Abstract
European eel (Anguilla anguilla (L.)) stocks are in decline in most of their geographical distribution and their status is considered below safe biological limits. Recently, there is an increasing awareness that spawner quality might be an essential element in the decline of the species since pollution by bioaccumulating chemical substances may have a large impact on the reproduction success of the eel. This review gives an overview of the literature on the effects of contaminants on the European eel and on the consequences on the biology and fitness of the eel in order to document the role of pollution in its decline. A variety of contaminants have been found to affect the eel. These contaminants may cause disturbance of the immune system, the reproduction system, the nervous system and the endocrine system and effects were reported on several levels of biological organization, from subcellular, organ, individual up to even population level. More extensive research is needed in order to evaluate how pollutants are detrimental to eel populations. Getting a comprehensive overview of the quality (including contamination levels, biomarker responses, lipid content and condition) of the silver eel population all over Europe seems to be an essential and urgent objective for the European eel management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The stocks of the European eel have declined in most of their geographical distribution and they are considered below safe biological limits for population survival (Dekker 2002a). There is evidence that anthropogenic factors e.g. fisheries, pollution, habitat deterioration (such as migration obstruction) and transfer of parasites, diseases as well as natural processes e.g. climatic and oceanic changes and predation have contributed to this decline (ICES 2002). A growing awareness points to the spawner quality as an essential element in the decline of the species. The quality of the silver eels, that start their migration towards the reproduction sites in the Sargasso Sea, seems to be seriously impaired by pollution, diseases and parasites. Due to specific ecological and physiological traits, eels are particularly prone to bioaccumulation of (especially lipophilic) contaminants. In Belgium, the INBO Eel Pollutant Monitoring Network database (network covering Flanders, northern part of Belgium, http://visapp.milieuinfo.be/pages/welcome.do), demonstrates a considerable variety in distribution and levels of contamination in eel, with, at specific sampling sites, extremely high values of specific substances (Goemans et al. 2003; Belpaire and Goemans 2007; Maes et al. 2008). Some of these contaminants accumulate in the fat tissue of eels during their feeding stage (yellow eels), even to levels that make them unsuited for consumption (Harrad and Smith 1999; Bilau et al. 2007). On a European scale the collation of data on the contamination of the European eel within several other countries allow similar conclusions (ICES 2007, 2008).
Because of their toxicity and persistence in the environment, many substances have been banned in Europe in the 1970s. Yet, more than 30 years later, e.g. residues of DDT (dichlorodiphenyltrichloroethane) and other organochlorine pesticides continue to be detected in air, rain, soil, surface water, river bed sediment, and aquatic as well as terrestrial biota. Moreover, research suggests that low levels of some contaminants have the potential to affect the development, reproduction, and behaviour of fish and wildlife, and possibly of humans as well (Nowell et al. 1999). Adverse impacts of contaminants have been described in several fish species in many fresh water habitats and the effects of contaminants on fish and fisheries are relatively well studied (for reviews see e.g. Jobling et al. 1998; Nowell et al. 1999; Jobling et al. 2002a, b, 2006; Lawrence and Elliott 2003; Di Giulio and Hinton 2008). Pollution impacts include effects on a variety of levels of biological organisation, from the subcellular and molecular level, through organism to population and community levels and subsequently to socio-economic consequences. Impacts are highly determined by the type of contaminant, and eventually by synergetic processes associated through the combination of a mixture of chemicals. The type of response will also depend on the developmental stage of the fish and will be influenced by other environmental factors (e.g. temperature, salinity, oxygen, pH; Lawrence and Elliott 2003).
Also in eel a variety of effects have been demonstrated during recent years. Many specific responses to elevated contaminant pressure have been described on subcellular level. Even under natural circumstances chemicals affect vital organs, such as liver. But even more concerning are reports of effects on lipid metabolism, genetic diversity and detrimental effects on reproduction and viability of offspring. Therefore it is important to obtain insight in the harmful effects of contaminants. Within the eel, reviews of the possible effects of various contaminants on the reproductive biology and physiology, have been elaborated before, e.g. Bruslé (1991), Knights (1997), and Robinet and Feunteun (2002). Bruslé (1991) gave an overview of eel contaminated with organic pollutants; Knights (1997) and Robinet and Feunteun (2002) on the possible toxic effects on eel.
Quality issues on the eel benefit of increasing attention in the framework of the international conservation measures for restoring eel stocks. ‘Quality’ is considered as the ‘quality of spawners’ describing the capacity of silver eels to reach spawning areas and to produce viable offspring (ICES 2006). The Joint EIFAC/ICES Working Group on Eel (ICES 2006) recommended to identify areas producing high quality spawners with low contaminant burdens in order to maximize protection for these areas. Attention should be paid to pollution monitoring within the Eel Management Plans and within the evaluation of the chemical status under implementation of the Water Framework Directive. In 2007, a European Eel Quality Database (EEQD) was set up to collate information on contamination in eels over Europe (ICES 2007, 2008). ICES (2007) recommended to develop and maintain this database and to initiate harmonized monitoring strategies for eel within member countries. As a consequence of the increased international concern about the decline of the stocks, recent research has paid increasing attention to measure contaminants in the eel and to investigate the effects of these substances in the eel. As a result a large and growing quantity of information became available. The objective of this paper is to review these new insights on the effects of pollutant contamination in the European eel and to document the role of pollution in the decline of the species. It should further underpin eel management and restoration plans.
Evidence for exposure and contamination
Eels accumulate lipophilic xenobiotics in the fat. They often reside in contaminated sediments accumulating high levels of lipophilic compounds through gills, skin and contaminated food. Eel is semelparous, carnivorous in its yellow stage, benthic, and often burrowed in the sediment. Yellow eel seems quite resistant, surviving in poor water conditions, and often living in habitats polluted by diverse contaminants. In the yellow stage, eels are sub-adults, living in freshwater conditions in which they do not reproduce. Therefore, body burdens are not seasonally affected by a reproduction cycle neither by associated changes in lipid metabolism. Unlike iteroparous species, there is no loss of contaminants, specifically associated with annual reproductive processes (fat metabolisation and production and release of gametes). They can stay for a prolonged period in freshwater (on average 5.9 years for males and 8.7 years for females; Vollestad 1992), during which they continuously bioaccumulate xenobiotics. Concentration levels generally increase with age, reaching a maximum just prior to silvering and emigration. van Ginneken et al. (2009) report fat percentages in silver eel reaching up to 27–29% before the onset of migration, meaning that eels indeed accumulate lipophilic contaminants in large quantities. They generally show life-long accumulation and low depuration rates (Larsson et al. 1991; De Boer et al. 1994; Tulonen and Vuorinen 1996; Knights 1997; Daverat et al. 2006). In an 8-year study by De Boer et al. (1994) for example, it was demonstrated that elimination half-lives of PCBs are in the order of years. For the higher chlorinated PCBs no elimination was found at all.
