via gills but also insufficient hydrolytic enzymes for pyrethroids in fish (Aydin et al. 2005). These pesticides are metabolized to sulphates and glucuronides after distribution to the kidney, bile, liver and blood cells where significant adverse effects can cause multiple damage to fish meat quality and even the survival of these fish (Richterova and Svobodova 2012; Gautam and Gupta 2008; Yang et al. 2014).
Fish components can be used for environmental monitoring because they can accumulate the contaminants directly from diet and water (Chaudhry and Jabeen 2011; Kafilzadeh et al. 2012). Therefore, this study aimed to involve replicated samples of three freshwater fish species namely Labeo rohita (rohu), Channa marulius (giant snakehead or soal) and Cyprinus carpio (common carp) to monitor the extent and level of the presence of different pesticides in fish specimens from different sites of the Indus River in Mianwali District of Pakistan for potential health risks to the consumers.
L. rohita is a very popular and tasty fish in Pakistan, India, Thailand and Bangladesh. Commonly, it is known as Dumbra or Rohu and it belongs to the major carp family. In adult stage, it mostly depends upon aquatic vegetation being found in freshwater lakes and rivers in South-East Asia (Chaudhry and Jabeen 2011; Rasool et al. 2013). Ch. marulius or giant snakehead fish has high market value due to its high growth and consumption rate (Khan et al. 2012). It is commonly found in standing waters of lakes, canals, swamps and streams. It is strictly carnivorous as it feeds on earthworms, shrimps, tadpoles, fish and other aquatic organisms (Riaz 2008). Among the cultured species, C. carpio is important due to its easy maintenance, rapid growth, taste and omnivorous nature. It is commonly found in ponds, natural lakes and slow flowing deep rivers having abundant vegetation and detritus bottoms (Gul et al. 2010).
The main aim of this study was to investigate the pesticide residues in the above-mentioned fish species which are commonly consumed in the study area. Such determination of the chemical quality of freshwater fish is needed to avoid the possible risks to their meat quality and ultimately human health. For this reason, we examined the presence or absence of some commonly used and newly introduced pesticides in replicated samples of muscles from L. rohita, Ch. marulius and C. carpio representing different trophic levels alongside samples of sediments and water of the Indus River. The studied pesticides included pyrethroids (cypermethrin, lambda cyhalothrin and deltamethrin), carbamates (carbofuran) and neonicotenoids (imidacloprid, acetamiprid, thiacloprid, thiamethoxam, 6-choloronicotinic acid).
This study may be helpful in determining the extent of accumulation of these compounds in the aquatic biota to help understand the behaviour and fate of these chemicals in fish specimens (Kafilzadeh et al. 2012). This study, therefore, strives to provide crucial information on the levels of newly introduced pyrethroid, carbamate and neonicotinoide residues in fish, water and sediments for the first time from this study area around Indus River. The information about pesticide residues should facilitate the scientific assessment of the impact of pesticides on fish composition, public health, agriculture and the environment in this area and beyond.
Materials and methods
Study area and its significance
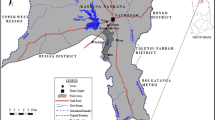
The study was conducted in the Mianwali District of Punjab, Pakistan, which is located along the bank of the Indus River. Mianwali is one of the northwestern cities in the Punjab Province of Pakistan with an area of 5840 km2. The city is located at 32.58° latitude and 71.55° longitude with an altitude of 209 m. This district is a rich source of minerals, coal, gypsum, clay, rocks, limestone and salts, which are commercially excavated as previously reported by Jabeen and Chaudhry (2010a, b). The inland fisheries in Pakistan are greatly dependent upon the Indus River, and it is one of the world’s largest rivers for its area of drainage basin (970,000 km2), sediment and discharge load because it flows from the northern mountains to the southern plains before it falls alongside other rivers into the Indian Ocean in the Sindh Province of Pakistan (Jabeen and Chaudhry 2010a, b).
Site selection
The study covered three sites around the Indus River where Kukranwala (KW = S1) was designated as the reference or low polluted site because of relatively less human activities in its surrounding area. The other two sites called Rokhri (RK = S2) and Ballo Khel (BK = S3) were designated as medium and heavy polluted sites, respectively, because these sites were receiving different types and amounts of pollutants from adjacent areas due to agricultural practices in the neighbourhood. However, S3 is more famous for the production of vegetables where more insecticides are used to control pests. These sites were 45 km apart from each other and used as commercial fish sale points. The neighbouring farmers are regularly using newly introduced pesticides like pyrethroids, carbamates and neonicotenoids on cash crops (wheat, oat, barley and legumes) and fruits plus vegetables to control insects and other pests in order to get better crop production in the study area. Therefore, it was logical to determine the presence of pyrethroids (cypermethrin, lambda cyhalothrin, deltamethrin), carbamates (carbofuran) and neonicotenoids (imidacloprid, acetamiprid, thiacloprid, thiamethoxam and 6-choloronicotinic acid) in selected fish species alongside samples of river water and sediments.
Experimental design, fish sampling and morphometric observations
This study involved three fish species of different trophic levels namely C. carpio as an omnivorous, L. rohita as mostly a herbivorous and Ch. marulius as a carnivorous species. A 3 × 3 factorial arrangement was used to collect replicated samples of three fish species from three predetermined sampling sites. The fish specimens were collected concomitantly with the samples of water and sediments. The fishing was performed by using conventional gill nets in synchronization with the local fishermen as previously described by Jabeen and Chaudhry (2010a, b). The total fish catches were harvested from three nets per site. Nine fish samples of each fish species of around 2 kg from each site were collected by using three fish samples per species from each net before their transfer to large buckets filled with the river water. The selected fish weights represented those that the fish consumers commonly used in the study area. The fish were immediately sacrificed under the local ethical guidelines before their morphometric measurements were performed on the spot. The selected fish samples were transported to the research laboratory of the GC University Faisalabad (GCUF) where each fish was dissected and muscles from mid dorsal side were stored at −20 °C until their use. The analysis of fish muscles for pesticide residues was carried out through high-performance liquid chromatography (HPLC) by using the research facility of the Nuclear Institute of Agriculture and Biology Faisalabad, Pakistan.
Sampling of river sediments
Replicated samples of river sediments were collected from the same three selected sites of the Indus River with the help of a boat by using a stainless steel Wildco hand core sampler (Thermo Fisher Ltd, UK) via self-gravity diving and suction. The samples were sieved to remove gravel, shells and other particles that were larger than 2 mm. The sieved sub-samples from each site were pooled, homogenized and transported in glass containers with Teflon lids to the research laboratory of GCUF at 4 °C. The sediment samples were air dried and frozen at −20 °C until their analysis. All the apparatus used for sample collection, transport and preparation were free from all types of pesticides.
Sample collection and physicochemical analysis of river water
Representative samples of about 1-l water were collected in glass bottles that were washed with distilled water and then with the river water from the sampling sites. The water samples were collected in September 2012 at midday from nine locations as three replicates from selected sites at around 30-cm depth. Physicochemical analysis of river water including dissolved oxygen, pH, temperature, conductivity, total dissolved solids, salinity and oxidative reductive potential (ORP) was carried out by using HI 9828 multi-parameter instrument (Hanna Instruments, USA).
Analysis of pesticide residues by HPLC
Chemicals and equipment
Ethyl acetate, cyclohexane, acetone, acetonitrile, methanol, n-hexane and sodium sulphate anhydrous were purchased from Bio-Rad, UK. All the solvents were glass redistilled by fractional distillation apparatus before use.
The following equipment were used in various analytical procedures: Topload (0.1–3000 g) and analytical balances (Sartorius, Germany), water distillation unit (Bibby England), fume hood (Fisher scientific Co., USA), micropipette 200 μL–1 mL (Oxford, Ireland), fractional distillation apparatus (Gellenkamp, UK), rotary evaporator (Buchi, model 011, Switzerland), sonication apparatus (Ogawa Seiki Co., Japan), gel permeation chromatography (Type: KL-SX-3, Radamed, Hungary) system. Shimadzu HPLC system comprising LC pump, 10 AS, system controller unit SCL-10A, column oven CTO-10A, UV-Vis detector SPD-10A, communication bus module CBM-101, column supelcosil TM LC-18, 25 × 0.45 cm, 5 μm and acquisition software class LC-10A was used for the analysis.
Extraction procedure for sediments
About 50-g dried sample was mixed with 2.5 g NaCl, 10 g sodium sulphate anhydrous and 70 mL ethyl acetate in a conical flask at about 60 rpm for 1 h. The solvent layer was collected, centrifuged at 2500 rpm for 5 min, and the supernatant was dried on a heating block under nitrogen stream through an air nitrogen generator (ANG 2381HC, CryoService, UK). Each dried sample was dissolved in 0.5 mL acetonitrile before the HPLC analysis was carried out.