Eel is used as a bioindicator of contaminants (De Boer and Hagel 1994; Belpaire and Goemans 2007; Belpaire et al. 2008) and many authors have reported (high) levels of a variety of xenobiotics in the eel. Belpaire and Goemans (2008) compiled an overview of recent reports describing bioaccumulation of various chemicals in eel within EC countries. The most studied chemicals in eels are polychlorinated biphenyls (PCBs), pesticides and heavy metals. However, also polycyclic aromatic hydrocarbons (PAHs), perfluoro-octanesulphonic acids (PFOS), brominated flame retardants (BFRs), dioxins and furans, and volatile organic compounds (VOCs) were reported. Persistently elevated contamination levels, above human consumption standards, are reported in many European countries. In 1990, Bruslé (1990) listed the concentration values and effects of heavy metals and in 1991 (Bruslé 1991) the list was completed with pesticide and (observed) PCB concentrations and biological effects on eel. He also listed experimental concentrations of PCBs, phenols and pesticides in different stages of the eel life cycle. In their review, Robinet and Feunteun (2002) tabled examples of reported mean concentrations or concentration ranges of some PCBs and pesticides in yellow European and American eel tissues. In 2008, Maes et al. (2008) reported concentrations of PCBs, organochlorine pesticides and heavy metals in Belgian eels, and compared them to bioaccumulation data from other countries. Since the increasing attention to eel quality and fitness parameters (within Eel Management Plans), the growing awareness for monitoring environmental contaminants in aquatic biota, and a more stringent food control regulated by recent new European legislation (such as for dioxin-like contaminants), the amount of information about the accumulation of contaminants in eel is increasing rapidly and warrants the compilation of this data in a comprehensive way. The most recent overview of data on contaminants, parasites and fat levels in European eel over its geographical distribution can be found in the European Eel Quality Database of the Joint EIFAC/ICES Working Group on Eel (unpublished results). This database compiles data from 14 European countries. Results show persistently elevated contamination levels above human consumption standards in many European countries and, demonstrate highly variable data within river basin districts, according to local anthropogenic pollution, linked with land use (ICES 2008). Especially the lipophilic contaminants (such as PCBs) may attain a very high level in eel. Figure 1 gives an overview of the mean PCB 180 (ng/g BW) data in European eel muscle for 11 countries and the mean Hg (ng/g BW) data for 7 countries as cited in a number of reports presented in the EEQD. This figure shows that in many European countries PCB levels in eel are at levels of concern. In general, PCB levels are considerably higher than in other fish species (Lindell et al. 2001; van Leeuwen et al. 2002; Maes et al. 2008). The situation is better for the heavy metal mercury where only in Spain concentrations exceed the European consumption limit (1000 ng/g BW). However, the number and choice of sampling sites in the countries is not always representative for the whole country. So results must be considered carefully. Considering the high levels of hazardous compounds accumulating in eel, it is reasonable to assume that toxic effects in the eel will be more obvious compared to other species. For a variety of chemical compounds reports have described the impact on the health of the eel. Most of them describe sublethal biological effects, however also pollution induced mortalities have been reported.
Mean concentration of PCB 180 (ng/g b.w.) in 11 countries (a) and mean concentration of Hg (ng/g b.w.) in 7 countries (b) in European eel muscle as reported recently (Belgium: INBO Eel Pollution Database http://visapp.milieuinfo.be/pages/welcome.do); Denmark: Erichsen et al. (2000); France: Durrieu et al. (2005), Tapie et al. (2006); Germany: Gaumert et al. (2001, 2002), Krinitz et al. (2002), Gaumert et al. (2003), Bergemann and Gaumert (2005); Ireland: Santillo et al. (2005); Italy: Orban et al. (2004), Mancini et al. (2005), Storelli et al. (2007); Norway: Knutzen et al. (1998, 1999, 2001); Portugal: Bordajandi et al. (2003), Santillo et al. (2005); Spain: Bordajandi et al. (2003), Usero et al. (2003), Santillo et al. (2005), Alcaide and Esteve 2007; The Netherlands: Pieters et al. (2005), Hoogenboom et al. (2007), Hoek-van Nieuwenhuizen and Kotterman (2007); United Kingdom: Foster (2005). The number of sites is indicated (N); The European consumption limit for Hg (1,000 ng/g b.w.) is indicated in b
Direct mortalities due to contaminants
Direct lethal effects of contaminants have been reported, both under field circumstances where fish kills due to some specific spills or accidents have been documented, and under laboratory conditions where the lethal effects of specific chemicals on the survival of the eel have been studied.
As an example of pesticide-driven events Nowell et al. (1999) mention the number of fish kills attributed to organochlorine and organophosphate insecticides, DDT, and pentachlorophenol. Fish kills on a local scale often result from inadvertent management of land users (e.g. spill) but severe fish kills often result from accidental discharge or leakage on industrial sites producing or processing pesticides or other chemical compounds. In 1986 for example, incidents with atrazine were reported at Ciba Geigi-Bazel, with pesticides at BASF-Ludwigshafen, with chlorobenzol at Hoechst-Frankfurt (in the River Main), with methanol at Bayer-Leverkusen, with desinfectants at Bayer-Krefeld-Uerdingen, and with ethylene glycol at BASF. Fire-fighting water used to extinguish a fire in an agrochemical warehouse of the Basel chemical company Sandoz, contaminated by a variety of pesticides entered in the Rhine on November 1, 1986. Approximately 6–22 tons of pesticides are estimated to have been discharged into the river (about 1–3% of the inventory). This caused extensive pollution of the river due to pesticides and insecticides, including mercury-based and zinc-based pesticides. Levels of mercury in the Dutch section of the river were reported to be 3 times the normal limits. As a consequence half a million eels (ca. 200 tonnes) were killed, and the eel population was affected for years up to 650 km downstream (Christou 2000). Following Balint et al. (1997) deltamethrin (the active ingredient of the insecticide K-OTHRIN 1 ULV) contributed to the severe eel devastation that occurred in Lake Balaton in 1991 and 1995, killing respectively 300 and 30 tons of eels. It seems that when eel kills occur, it is very hard to correlate these mortalities with precise chemical factors, because of the complexity of the pollution load (including a variety of contaminants which may interact) in many polluted areas (Anonymous 1987). In 2007, 25 ton of fish (among which numerous eels) were killed in the River Meuse due to the discharge of 64 kg chloropyriphos and 12 kg cypermethrin, two components of pesticides.
Specific toxicity studies under controlled conditions reported lethal concentrations of specific compounds on the European eel. Table 1 gives an overview of lethal toxicological studies from the primary scientific literature, including LC50 values. A more extensive overview of study results can be found in the PAN Pesticide Database (Kegley et al. 2008).
Sublethal biological effects
In most cases contaminants will not result in direct mortality, but will induce sublethal effects. Apart from the specific toxicity of the contaminants involved, the exposure time and concentration, effects are influenced by a variety of environmental factors for example, pH, oxygen concentration, temperature and salinity. The potential effects of a contaminant can also be reinforced synergetically if other contaminants or diseases and parasites are involved, putting additional pressure on the individual. Many reports are available mostly from laboratory experiments, describing the effects of pollution agents on a variety of biological functions. An overview of these studies, reporting on the effects of a variety of chemicals on the European eel is given in Table 2 and a selection of known responses are discussed below.
Histological and subcellular effects
Exposure to different chemicals may result in chronic effects which cause organ injuries and tissue damage. In eel, these effects have been described for PCBs, pesticides, heavy metals, and PAHs, phenols and PFOS. For example, Svobodova et al. (1994) described considerable histopathological changes in liver, spleen and kidney from fish as long term effects of exposure to PCBs both in nature and under laboratory conditions.
Pesticides are known for their ability to disrupt the structural integrity of fish gills and it may be assumed that as a result of the reduced efficiency of the damaged gills to respiration, the tissues receive less oxygen (Sancho et al. 1997). Pesticide induced hepatic megalocytosis, necrosis, cellular proliferation, aneurysms, disorganization of second lamellae, loss of hepatocyte limit etc. has been recently reported in other fish species (Dutta and Meijer 2003; Marty et al. 2003; Akaishi et al. 2004; Brown and Steinert 2004). Exposure to a pure PAH, phenanthrene, also denotes liver cell injury in a tropical fish (Oreochromis mossambicus; Shallaja and D’Silva 2003). Roche et al. (2002) indicated that tumours in liver and spleen of eels resulted from long-lasting exposure to a combination of potentially carcinogenic pollutants (such as PAH, lindane and dieldrin).