Extraction of pesticide residues from water
About 500 mL of water sample was filtered through Whatman 1 filter paper. The filtered samples were passed through a preconditioned solid phase extraction (SPE) cartridge (Lichrolute C18 silica cartridge, Merck) at a flow rate of 1 mL/min under pressure of 1.33 kPa. The cartridge was dried under vacuum of 3.99 kPa for 1 h to complete the removal of water from adsorbent. The pesticide residues were eluted with a methanol/acetonitrile/ethyl acetate mixture, and the cumulated volume was reduced to dryness on a heating block under a nitrogen stream. Each dried sample was dissolved in 0.5 mL acetonitrile before the HPLC analysis was carried out.
Extraction of pesticide residues from fish meat
The frozen samples were thawed, and then 50 g of each of the four replicates of each fish muscles was mixed in a Pyrex extraction flask with 70 mL ethyl acetate, 2.5 g NaCl and 1 g sodium sulphate anhydrous at 50 rpm and 37 °C for 1 h. The extracts were then filtered in individual conical flasks before their evaporation using rotary evaporation apparatus. The concentrated samples were then dried in a heating block under nitrogen stream as described earlier. Each dried residue was dissolved in 0.5 mL acetonitrile before analysis by an HPLC.
Clean-up of extract
Gel permeation chromatography (GPC), which is a common and convenient technique for post-extraction sample clean-up prior to analysis by HPLC, was used for the clean-up of sediments and fish tissue extracts. GPC clean-up performs selective removal of high-molecular weight components such as lipids, pigments, proteins, humic acids and plasticizers while maintaining sample integrity.
The GPC was operated with constant nitrogen pressure (50 kPa) where the reservoir was filled with 1000 mL of ethyl acetate + cyclohexane mixture (1:1). About 0.5 mL of sample extract was mixed in 0.5 mL ethyl acetate + cyclohexane (1:1) and 500 μL of above mixture (sample extract + ethyl acetate + cyclohexane) was injected into the GPC column. About 12-mL elute for oil was collected first and then 10-mL fraction containing pesticides was collected in separate containers. This process was repeated twice for each sample. Each pesticide fraction was evaporated under nitrogen stream to its dryness.
High-performance liquid chromatography
The standards and samples were analysed for pesticide residues by using the reverse phase HPLC having UV-Visible detector. Identification of pesticides was carried out by comparing sample peak relative retention times with those obtained for standards from Dr. Ehrenstorfer (Augsburg, Germany). The area of sample peaks was compared with those of the standards to quantify the presence of different pesticides. All the glassware was washed with detergent, rinsed with purified water and heated at 180 °C for 2 h before their use. We tested different wavelengths falling between 200 to 500 nm for the optimum identification of pesticides in different samples of this study. However, the wavelengths of 260 and 232 nm were observed to be the optimum alongside other sets of conditions for detecting different groups of pesticides. These conditions are presented in Table 1.
Risk assessment
To assess the risk of pesticide intake through fish meat consumption by the local population, it was assumed that one adult person would eat 100 g of fish per day. By multiplying the fish intake with the pesticide concentrations in micrograms per gram of muscle yielded the estimated daily intake per person for each pesticide in micrograms. Then, daily intake was normalized for an average human weighing 60 kg (WHO 1997) to give intake values in micrograms per kilogram of body weight. To compare pesticide intake with acceptable daily intakes (ADI) mentioned by WHO (2004), daily intakes of different pesticides are summed to calculate a toxic unit. This was estimated by dividing the sum of the intake per pesticide by its corresponding ADI. The ADI values as defined by the World Health Organization (WHO 2004) are 100, 2 and 15 μg/kg body weight/day for deltamethrin, carbofuran and cypermethrin, respectively. The toxic unit was calculated to estimate the associated risk of consuming fish containing a mixture of different pesticides. When the sum of the toxic units approached or exceeded a value of 1.0, it was used as an indicator of a health risk for the local population.
Statistical analysis
The data were statistically analysed by using Minitab16 software to test the main effects of sampling sites (site), fish species (species) and site × species on each of the studied parameters of fish muscles, river water and sediments. These effects were declared significant if P < 0.05 and highly significant if P < 0.01. Tukey’s test was used if there were more than two means to compare for a significant difference at P < 0.05.
Results
Physicochemical parameters
The mean values of physicochemical parameters of river water from selected sites are shown in Table 2. The dissolved oxygen in water from three sites (S1, S2 and S3) was recorded as 8.14 ± 0.33, 6.77 ± 0.25 and 5.21 ± 0.36 ppm, respectively. The pH at these sites (S1, S2 and S3) was observed as 8.88 ± 0.33, 8.37 ± 0.55 and 8.29 ± 0.16, respectively. The mean temperatures at sites S1, S2 and S3 were recorded as 20.39 ± 0.70, 19 ± 0.67 and 20.06 ± 1.24 °C, respectively. Electrical conductivity at these sites (S1, S2 and S3) was recorded as 226 ± 21.52, 315.3 ± 23.76 and 452.7 ± 22.53 μS/cm, respectively. Total dissolved solids at three sites (S1, S2 and S3) were recorded as 113.7 ± 12.06, 182 ± 15.38 and 285.3 ± 8.02 ppm, respectively, whereas salinity was 0.11 ± 0.02, 0.18 ± 0.03 and 0.19 ± 0.07, respectively, and ORP was 131.7 ± 2.76, 155.8 ± 7.83, and 193.5 ± 12.32, respectively, at these sites. Most of the physicochemical parameters differed significantly (P < 0.05) among selected sites except temperature which did not differ significantly (P > 0.05).