Cadmium effects in European eel were reported by Gony (1990), who describes structural or functional perturbations in the gills like swelling of the primary and secondary lamellae caused by epithelium hypertrophy and accumulation of secondary lamellae after 2 h exposure to 5 μg/L cadmium (Cd; Table 2). At the same moment, melanism appeared in the gill blood vessels. Dependent of the individual response on cadmium exposure also other injuries appeared like exfoliation of the epithelium and the collapse and merging of lamellae. Also liver tissue was influenced by cadmium exposure.
Copper (Cu) exerts a wide range of physiological effects in fishes, including increased metallothionein synthesis in hepatocytes, altered blood chemistry, and histopathology of gills and skin. Sublethal exposure suppresses resistance to viral and bacterial pathogens. Rate and extent of copper accumulation in fish tissues are extremely variable between species and are further modified by abiotic and biological variables (Eisler 1997).
Santos et al. (1990) reported skin disruption in adult eel due to bleached kraft pulp mill effluent (BKPME; Table 2) and Howard et al. (1971) reported a favourable fish adaptation capacity in their study with pulp mill effluents. DHAA (dihydroabietic acid), a resin acid, also caused structural changes in the gills impairing oxygen uptake by causing circulatory vasoconstriction and by increasing the diffusion distance (Tuurala and Soivio 1982; Pacheco and Santos 2002a). These histopathological alterations may have important adverse consequences on fish health, particularly due to the obstruction of oxygen diffusion across the gills and the impairment of the osmoregulatory function (Pacheco and Santos 2002a). Also glomerular injury was observed, impairing glomerular filtration, just as histological alterations in the renal tubules of BKPME-exposed eels (Santos et al. 1990).
In 1991, Bruslé (1991) reported that acute toxicity of phenols to eels is characterised first by an exciting phase, then ataxia, followed by death. Grauby et al. (1973) tested the toxicity of effluents from a refinery and from chemical plants and found that the high concentrations of phenols (17.5–19 mg/L) are responsible for fish kills. Due to a high fat content of muscle tissue, eel was one fish with a higher intake (49 ppm in the flesh) than other species. Neff (2009) on the other hand, reported that phenols are not very toxic in marine organisms but that toxicity seems to increase with higher taxonomical position. Phenol can accumulate in tissue lipids to concentrations that cause tissue disruption and several chlorinated phenols and nitrogen-substituted phenols are uncouplers of oxidative phosphorylation.
PFOS induced alterations in the liver have been demonstrated for eels under natural conditions in Belgium (Hoff et al. 2005).
Effects on blood and organs
It is known that as long as the contaminants are stored in the fat reserves, toxic effects are minor. But, at the start of the migration, when the lipids are oxidized and the PCBs released, PCB-levels in the blood plasma may increase up to toxic levels. van den Thillart et al. (2005) investigated the influence of PCBs on the physiology and gonad development of silver eel during a simulated migration through experiments in swimming tunnels. They reported a significant 1.5-fold higher weight loss in the PCB-loaded groups, which can not be ascribed to the refusal of food but may be the result of PCB effects on the intermediary metabolism. In the PCB-loaded groups hypoglycemia was observed. PCB exposure, in combination with the swimming protocol, is not stressful as none of the secondary indicators of a stress response (a rise of lactic acid, an increase of potassium and an increase of glucose) increased in PCB loaded and/or swim groups (van Ginneken et al. 2002). From other PCB-studies it can be concluded that PCB-exposure leads to a lowering of the adrenocortical function (van den Thillart et al. 2005, Table 2).
Sancho et al. (1997) investigated the sublethal effects of the organophosphate insecticide fenitrothion (o,o-dimethyl-o-3-methyl-4-nitrofenyl phosphorothioate) which is extensively used in agriculture for crop protection. A constant sublethal concentration of fenitrothion in the surrounding water for 96 h appeared to be physiologically stressful to the European eel. A consistent hyperglycemia was seen and a spectacular increase of lactate levels occurred in blood, liver and gill while protein levels decreased significantly (Table 2). Sancho et al. (1997) suggested that the development of such internal hypoxic conditions may be ultimately responsible for the shift to the less efficient anaerobic metabolism, indicated by the observed changes in lactic acid contents. These results agree with those from Ferrando and Andreu-Moliner (1991a), b) and Gimeno et al. (1994) for A. anguilla exposed to pesticides (Table 2). Sancho et al. (1997) also found that the fenitrothion-treated eels exhibited no significant change in liver glycogen levels after 5 days of exposure, but protein content decreased significantly and hepatomegaly was observed. Holmberg et al. (1972) found similar results in eel exposed to pentachlorophenol (PCP) for 8 days. They also found an increased hepatosomatic index which can be explained by an enlargement of the liver as a result of the pesticide action. The decrease in protein content of fenitrothion-intoxicated fish also indicates the physiological adaptability of the fish to compensate for pesticide stress. To overcome the stress the animals require high energy. This energy demand might have led to the stimulation of protein catabolism (Sancho et al. 1997) and might disturb fat metabolism (see below).
A 30-days exposure of eels to DHAA causes splenic hemosiderosis (a form of iron overload disorder resulting in the accumulation of hemosiderin) pointing to erythrocytic catabolism which may result in a decrease in the number of mature erythrocytes in the circulating blood (Hibiya 1992). DHAA exposure for 90 and 180 days revealed regression of the hemosiderosis, indicating erythocytic catabolism levels close to the control level. This fact may be interpreted as a tendency for splenic recovery (Pacheco and Santos 2002a).
Pesticides (Azzalis et al. 1995), as well as heavy metals (Stohs and Bagchi 1995) and PAHs (Ibuki and Goto 2002) are associated with increased free radical concentrations within the cytosol. These oxidative forms may increase programmed cell death or disturb cell homeostasis and cellular necrosis. Also, prenecrotic areas suggest another necrosis event where the invasion of blood cells in the tissue is evidence of cell injury. Individuals with high incidence of necrosis also displayed prenecrosis, strongly suggesting a continuous exposure to the related xenobiotic compounds present in the environment (Oliveiro Ribeiro et al. 2005).
Santos and Hall (1990) studied the influence of inorganic lead (Pb) on the biochemical composition of eel blood by exposing eels (mean weight 50 g) for 30 days to 300 μg Pb/L (Table 2). A counting of the white blood cells showed an increased number of lymphocytes and increased plasma lactate levels in lead-treated eels due to metal pollution and stress. Lymphocytes are known to be immunocompetent, relating the immune system by transforming themselves into antibody-producing cells or their precursors. A significant lymphocytosis indicates the lasting action of the toxicant on the immune system.
Golovanova (2008) reported that visceral distribution of mercury (Hg) in organs and tissues of fish often shows the following order: muscles > liver > intestine > spleen > brain > gonads. This is due to the high content of functional proteins (–SH, –NH2, –COOH, –OH) in muscles, having high affinity to Hg. Immunotoxic effects following Hg exposure range from depressed haematopoiesis and enzyme activity to enhanced immune cell death. Investigators have observed a continuum of effects ranging from low-dose activation to high-dose inhibition of fish immune cell function following Hg exposure (Carlson and Zelikoff 2008, Table 2). Relatively low concentrations of Hg have been shown to increase lymphocyte mitosis and intracellular calcium levels whereas higher concentrations suppressed DNA synthesis, induced rapid calcium influx, and altered phosphorylation of cell proteins (Carlson and Zelikoff 2008). Sanchez-Galan et al. (2001) found that injected mercury induced micronuclei expression in eels, the concentration tested being 1.7 μg metal/g body weight and the micronuclei induction being 2.64 and 2.35 micronuclei per 1,000 cells (Table 2).