Pesticide residue analysis
Table 3 presents the mean peak areas, quantity, retention time and response factors for the nine pesticide standards that were run for deltamethrin, lambda-cyhalothrin, cypermethrin, imidacloprid, acetamiprid, thiacloprid, thiamethoxam, 6-choloronicotinic acid and carbofuran through HPLC.
To calculate the concentration of each pesticide in each tested sample, the following formula was applied:
Deltamethrin
The presence of deltamethrin was detected in muscles of C. carpio and Ch. marulius and river sediments from only the polluted (S2 and S3) but not the reference site (S1), whereas it was not detected (ND) in L. rohita and any of the water samples (Table 4). The mean deltamethrin concentrations at sites S2 and S3 were 0.490 and 0.839 μg/g wet weight (WW) in C. carpio whereas 0.051 and 0.283 μg/g WW in Ch. marulius. Mean concentrations of deltamethrin in river sediments at S2 and S3 were 0.214 and 0.318 μg/g WW, respectively. The concentration of deltamethrin was higher (P < 0.05) in fish tissues and the sediments from S3 than S2 as presented in Table 4.
Carbofuran
Table 5 shows that carbofuran was detected in muscles of L. rohita and Ch. marulius and river sediments from only the polluted (S2 and S3) but not the reference site (S1), whereas it was not detected in any of the water samples. Mean carbofuran concentration was higher at heavy (S3) than the moderately polluted (S2) site. Mean concentration of carbofuran in L. rohita at S2 and S3 was reported as 0.0425 and 0.066 μg/g WW, respectively. Conversely in Ch. marulius, it was detected as 0.613 and 0.946 μg/g WW from S2 and S3, respectively. Mean concentration of carbofuran in L. rohita and Ch. marulius was higher at S3 than S2 (P < 0.05). Mean concentration of carbofuran in river sediments was higher (P < 0.05) at S3 than S2, and it was observed as 0.082 and 0.069 μg/g WW at S3 and S2, respectively.
Cypermethrin
Mean concentration of cypermethrin in Ch. marulius at S2 and S3 was 0.141 and 0.174 μg/g WW, respectively, and it was higher (P < 0.05) at S3 than S2 (Table 6). Mean concentration of cypermethrin in river sediments at S2 and S3 was observed to be 0.183 and 0.197 μg/g WW, respectively. No cypermethrin was detected in fish muscles or sediments from S1 of the Indus River. The muscle samples of L. rohita and C. carpio and water did not show cypermethrin at any of the sites.
Risk assessment
To determine the potential risk of pesticide residues in fish for the human population around the Indus River, intake of three groups of pesticides (deltamethrin, carbofuran and cypermethrin) from fish consumption was estimated as shown in Table 7. For all fish species, the sum of toxic units (∑TU) was far lower than 1.0, thus indicating that the risk of pesticide intake for humans through fish consumption was relatively low.
Discussion
The Indus River in the study area is surrounded by agricultural lands. A large amount of fertilizers and pesticides are used by agricultural farmers which can enter the river through running waters and tributary canals. Also, garbage and untreated wastewaters are discharged into the river by neighbouring inhabitants. All these factors may lead to the contamination of the Indus River. Representative samples of three fish species (L. rohita, Ch. marulius and C. carpio), water and sediments were collected from three variably polluted sites (S1, S2 and S3) receiving agricultural run-offs from the surrounding areas. At S1 (low polluted reference site), no pesticide residues were detected in any fish, water and sediment samples. Conversely, pesticide residues were detected in fish and sediments sampled from S2 and S3 sites.