Oliveira et al. (2008) indicated that Cu at environmentally realistic levels may pose a serious ecological risk to fish. After a 7 days exposure to Cu, European eel revealed a significant metallothionein (MT) induction response in liver, and the erythrocytic nuclear abnormalities frequency increased significantly in the Cu exposed group (Table 2).
Teles et al. (2005) studied the sequential exposure to PAHs and heavy metals by exposing eel for 24 h to chromium (Cr) or copper, with or without a 24 h pre-exposure to β-naphthoflavone (BNF; Table 2). Single exposures to Cr decreased plasma T4 (free thyroxin) in eels, and to Cu resulted in elevated plasma cortisol and glucose (2.5 μM), as well as plasma lactate (1 μM), whereas a T4 decrease was found for both concentrations. In general, plasma T4 was the most affected hormone, as it responded to all Cr and Cu exposure conditions (Teles et al. 2005). The interference of BNF pre-exposure on Cr effects was observed as a significant plasma glucose increase. BNF pre-exposure prevented plasma cortisol and lactate increases; however, a greater T4 decrease was observed in eels exposed to 2.5 μM Cu.
Effects on osmoregulation
Already in 1971 it was accepted that the insecticide DDT exerts a direct toxic effect on the nervous system in both vertebrates and invertebrates. Janicki and Kinter (1971) reported that DDT impairs fluid absorption in intestinal sacs from eels adapted to seawater. Furthermore, this functional impairment has an enzymatic basis; DDT also inhibits the Na+ and K+ activated, Mg2+-dependent adenosine triphosphatase (ATP) in homogenates of the intestinal mucosa and gill filaments (Kinter et al. 1972). Thus, the extreme sensitivity of teleosts to organochlorine pollutants may involve the disruption of osmoregulatory transport mechanisms. Moreover, other organochlorine insecticides, including endrin also inhibited Na+-K+-ATPase from fish brain and endrin has been observed to disrupt osmoregulation in both marine and freshwater teleosts (Kinter et al. 1972).
Cadmium also has a dose-dependent inhibition on in vitro activities of Na+-K+-ATPase and carbonic anhydrase on the intestines and gills of eels. Lionetto et al. (1998) experienced that these activities were inhibited by increasing cadmium concentrations with a maximum inhibition (±80%) at 5 and 50 μM CdCl2 for gill and intestines Na+-K+-ATPase (Table 2). Carbonic anhydrase activities, measured in gill homogenate and in cytosolic and brush border membrane fractions isolated from intestinal mucosa, were significantly inhibited by pre-incubation (1 h) with CdCl2. Maximal inhibition (about 80%) of branchial carbonic anhydrase was noted at approximately 60 M; higher concentrations evoked no further significant inhibition. Intestinal carbonic anhydrase isoforms, cytosolic and membrane-bound, exhibited lower sensitivity to the heavy metal with respect to the branchial carbonic anhydrase activity, since the highest concentration of CdCl2 tested produced an inhibition of about 30 and 50% respectively. These results suggested that cadmium, by inhibiting the activity of carbonic anhydrase and Na+-K+-ATPase enzymes in intestine and gills, could alter both acid–base balance and osmoregulation in eel (Lionetto et al. 1998). Fabbri et al. (2003) reported that Cd2+ and Hg2+ may impair a crucial intracellular transduction pathway involved in the adrenergic control of glucose metabolism, but also in several other routes of hormonal regulation of liver functions. Micromolar concentrations of both heavy metals significantly reduced the epinephrine-modulated cAMP levels in isolated eel hepatocytes, in good agreement with the reduction of glucose output. DHAA can obstruct oxygen diffusion across the gills and impair the osmoregulatory function as reported by Pacheco and Santos (2002a).
Oxidative stress and biomarkers
Organisms dispose of a mono-oxygenase system which helps them to detoxify contaminants, to remove them from tissues and to excrete them. Available data indicate that most of the PCBs are only slowly metabolised, if at all, in fish. So, an effective way to reduce the toxicity of PCBs is to store them in lipid tissue, a metabolically relatively inactive compartment. However, these contaminants are put into circulation again when eels start migrating and lipid reserves are consumed. The mono-oxygenase system can be partially inhibited by hormones e.g. steroid hormones, but also by temperature, sex, age and food. Reproductive hormones regulate seasonal changes in mono-oxygenase activity. Before the spawning season, this activity decreases by natural factors in many fish species (Walton et al. 1983). But the detoxification mechanism of fish is also stimulated by foreign chemicals like PCBs. Several studies have demonstrated that fish P-450-mediated enzyme activity can be stimulated by PCB mixtures (Melancon et al. 1981; Ankley et al. 1986; Kleinow et al. 1987). This is supported by field observations of high levels of mono-oxygenase activity in fish liver in the Rhine downstream a PCB-incinerator (Monod et al. 1988). The induction of the mono-oxygenase activity during spawning can influence the breeding success of eel and can lead to a decrease in numbers of young eels (van Ginneken et al. 2009), because steroids are endogenous substrates for certain types of mono-oxygenases. The overall effect will result in a reduction of circulating steroid levels (Sivarajah et al. 1978). An increase in mono-oxygenase activity is related to a reduction of gamete viability and embryological development (Spies and Rice 1988). Starving fish exhibit lower levels of mono-oxygenase activity than well-nourished fish (Jimenez et al. 1988). However, as in maturing animals, pollutants increase mono-oxygenase activity in starving fish (Jimenez and Burtis 1989). Eels are thought to starve for as long as their migration endures, which is approximately 6 months. It is likely that during this period the mono-oxygenase activity increases when PCB levels in the body increase due to fat oxidation (van Ginneken et al. 2009).
In the past 25 years, numerous biomarkers have been developed with the objective to apply them for environmental biomonitoring exposure and effects of pollution (Whyte et al. 2000; Zhu et al. 2006). A biomarker is a biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition. The selection of an appropriate biomarker for the contaminant-effect/low dose-response relationship is frequently a controversial issue, especially when there is insufficient information concerning the mechanism by which a contaminant acts (Zhu et al. 2006). Whyte et al. (2000) describe EROD activity (a catalytic measurement of cytochrome P4501A induction) as a biomarker in fish. It already has proven its value as biomarker in a number of field investigations of bleached kraft mill and industrial effluents, PAH and PCB contaminated sediments, and chemical spills (Aubry et al. 2007b).
Several fish studies have reported benzo[a]pyrene (BaP; Wolkers et al. 1996; Pacheco and Santos 1998, 1999, 2001), DHAA and BKPME (Martel et al. 1994; Pacheco and Santos 1999) as EROD inducers and strong fish mutagens. Pacheco and Santos (2002a) studied the biotransformation response of eel on the toxicity of these compounds (Table 2). The results were unexpected because BaP nor BKPME exhibited considerable total EROD induction. The response to shorter DHAA exposures also did not corroborate their previous results, despite the unequivocal total EROD induction exhibited by 180-day DHAA exposed fish. Pacheco and Santos (2002a) explained the discrepancy between results either by differences between fish lots or by a relative lack of sensitivity of EROD methodology concerning the measurement on the whole body. They also discovered that 30-day DHAA treated eels have epidermis exfoliation probably due to the abrasive effect of resin acids. Previous studies (Bushnell et al. 1985; Toivola and Isomaa 1991) pointed to the fact that an eventual detergent-like action and consequent cell breakdown directly affecting the eel’s body surface also has to be considered. Resin acids are important components of pulp mill effluents; therefore, DHAA may also be partially responsible for the same histological alteration observed in BKPME-treated fish (Pacheco and Santos 2002a). A study by Oliveira et al. (2003) on the effects of chromium on liver organ culture after 24 h demonstrated a serum’s protective effect against chromium EROD inhibition in liver organ culture (Table 2).