After extraction and the HPLC analysis, three pesticides representing pyrethroids and carbamates were detected in fish muscles and sediments from the selected sites of the Indus River. New pesticides are recently introduced for their general use in the environment after the ban on the chlorinated hydrocarbon pesticides. The pesticides containing pyrethroids are classified as “limited use pesticides”, by the Environmental Protection Agency, due to their toxicity to fish (Aydin et al. 2005; Saha and Kaviraj 2009). Pyrethroids are synthetic insecticides which present a wide activity range, fast action and efficiency on low dosage, low residual power and low toxicity to mammals, when compared to other insecticides (WHO 1990). Pyrethroids are largely used as terrestrial and aquatic pesticides; they act on the functioning of the nervous system, through ion channels on nerve cells, causing hyperactivity and subsequent lack of control of the normal functions. Pyrethroids are virtually non-toxic to mammals and birds but are highly toxic to fish and aquatic invertebrates. The main reason is that the metabolism and elimination of such compounds are significantly slower in fish than in mammals and birds (Yilmaz et al. 2004; Begum 2005; Yang et al. 2014).
Deltamethrin belongs to pyrethroid group of insecticides. In this study, deltamethrin was observed in C. carpio, Ch. marulius and sediments from S2 and S3 sites. In this study, deltamethrin was not detected in Indus River water, but other authors did report the presence of deltamethrin in water sampled from Ebro River Delta (Feo et al. 2010) where its concentration in sediments ranged from 0.214 to 0.318 μg/g. Higher concentration of pesticide residues in sediments as compared to water in this study is in agreement with the findings of Ezemonye (2005) and Upadhi and Wokoma (2012) who reported that sediment are known to act as a sink for pollutants and therefore have the tendency of accumulating pesticides. In this study, Deltamethrin residues ranged from 0.051 to 0.839 μg/g WW in fish muscles, and the concentration of deltamethrin in C. carpio at polluted site exceeded the maximum residual limit of 0.5 μg/g WW by FAO-WHO (2013). It is recognized that the consumption of fish contaminated with deltamethrin may pose health problems for the fish consumers. High concentration of pesticides in fish samples could be due to the fact that fish are mobile, so the fish may have been exposed to compounds in other parts of the hydrologic system and also due to the presence of fat content in fish tissues, which is in good agreement with the findings of Upadhi and Wokoma (2012).
Cypermethrin is a widely used pyrethroid synthetic insecticide, but it is highly toxic to aquatic invertebrates and fish populations. It is commonly used to control pests in cereals, cotton, fruits, vegetables and parasite and vectors in animals (Gautam and Gupta 2008). In this study, cypermethrin was detected in Ch. marulius and sediments from the S2 and S3 sites around Indus River. In Ch. marulius, it ranged from 0.141 to 0.174 μg/g, while in sediment samples, it ranged from 0.183 to 0.197 μg/g. Overall, the cypermethrin concentration ranged from 0.141 to 0.197 μg/g in fish and sediments sampled from the Indus River. Other authors also determined the presence of cypermethrin in water and soil sampled from Ebro River Delta (Feo et al. 2010) who reported the cypermethrin concentrations of 0.0083–0.072 μg/g in soil samples. Lao et al. (2010) determined pyrethroids in sediments from an urban estuary. Sediments contained high concentrations of pyrethroid that were 0.473 μg/g alongside cypermethrin that was present in abundant amounts. Vryzas et al. (2011) also analysed the high concentration of cypermethrin in three trophic levels comprising algae, fish and aquatic invertebrates. In this study, cypermethrin residual concentration was under the maximum residual limits of FAO-WHO (2013). Hence, the fish from that study area may be safe for human consumption due to the lower cypermethrin levels.
Carbofuran is a systemic insecticide, acaricide and nematicide among carbamates for its use worldwide. Due to its widespread use, it has been detected in surface, ground and rain waters. Carbofuran is extremely toxic for aquatic organisms such as fish (Ensibi et al. 2012). In our findings, carbofuran was detected in L. rohita, Ch. marulius and sediments sampled from the Indus River around Mianwali. Other authors also determined the presence of carbofuran in water samples collected from Pirgach Thana, Bangladesh (Chowdhury et al. 2012). Akhtar et al. (2012) also reported the presence of low concentrations of carbofuran in sediment samples from Ravi River. Carbofuran residues were also detected by Vryzas et al. (2011) in fish samples collected from North Eastern Greece. In this study, carbofuran concentration detected in Ch. marulius at polluted site exceeded the maximum residual limit of 100 μg/kg being suggested by the FAO-WHO (2013). So, this kind of fish contaminated with carbofuran may pose health problems for the fish-consuming people of this and other regions.