Teles et al. (2003) showed, in agreement with results from a study of Pacheco and Santos (2002b), an early significant EROD-activity inhibition after eel exposure to naphthalene (NAP) and BNF, especially for the highest NAP concentrations at 2–6 h exposure and for BNF at 2 h exposure (Table 2). However, a significant EROD activity increase was detected from 16 to 72 h exposure for NAP and from 4 to 72 h exposure for BNF. The highest BNF concentrations were demonstrated to induce histopathological liver alterations and to impair the EROD activity response. During the first 8 h exposure to NAP, erythrocytic nuclear abnormalities (ENA) were observed, pointing to an increased genotoxic response. However for longer exposures the ENA frequency returned to control levels, reflecting a considerable DNA repair capacity and/or a metabolic adaptation providing an efficient NAP biotransformation and consequent detoxification.
In eel and carp (Cyprinius carpio L.) Hoff et al. (2005) observed a significantly and positively related hepatic PFOS concentration with the serum alanine aminotransferase (ALT) activity and a negative correlation with the serum protein content. The hepatic PFOS concentration in carp and eel correlated significantly and positively with the serum ALT activity, a marker for hepatic damage, showing that PFOS may induce liver damage by disturbing the membrane structure. A decrease of the total serum protein content and an increase of hematocrit levels were suggested to be PFOS mediated. Moreover, PFOS has been shown to induce hepatocyte damage in vivo (Hoff et al. 2003) which might be indicative for a more general cytotoxic capacity, that of the gills included.
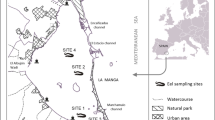
Metallothionein is generally considered as a storage and supply site for essential metals such as Zn and Cu which are utilized in protein synthesis, nucleic acid metabolism and other metabolic processes (Langston et al. 2002). It has high affinity to bind Ag, Cd, Cu, Hg, and Zn and, consequently it has been proposed as a specific biomarker for studying metal exposure (Zhu et al. 2006). In addition to this regulatory function, MT may also play a role in metal detoxification. Oliveira et al. (2008) reported that MT induction was insufficient to prevent endocrine and metabolic alterations as well as genotoxicity/clastogenicity in blood. Langston et al. (2002) found that MT levels in eels are a direct function of metal concentration in surrounding sediment or water. This has recently been further studied by Van Campenhout et al. (2008) in a field study in Belgium. They studied the effect of metal exposure on the accumulation and cytosolic speciation of metals in livers of European eel measuring MT induction. Four sampling sites in Flanders with different degrees of heavy metal contamination (Cd, Cu, Ni, Pb and Zn) were selected for this purpose. The cytosolic concentration of Cd, Ni and Pb increased proportionally with the total liver levels. However, the cytosolic concentrations of Cu and Zn only increased above a certain liver tissue threshold level. Cd, Cu and Zn, but not Pb and Ni, were largely associated with the MT pool in correspondence with the environmental exposure and liver tissue concentrations. It was concluded that the metals, rather than other stress factors, are the major factor determining MT induction.
Both the redox cycling of heavy metals as well as their interaction with organic pollutants are a major contributor to oxidative stress resulting from aquatic pollution. Oxidative stress is caused by an imbalance between the production of reactive oxygen and a biological system’s ability to readily detoxify the reactive intermediates or easily repair the resulting damage. All forms of life maintain a reducing environment within their cells. This reducing environment is preserved by enzymes that maintain the reduced state through a constant input of metabolic energy. Disturbances in this normal redox state can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Ahmad et al. (2005) studied the oxidative stress response of European eel for 24 h to copper exposure and to BNF with or without pre-exposure to BNF (Table 2). Eel gill and kidney oxidative stress biomarker responses are lipid peroxidation (LPO), glutathione peroxidase (GPX) and catalase (CAT). Exposure to copper or BNF induces nor in the kidneys neither in the gills LPO. Double BNF exposures potentiated the risk of peroxidative damage occurrence in both organs. BNF/Cu interference on antioxidant responses differs between the studied organs. In gill, antagonistic effects were denoted with probable reflex in terms of peroxidative damage increase. In kidney, BNF pre-exposure prevented CAT and GPX inhibition by copper; nonetheless, no advantage of this effect was perceptible as defence against LPO generation (Ahmad et al. 2005). Ahmad et al. (2006) also examined the oxidative stress and genotoxic effects of Cr in gill and kidney of eels (see below).
Pierron et al. (2007b) also investigated the expression level of various genes involved in the mitochondrial respiratory chain, in the cellular response to metal and oxidative stress of glass eels. Their results showed that hypoxia enhanced ventilation of glass eel and Cd accumulation in gills only at the lowest metal concentration tested (Table 2). At the gene level, Cd exposure mimicked the effect of hypoxia since they observed a decrease in expression of genes involved in the respiratory chain and in the defence against oxidative stress.
Endocrine disruption
The endocrine system is a tightly regulated system comprised of a number of specialized glands which synthesize and secrete hormones under control of the hypothalamic-pituitary axis. It is instrumental in regulating physiological processes such as metabolism, growth and the homeostatic control, reproduction, energy production and osmoregulation.
Several contaminants are known to induce endocrine disruption. Endocrine disruption in freshwater fish presenting intersex individuals with ovotestes has now been reported from many places and in many freshwater and marine fish species (of which the reproduction can be studied; Jobling et al. 1998). Due to the high contaminant load in eels, researchers suspect endocrine disruption also will occur in eels (Versonnen et al. 2004). Versonnen et al. (2004) investigated plasma vitellogenin (VTG) content, measured in 142 eels sampled at 20 different locations in Belgium, in relation to the internal pollution levels (PCBs, organochlorine pesticides, metals; Table 2). No correlations were found between VTG content and weight, length, condition, fat content, contaminants or date of sampling. Plasma VTG content of eels from the field study was very low, despite a very high internal load of endocrine disrupters. These results, together with previously published studies of eels sampled at different locations in the UK during different seasons, suggest that immature yellow European eel might not be the best sentinel species to study the effects of estrogenic compounds on VTG levels of wild fish populations (Livingstone et al. 2000; Peters et al. 2001). The fact that yellow eel might be relatively insensitive (regarding VTG levels) to waterborne endocrine disrupters is also confirmed by Burzawa-Gerard and Dumas-Vidal (1991) and Luizi et al. (1997) who found that high doses of (injected) 17β-estradiol (E2) during 12 days; Table 2) were needed to induce VTG production in immature eels. The onset of maturation in the European eel only takes place during a period of prolonged swimming which might be a necessary physiological stimulus (van Ginneken et al. 2007). It is therefore to be expected that endocrine disrupting effects of pollutants will only become apparent during the starvation period while migrating or during the spawning itself (Versonnen et al. 2004).
Two studies by Maria et al. (2006) and Teles et al. (2007) showed that agricultural chemicals such as fertilizers and pesticides, domestic sewage, as well as heavy metals from electroplating industries, have endocrine, metabolic and genotoxic responses in eel caged for 48 h at sites, differing in their distances to the main known pollution source. PCB mixtures and individual chlorobiphenyl (CB) congeners have various endocrine-disrupting effects, but ultimate responses may be altered by concurrent effects on enzyme levels and enzyme activities (Maria et al. 2006). Evidence suggests that both individual congeners and mixtures of PCBs and PAHs directly interfere with thyroid hormone metabolism, with enzymes such as uridine-diphosphate-glucuronyl transferases (UGTs), iodothyronine deiodinases (IDs) and sulfotransferases (SULTs) in liver and brain and with the plasma transport system of thyroid hormones (Brouwer et al. 1998). PCBs interfere with neuroendocrine systems, partly by exerting estrogenic effects (Soontornchat et al. 1994). PCBs may interfere with corticosteroidogenesis and corticosteroid action in a similar manner as described for gonadosteroidogenesis (Barron et al. 1995) and thyroid hormone synthesis (McKinney and Waller 1994; Murk et al. 1994).
A study by Teles et al. (2003) also revealed endocrine disruption due to a cortisol-impaired response from 4 to 24 h NAP exposure. However, an adaptation process seems to occur after 48 h, since the plasma cortisol had a tendency to increase.
Alkylphenols (including octylphenol an nonylphenol, bisphenol-A, some phthalate esters, several phytoestrogens of natural plant origin, several PCB congeners, and some pesticides (kepone, lindane, o,p′-DDT)) have been reported to be estrogenic, causing endocrine disruption in fish but only one study on eel was found (Miura et al. 2005). They studied the effects of para-nonylphenol (p-NP) on spermatogenesis of Japanese eel (Anguilla japonica), and compared it with the action of E2, using an eel testicular organ culture system. A clearly effect on Sertoli-cells was seen in the presence of an androgen (11-KT), potentially disturbing 11-KT-induced spermatogenesis. Due to the knowledge gaps on eel studies on endocrine disruption, results from studies on other fish or eel species and other pollutants than pesticides are mentioned below.
Neff (2009) reports that by the binding of environmental estrogens, octylphenol or nonylphenol to the estrogen receptor in fish elicits a variety of specific biochemical responses, including the synthesis of the egg yolk protein, in the liver.
Schwaiger et al. (2002) used rainbow trout (Onchorynchus mykiss) as a test organism to evaluate the estrogenic effects of nonylphenol (NP). The histological examination revealed no alteration in sex ratios. But in single cases, intersex occurred in both male and female offspring of exposed fish. The analysis of sex steroid levels revealed a 2-fold increase of estradiol in the plasma of male offspring and a 13-fold elevation of testosterone in the plasma of female progeny. The present findings indicate that NP, in an environmentally relevant concentration range, acts as a weak estrogen in directly exposed adult male rainbow trout as indicated by elevated plasma vitellogenin levels. Reproduction success was reduced as indicated by decreased hatching rates. Hormonal imbalances detected in the offspring of exposed fish indicate a transgenerational effect mediated by the endocrine system.
Hasselberg et al. (2004) report effects of alkylphenols on the redox status in first spawning Atlantic cod (Gadus morhua). Alkyphenols and polyethoxylates bind to the estrogen receptor resulting in the expression of several responses both in vitro and in vivo, including the induction of vitellogenin. The threshold for vitellogenin induction in fish is 10 μg/L for NP and 3 μg/L for octylphenol (OF). Polyethoxylates also affect the growth of testes, alter steroid metabolism, disrupt smoltification and cause intersex (ova-testes) in fish. The available literature suggests that the ability of alkyphenols and polyethoxylates to bioaccumulate in aquatic biota in the environment is low to moderate.
Genetic variability and genotoxic effects
Cajaraville et al. (2003) reviewed molecular and cellular impacts of pollution in fish, including genetic damage. Direct genetic damage may occur by mutagenic chemicals or radiation, and may affect a wide range of cellular functions. There is however, limited quantitative knowledge about pollution induced molecular and genetic damage in the eel, and their consequences on individual health, fecundity and population productivity and viability.
Maes et al. (2005) assessed whether the genetics of Belgian yellow eels under natural conditions could be linked to fitness and pollution pressure. A significant negative correlation was observed between the level of heavy metal (Hg, Cd, Pb, Cu, Zn, Ni, Cr, As and Se) bioaccumulation and condition suggesting an impact of pollution on the health of sub-adult eels. In general, there was a reduced genetic variability in strongly polluted eels, as well as a negative correlation between bioaccumulation level and allozymatic multi-locus heterozygosity. No pollution related differences were shown for microsatellites, suggesting a differential response at metabolic enzymes and possibly direct overdominance of heterozygous individuals (Maes et al. 2005). The level of bioaccumulation of toxicants is not only dependent on the level of pollution, but also on the individual’s capacity for detoxification, which correlates with the individual’s level of heterozygosity for these enzymes (van Ginneken et al. 2009). Species with a high effective population size (mostly marine) generally exhibit high levels of heterozygosity and are expected to be more resistant to pollution; multi-locus heterozygotes often show an increased fitness over homozygotes (Nevo et al. 1986). Maes and Volkaert (2007) suggested that locally polluted rivers would only have a low impact on the entire population because of the lack of spatial genetic structure at a local level. Nevertheless, selection during each generation may erode local genetic variability differentially, slowly reducing overall genetic variability.
Several authors described genotoxic effects of specific chemicals. BaP is a very carcinogenic compound which toxic potential is already demonstrated. Fish liver is a common target of BaP-induced carcinogenicity, and CYP1A appears to be primarily responsible for converting BaP to the 7,8-dihydrodiol and the ultimate carcinogen 7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydro-benzo[a]pyrene (BPDE; Van Veld and Nacci 2008). Maria et al. (2002) described a decrease in blood and liver DNA integrity and an increase in the frequency of erythrocytic nuclear abnormalities, CYP1A protein levels, EROD activity and PAH metabolites in bile from European eel exposed to BaP (Table 2). Nigro et al. (2002) and Nogueira et al. (2006) observed an elevated DNA damage and a significant induction of apoptosis (cell death) in eel, after exposure to BaP (Table 2). Jha (2004) concluded that BaP induced DNA breaks could lead to induction of chromosomal aberrations which are also associated with initiation and promotion of cancer. Induction of heritable mutations in germ cells could have long term detrimental effects on population survival (Jha 2004).
The combination of several contaminants may act synergetically and reinforce effects. The study on the sequential exposure to PAHs and heavy metals of eel by Teles et al. (2005) revealed that the pre-treatment with BNF was crucial for genotoxicity expression because only BNF + 2.5 μM Cu-exposed eels exhibited significant induction of erythrocytic nuclear abnormalities.
Gravato et al. (2006) did a similar experiment and found that BNF pre-exposure promoted a significant increase in liver EROD activity, but did not change the other responses investigated in eels. Liver total glutathione, reduced glutathione (GSH) and GSH/oxidized glutathione levels were slightly decreased, liver glutathione reductase and catalase activities were significantly inhibited, and liver DNA integrity decreased by 1 and 2.5 μM Cu in eels pre-exposed to BNF (Table 2).
Ahmad et al. (2006) examined the oxidative stress and genotoxic effects of Cr in gill and kidney of eels (Table 2). They demonstrated that in gills, GSH played a crucial role in genotoxicity and that sporadic induction of antioxidant enzymes was not effective in the protection against genotoxicity. A different mechanism occurred in kidney, since the loss of DNA integrity detected for all exposed groups was not accompanied by alterations in antioxidant levels. The interference of BNF pre-exposure with the response of organs to Cr showed a marked dependence on the Cr concentration. The lowest Cr concentration induced an increase on LPO and GPX as well as on CAT and GSH decrease in gills, and an LPO increase and GSH decrease in kidney. For the highest concentration, an additive effect on the decrease of DNA integrity and an antagonistic effect on the increase of GPX were observed in gills, as well as a CAT and GST decrease in kidney. In contrast, an antagonistic action was observed on DNA integrity loss for both Cr concentrations (Ahmad et al. 2006).