Neonicotinoids are a relatively new class of insecticides that share a common mode of action that affect the central nervous system of insects, resulting in paralysis and death. They include imidacloprid, acetamiprid, clothianidin, dinotefuran, nithiazine, thiacloprid and thiamethoxam. In this study, five neonicotinoids (imidacloprid, acetamiprid, thiacloprid, thiamethoxam and 6-choloronicotinic acid) were determined in fish muscles, water and sediments. Thankfully, none of these neonicotenoids were detected in any sample of fish, sediments and water. Miranda et al. (2011) determined the distribution and leaching potential of neonicotenoids which are registered for agricultural use in Brazil. Imidacloprid especially appeared to be high in the Brazilian samples. In this study, the absence of neonicotinoids in fish, river water and sediment samples showed that farmers around the study area perhaps have not used those yet in their farming activities.
The range of physicochemical variables investigated in this study was also compared with the WHO (2006) and EPA (2006) criteria for aquatic life. The mean values of electrical conductivity, total dissolved solids and dissolved oxygen remained within the permissible range of these parameters as described by WHO (2006) and EPA (2006). The proposed value of electrical conductivity by WHO (2006) and EPA (2006) is 1500 μS/cm as compared to 226–452.7 μS/cm that was observed in this study. The acceptable value of total dissolved solids by WHO (2006) and EPA (2006) is 1000 mg/L as compared to 113.7–285.3 mg/L being noted in this study. The proposed value of dissolved oxygen by WHO (2006) and EPA (2006) is >5 ppm, as compared to 5.21–8.17 ppm in this study. The mean pH values remained slightly higher at S1 (8.88) as compared to 6.5–8.5 proposed by WHO (2006) and EPA (2006), while at S2 and S3, it remained within the recommended range suitable for the aquatic life. Singh et al. (2010) compared some physicochemical parameters with the WHO standards for sustainable existence of life in a river system. Abowei (2012) also measured the dissolved oxygen, temperature, salinity and pH in the Nkoro River located in the Niger Delta area of Nigeria. In his findings, there were non-significant differences in the pH and salinity between different sites, while dissolved oxygen and temperature showed highly significant differences among the river sites which is in line with the results of this investigation.
In general, deltamethrin, cypermethrin and carbofuran were detected in fish samples and sediments but not in the river water. This may be because these pesticides were less soluble in water due to their hydrophobic nature that made their presence in water to be at very low level and so their precise determination was difficult. The adsorption of these compounds to sediments and other particulate matter is an important mechanism for their removal from the water column, and consequently, the sediment component of aquatic ecosystems can be the ultimate sink of these pesticides. However, these pesticides can accumulate in fish tissues due to their lipophilic nature when they are discharged into water bodies. The highest pesticide concentration at S3 may be due to the abundance of agricultural lands that were involved in vegetable production around this site which might have facilitated the entry of these pesticides via sharp slopes into this part of the river. However, less or no pesticide detection at S1 site may be because there were least agricultural or other human activities with less or no use of chemicals around that site with relatively clear waters. Ch. marulius, because of its carnivorous feeding habit, appeared to be more vulnerable to pesticide residue accumulation than the two other selected fish species. The order of pesticide accumulation in selected fish species was Ch. marulius > C. carpio > L. rohita. Although limited pesticide residues were detected in L. rohita of selected polluted sites of study area, their concentration was well below the maximum residual levels of WHO for food fish. This could be attributed to its feeding habit as a juvenile, or adult fish is a herbivorous column feeder, eating mainly phytoplankton and submerged vegetation. The higher level of deltamethrin in C. carpio could be due to its bottom feeding habit which may cause the consumption of sediments alongside its aquatic feed from the river bed.
As illustrated in Table 7, the daily fish consumption was estimated to be around 100 g per person which is higher than average per capita consumption of 2-kg fish per annum in Pakistan. However, it can be concluded that the total pesticide intake via fish consumption is rather low around the study area. Also, it appears from Table 7 that the toxic units for different pesticide were lower than 1. It may therefore be concluded that the human population consuming fish from the Indus River is not at risk due to the studied pesticide pollution of the fish. However, the detection of these compounds even at non-toxic levels has perhaps confirmed the perceived view that unregulated use of pesticides has affected the quality of Indus River water and consequently the health of fish and people of the study area. Therefore, further research is needed to investigate the potential presence of a wide range of multiple pesticides in freshwater systems and their ultimate risks to the fish-consuming human population living along the Indus River.
Conclusions
This study revealed that pesticide residue levels of deltamethrin and carbofuran in the fish samples were above the maximum residue limits at the polluted sites and this could be an important source of pesticide transfer to humans via fish consumption. However, a preliminary risk assessment indicated that the daily intake of detected pesticides by the fish-consuming people around the Indus River is low and does not present an immediate risk. While the pesticide use for pest control may be unavoidable, its unlimited use may have implications for the fish and its consumers. Therefore, regulatory and awareness means are needed to control the use and possible seepage of these pesticides into the freshwater systems. Such efforts may help us protect the quality of fish as a food for human beings of this and other regions for the foreseeable future.