Genotoxic effects of pollution in eel have also been described under field conditions. Two field studies presented by Maria et al. (2006) and Teles et al. (2007) demonstrated increased plasma cortisol and glucose concentration levels in eels at three exposure sites close to a pollution source. The three exposure sites, are polluted by genotoxic compounds. The genotoxic effects induced in eel suggested a differential level in contamination by genotoxic chemicals at the exposure sites (Maria et al. 2006) in function of the distance to the source.
Ethology
Recent research (Bureau Du Colombier et al. 2007) demonstrates contaminant induced changes in behaviour in glass eel. The activity of glass eel with different load of metals was assessed in flume experiments. Active glass eel had significant lower body concentrations of Pb and Zn than inactive ones (Bureau Du Colombier et al. 2007). Bureau Du Colombier et al. (2007) explain the difference in body concentrations from different levels of contamination in glass eels entering the estuary and/or different accumulation levels when exposed to micro-pollutants during their estuarine migration. Contamination might then affect migratory efficiency, for example throughout swimming performance, resulting in the different levels of activity observed in the flume.
Contaminants and parasites
There may be considerable interaction of contaminants and disease agents with respect to their effect on the eel. These interactions may be complex. Contaminants may induce disease outbreaks, and can influence the immune system of the eel. Some parasites may bioconcentrate specific contaminants and hence may suppress the detrimental effect of these chemicals on the eel.
In 1977 Rødsæther et al. (1977) exposed eels to copper-contaminated freshwater and reported mortality due to vibriosis (Vibrio anguillarum). Eels kept in non-contaminated freshwater (<6 ρg Cu/L) remained healthy. Rødsæther et al. (1977) suggested that V. anguillarum is a common inhabitant of eels, and copper can change a commensal association between fish and bacterium to one of pathogenicity. Probably this illustrated a decrease in immune response induced by copper (Table 2).
Toxicants like PCBs have been related to resistance to diseases, viruses and parasites. Sures (2006) demonstrated that parasites: (1) may influence the metabolism of pollutants such as PCBs, in infected eels, (2) interact with pollution in synergistic or antagonistic ways, and (3) may induce physiological reactions in eels which were thought to be pollutant-induced. Experimental studies showed that alterations in pollutant uptake and accumulation in different intermediate and final hosts due to parasites are very important in the field of ecotoxicology. Sures (2006) points that in addition to such alterations, there is a close interaction between the effects of pollutants and parasites which seems to be mediated at least partly by the endocrine system, which itself is closely related to the immune system in fish.
Fat oxidation (e.g. as a result of contamination, see later) could lead to immuno-suppression, thus to reduced resistance to diseases, viruses and parasites. In combination with swimming, the EVEX virus causes haemorrhages and anaemia resulting in the death of the animals after 1,000–1,500 km (van Ginneken et al. 2005). Sures and Knopf (2004) showed that in eels 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) suppresses the humoral response and that it increased the incidence of infection by the swimbladder nematode Anguillicoloïdes crassus. Experimentally infected eels with Anguillicoloïdes crassus and exposed to sublethal concentrations of Cd and PCB 126 showed a significant increase of Anguillicoloïdes-specific antibodies in the peripheral blood 61 days p.i., indicating that it was not the invasive larvae but the adult worms which elicit the antibody response (Table 2). The exposure to PCB 126 resulted in a complete suppression of the antibody response. Sures and Knopf (2004) also indicated that apparently the Cd concentrations (21.7 ± 12.8 μg/L (mean ± S.D.)) applied in their study were not high enough to suppress the immune response of European eels. Furthermore, as eels are able to withstand environmental pollution and tend to accumulate heavy metals to a very high degree (e.g. Mason and Barak 1990; Sures et al. 1994), it seems likely that this species is not sensitive enough to show alterations in its immune response at low levels of Cd pollution. The toxicity of PCB 126 seems to be related to a structural similarity to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most toxic of all halogenated aromatic hydrocarbons (HAH) (Regala et al. 2001). The degree of immunotoxicity of PCBs correlates with the degree of binding affinity to the cytosolic aryl hydrocarbonreceptor (Ahr; Kafafi et al. 1993), which is a well-described transcription factor for a variety of gene products, including cytochrome P4501A (Hahn and Stegemann 1994).
The lack of parasites or parasitic lesions in the skin of DHAA-treated animals indicated that the above reported abrasive action of DHAA also may be adverse to parasite fixation, preventing this kind of infestation (Pacheco and Santos 2002a).
Some parasites have been demonstrated to bio-concentrate specific contaminants such as metals, lowering the concentration of these chemicals in host tissues and hence suppressing the detrimental effects on the host. The intensity of the accumulation is parasite-specific and may be influenced by external factors. The effect of salinity and the mode of application (oral versus aqueous) on the lead accumulation in different eel tissues and its parasites Anguillicoloïdes crassus (Nematoda) and Paratenuisentis ambiguous (Acanthocephala) was investigated by Zimmerman et al. (1999). Waterborne as well as dietary lead exposure caused an increase in the metal levels of different eel tissues and its parasites. The mode of lead application had a significant influence on the distribution of lead in the fish tissues, and the resulting metal concentrations were approximately 20–2,000 times higher in P. ambiguus than in A. crassus. These differences may be due to the different microhabitats and nutrient uptake mechanisms of both parasite species (Zimmerman et al. 1999).
Eira et al. (2009) give evidence for the possible role of cestode infection in metal metabolization/storage processes in host tissues. They studied the effect of Proteocephalus macrocephalus on the accumulation of trace elements (Cr, Cu, Ni, Pb, Zn) in contaminated eel. Results showed decreased levels of Ni and Cr in kidney and liver tissue of eels while P. macrocephalus individuals accumulated these contaminants. Bioaccumulation of Cu, Cr and Pb in A. crassus varied according to eel co-infection with P. macrocephalus.
But A. crassus is also able to excrete mercury in detoxification processes as is shown by Palíková and Barus (2003) who found less mercury in A. crassus tissue compared to the tissue of its final host eel. Low efficacy of mercury accumulation (and also of other heavy metals) that they found in A. crassus enables to exclude in this parasite-host system, the influence of the parasite on decreasing the heavy metal concentrations in body tissues of infected eel specimens.
Evidence for biological effects on migration and reproduction
Lipid energy reserves and silvering
A number of studies have shown that the silvering process, the subsequent downstream and transoceanic reproductive migration, as well as gonad maturation, can only take place if a sufficient quantity of energy is stored as lipids. Under a critical fat mass in their yellow stage (28%), silvering may not even be initiated (Thurow 1959; Larsson et al. 1990). Boëtius and Boëtius (1980) estimated that the total stored lipids must exceed 20% of the body weight to cover the migratory needs of the European silver eel (female). van den Thillart et al. (2004, 2005) found that European eel is able to swim 5,500 km showing that energy reserves are, in principle, sufficient for migration to the Sargasso Sea. Fat consumption for a complete run would be 126.5 mg/g wet weight (van den Thillart et al. 2005) which corresponds to 60% of the total fat reserve of most silver eels. Calculations based on the number of oocytes and fat percentage in oocytes show that almost 40% of the total fat reserve is required for incorporation in oocytes. Thus animals with less than 13% (of the body weight) fat would not be able to swim 6,000 km. An additional 7% (of the body weight) is needed for oocytes. It is hypothesized that eels with insufficient fat content (<13% of the body weight) will not continue their migration activity but first linger downstream, only to continue their migration later. A value of 20% (of the body weight) fat is also the average for migrating silver eels, implying that only half of the silver eels is capable of successful migration and reproduction (van den Thillart et al. 2005). Establishing sufficient lipid energy is thus essential in the life cycle of the eel. However, a significant decrease of the muscle lipid contents of yellow eels in Belgium and The Netherlands have been observed recently (Belpaire et al. 2009). The magnitude of this decrease was considerable: from 20.0 to 12.3% over a 13-year period since 1994 in Belgium and from 20.6 to 13.1% in the Netherlands, now questioning the ability of these eels to start silvering and to achieve their spawning migration.