References
Abowei, J. F. N. (2012). Salinity, dissolved oxygen, pH and surface water temperature conditions in Nkoro river, Niger Delta, Nigeria. Advance Journal of Food Science and Toxicology, 2, 36–40.
Akhtar, M., Mahboob, S., Sultana, S., & Sultana, T. (2012). Assessment of pesticide residues in sediments collected from river Ravi and its tributaries between its stretches from Shahdara to Balloki headworks, Pakistan. Journal of Biology, Agriculture and Healthcare, 2, 127–133.
Amweg, E. L., Weston, D. P., You, J., & Lydy, M. J. (2006). Pyrethroid insecticide and sediment toxicity in urban creeks from California and Tennessee. Environmental Science and Technology, 40, 1700–1706.
Anampiu, J. M. M. (2011). Organochlorine pesticides residues in fish and sediment from Lake Naivasha. MSc thesis, University of Nairobi.
Aydin, R., Koprucu, K., Dorucu, M., Koprucu, S. S., & Pala, M. (2005). Acute toxicity of synthetic pyrethroid cypermethrin on the common carp (Cyprinus carpio L.) embryos larvae. Aquaculture International, 13, 451–458.
Begum, G. (2005). In vivo biochemical changes in liver and gill of Clarias batrachus during cypermethrin exposure and following cessation of exposure. Pesticide Biochemistry and Physiology, 82, 185–196.
Chaudhry, A. S., & Jabeen, F. (2011). Assessing metal, protein and DNA profiles in Labeo rohita from the Indus river in Mianwali, Pakistan. Environmental Monitoring and Assessment, 174, 665–679.
Chowdhury, A. Z., Jahan, S. A., Islam, M. N., Moniruzzaman, M., Alam, K. M., Zaman, M. A., et al. (2012). Occurance of organophosphorus and carbamate pesticides residues in surface water sample from the Rangpur District of Bangladesh. Earth and Environmental Science, 89, 202–207.
Ensibi, C., Moreno, D. H., Santiyan, M. P. M., Yahya, M. N. D., Rodriguez, F. S., & Lopez, M. P. (2012). Effects of carbofuran and deltamethrin on acetylcholinesterase activity in brain and muscles of common carp. Environmental Toxicology. doi:10.1002/tox.21765.
EPA (United States Environmental Protection Agency) (2006). Current National Recommended Water quality criteria. http://www.epa.gov/waterscience/criteria/wqcriteria.html.
Estevez, M. A., Perigo, E. L., Carballo, E. M., Gandara, J. S., Mejuto, J. C., & Rio, L. G. (2008). The mobility and degradation of pesticides in soil and the pollution of groundwater resources. Agriculture, Ecosystems & Environment, 123, 247–260.
Ezemonye, L. I. N. (2005). Polychlorinated Biphenyls (PCBs) levels and distributions in Ethiope and Benin rivers of the Niger Delta, Nigeria: surface water and sediment. International Journal of Environmental Studies, 62, 491–504.
FAO-WHO (2013). Codex, maximum residue limits for pesticides. FAO. www.codexalimentarius.net/pestres/data/pesticides/index.htmL. Assessed 01 Sept 2014.
Feo, M. L., Ginebreda, A., Eljarrat, E., & Barcelo, D. (2010). Presence of pyrethroid pesticides in water and sediments of Ebro river Delta. Journal of Hydrology, 393, 156–162.
Gautam, P. P., & Gupta, A. K. (2008). Toxicity of cypermethrin to the juveniles of freshwater fish Poecilia reticulate (peters) in relation to selected environmental variables. Natural Product Radiance, 7, 314–319.
Gul, A., Yilmaz, M., Kuscu, A., & Benzer, S. (2010). Feeding properties of common carp living in Hirfanli Dam Lake. Kastamonu Education Journal, 18, 545–556.
Jabeen, F., & Chaudhry, A. S. (2010a). Environmental impacts of anthropogenic activities on the mineral uptake in Oreochromis mossambicus from the Indus river in Pakistan. Environmental Monitoring and Assessment, 166, 641–651.
Jabeen, F., & Chaudhry, A. S. (2010b). Monitoring trace metals in different tissues of Cyprinus carpio from the Indus river in Pakistan. Environmental Monitoring and Assessment, 170, 645–656.
Kafilzadeh, F., Shiva, A. H., Malekpour, R., & Azad, H. N. (2012). Determination of organochlorine pesticide residues in water, sediments and fish from Lake Parishan, Iran. World Journal of Fish and Marine Sciences, 4, 150–154.
Khan, M. A., Khan, S., & Mian, K. (2012). Length-weight relationship of giant snakehead, Channa marulius and stinging catfish, Heteropaneustes fossilis from river Ganga, India. Journal of Applied Ichthyology, 28, 154–155.
Lao, W., Tsukada, D., Greenstein, D. J., Bay, S. M., & Maruya, K. A. (2010). Analysis, occurrence and toxic potential of pyrethroids and fipronil in sediments from an urban Estuary. Environmental Toxicology and Chemistry, 29, 843–851.
Miranda, R. B. G., Raetano, G. C., Silva, E., Daem, A. M., & Cerejeira, M. J. (2011). Environmental fate of neonicotenoids and classification of their potential risks to hypogean, epygean, and surface water ecosystem in Brazil. Human and Ecological Risk Assessment, 17, 981–995.
Mohamed, A. S. F. (2009). Histopathological studies on Tillapia zilli and Solea vulgaris from Lake Qarun, Egypt. World Journal of Fish and Marine Sciences, 1, 29–39.
Rasool, F., Qureshi, N. A., Parveen, S., Siddique, M., Hameed, A., Ashraf, M., et al. (2013). Principal component analysis for the variation among the population of Labeo rohita. Journal of Animal and Plant Sciences, 23, 487–492.
Riaz, A. M. (2008). Studies on the feasibility of raising Channa marulius with Tilapia by using different predator-prey stocking ratios in fertilized ponds. PhD thesis, University of Agricultural Faisalabad.
Richterova, Z., & Svobodova, Z. (2012). Pyrethroid influence on fish. Slovenian Veterinary Research, 49, 63–72.
Saha, S., & Kaviraj, A. (2009). Effects of cypermethrin on some biochemical parameters and its amelioration through dietary supplementation of ascorbic acid in freshwater catfish Heteropneustes fossilis. Chemosphere, 74, 1254–1259.
Singh, M. R., Gupta, A., & Beeteswari, K. (2010). Physicochemical properties of water sampled from Manipur river System, India. Journal of Applied Sciences and Environmental Management, 14, 85–89.
Upadhi, F., & Wokoma, O. A. F. (2012). Examination of some pesticide residues in surface water, sediment and fish tissue of Elechi Creek, Niger Delta, Nigeria. Research Journal of Environmental and Earth Sciences, 4(11), 939–944.
Vryzas, Z., Alexoudis, C., Vassiliou, G., Galanis, K., & Mourkidou, E. P. (2011). Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in Northeastern Greece. Ecotoxicology and Environmental Safety, 74, 174–181.
WHO (World Health Organization). (1990). Environmental health criteria 97—deltamethrin (1990th ed.). Geneva: International Programme on Chemical Safety (IPCS), World Health Organization.
WHO (World Health Organization) (1997). Guidelines for predicting dietary intake of pesticide residues (revised). Prepared by the Global Environment Monitoring System–Food Contamination Monitoring and Assessment Programme (GEMS/Food) in collaboration with the Codex Committee on Pesticide Residues. Geneva: Programme of Food Safety and Food Aid.
WHO (World Health Organization) (2004). Chemical Fact Sheets of the International Programme on Chemical Safety (ICPS) of the World Health Organization (WHO), Geneva. www.who.int/entity/water_sanitation_health /dwq/en/gdwq3_12.pdf. Accessed 9 Mar 2014.
WHO (World Health Organization) (2006). Wastewater reuse in agriculture, volume 2. World Health Organization.
Yang, Y., Huihui, M., Zhou, J., Liu, J., & Liu, W. (2014). Joint toxicity of permethrin and cypermethrin at sublethal concentrations to the embryo-larval zebrafish. Chemosphere, 96, 146–154.
Yilmaz, M., Gul, A., & Erbasli, K. (2004). Acute toxicity of alpha-cypermethrin to guppy (Poecilia reticulata, Pallas, 1859). Chemosphere, 56, 38–385.
Acknowledgments
The authors gratefully acknowledge the Higher Education Commission of Pakistan and British Council for funding the INSPIRE project SP-174 (2011-2014). Muhammad Abdullah Khan Niazi and Kaleemullah of GHS PAF Mianwali Pakistan, Naveed, Shahnam, Shaheer, Ibtisam, Aliya and Aima are acknowledged for their help and cooperation during the fish sampling from the Indus River around Mianwali, Pakistan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jabeen, F., Chaudhry, A.S., Manzoor, S. et al. Examining pyrethroids, carbamates and neonicotenoids in fish, water and sediments from the Indus River for potential health risks. Environ Monit Assess 187, 29 (2015). https://doi.org/10.1007/s10661-015-4273-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4273-4