Several authors described negative effects of specific contaminants on the lipid metabolism of the eel. Ceron et al. (1996) and Fernandez-Vega et al. (1999) demonstrated that lipid accumulation in eel was disturbed due to inhibition of the acetyl cholinesterase (AChE) activity after pesticide exposure. Ceron et al. (1996) studied the effects of the insecticide diazinon on the AChE activity in different eel tissues (brains, plasma and eye tissue) exposed to sublethal doses (Table 2). Fernandez-Vega et al. (1999) exposed eels to a sublethal concentration of the herbicide thiobencarb (S-chlorobenzyldiethylthiocarbamate). The results showed that thiobencarb is significantly limiting the plasma AChE-activity from the first contact with the poison (Table 2). ChE-activity, which is also a valid bio-indicator of organochlorine pesticides and PCBs exposure in fishes, affects lipidogenesis by inducing a fast lipid mobilisation by involuntarily and continuous muscular activity and subsequently reduce migration efficiency and breeding success (Corsi et al. 2005). Also fenitrothion was shown to affect lipid metabolism: eels under laboratory conditions showed an increased fat consumption in the presence of this organophosphate insecticide (Sancho et al. 1998a) and thus lower efficiency of lipid storage (Table 2).
Pierron et al. (2007a) investigated the possible impact of cadmium on the lipid storage efficiency of yellow eels. After a 1 month exposure to 0 and 5 μg/L Cd (Table 2), Cd toxicity was examined by studying the activity and expression level of several enzymes involved in liver lipolysis and lipogenesis and by determining lipid content in eel muscle. The observations suggested an increased fat consumption in presence of cadmium, which could compromise successful reproduction.
Hu et al. (2003) reported that perfluorinated compounds are known to affect lipid metabolism, through alterations in cell membrane properties in fish.
In Belgium, the relationships between lipid content and various environmental variables were studied by analysing an extensive dataset of contaminants. It was demonstrated that PCBs (especially the higher chlorinated ones) and DDTs had a negative impact on the lipid content of the eel (Geeraerts et al. 2007).
Endocrine disruption, presenting intersex individuals with ovotestes, has now been reported from many places and in many freshwater and marine fish species. Indirectly, endocrine disruption might also affect fat storage due to specific chemicals, some of them mimicking the steroid hormone oestrogen (Turner and Sharpe 1997).
Reproduction
A multitude of reports are available describing the detrimental effects of contaminants on the reproductive biology of fish. Contaminants may affect the endocrine system and disable the normal development of gonads, but also may interact with embryonic development, hatching and growth of larvae.
Due to the complex life cycle of the eel and the difficulties for artificial reproduction, reports on the pollution impact of contaminants on the eel are limited.
During maturation of female European silver eels, about 60 g fat per kg eel is incorporated in the oocytes (Palstra et al. 2007). According to the great importance of lipids in the egg-maturation process (Nassour and Léger 1989), a deficiency of lipid reserves available for gonad maturation may lead to a decrease of egg production with consequences on reproductive success (Henderson and Tocher 1987). In eel, 1.72 g eggs can be produced with one gram of fat (as presented in van Ginneken and van den Thillart 2000). Belpaire et al. (2009) described a potential decrease in fertility in eel, due to the observed lowered fat stores, presumably as a result of the impact of contaminants.
Together with the fat metabolisation during the migration, persistent organic pollutants such as dioxin-like polychlorinated biphenyls are metabolised too. The negative impact of highly contaminated lipid reserves in eel, explaining the stock decrease in Europe, has been suggested by Larsson et al. (1990): while the lipid reserves are depleted during migration, contaminants are released into the blood and may damage reproductive organs and affect embryogenesis.
Remobilization of pollutants to gonads might also occur with heavy metals, as demonstrated for cadmium. Pierron et al. (2008) reported that, after 30 days of Cd exposure, a significant metal accumulation was observed in the kidney, the liver, the gills and the digestive tract of Cd exposed eels (Table 2). Thereafter, during the maturation phase, which unfolded in Cd-free sea water, a significant increase in Cd content in gonads and kidney of Cd pre-contaminated eels was observed. This was associated in these animals with a significant decrease in Cd content of gills and digestive tract.
Palstra et al. (2006) observed a negative correlation between embryo survival time and TEQ (toxic equivalent) levels in the gonads implying TEQ-induced teratogenic effects. The disrupting effects occurred at levels below 4 pg TEQ/g wet weight gonad, which are below the EU maximum consumption limit for dioxin in food (Palstra et al. 2006, Table 2). PCB exposure also led to swelling of the yolk-sac and especially the pericardium (elevated wet weights of embryos/larvae), thus showing disturbed hydromineral balance (oedema). This indicates a disturbed water balance, which in adult fishes induces a stress response, partly controlled by the Hypothalamus Pituitary Interrenal (HPI) axis via cortisol (van Ginneken et al. 2009).
Many new contaminants such as BFRs, alkylphenols, phthalates, PFOS, etc. have been released and considering their demonstrated reprotoxic activities, cause now increasing concern. Studies on the effect of these compounds on the eel reproduction are missing. BFRs can cause developmental effects, endocrine disruption, immunotoxicity, reproductive, and long term effects, including second generation effects in chub (Leuciscus cephalus), bream (Abramis brama), and perch (Perca fluviatilis) (Hajslova et al. 2007). Norman et al. (2007) reported a dose related increase in number of atretic oocytes in female zebrafish exposed to a BFR mixture, which might indicate disturbed ovulation. Exposure to BFR at high dose (100 nM/g) resulted in a lowered spawning success. A reduced hatching success was seen in offspring from fish exposed to the BFR high dose. Uptake in adult fish and maternal transfer was shown for the BFR mixture in a parallel study (Rattfelt et al. 2008). To the best of our knowledge no studies are available on the effects of BFRs on (the reproduction of) the eel, but it was reported that in some rivers BFR bioaccumulates to very high levels in eels (e.g. in Belgium Belpaire et al. 2003; Roosens et al. 2008).
No studies were found on the effect of bisphenol A (BPA) on eel but on brown trout (Salmo trutta f. fario). Lahnsteiner et al. (2005) reported that in males exposed to estimated BPA concentrations of 1.75 and 2.40 μg/L semen quality was lower than in the control in the beginning of spawning (reduced sperm density, motility rate, and swimming velocity) and in the middle of spawning (reduced swimming velocity, at 2.40 μg/L BPA also reduced sperm motility rate). Therefore, production of high quality semen was restricted to the end of the spawning season and delayed for approximately 4 weeks in comparison to the control. At BPA exposure levels of 5.00 μg/L only one of eight males gave semen of low quality (reduced semen mass, motility rate, and swimming velocity). The percentage of ovulated females was similar for the control group and the groups exposed to estimated BPA concentrations of 1.75 and 2.40 μg/L, whereas at 5.00 μg/L BPA females did not ovulate during the investigation. Therefore, the tested BPA concentrations affected the percentage of ovulated females and the time point of ovulation. No effect was observed on the quality of eggs (egg mass, percentile mass increase during hardening, egg fertility).
Conclusions
It is obvious that eel is not as resistant as is generally assumed. Due to its apparent robustness to fluctuations in temperature, salinity, food availability, oxygen and even temporary emersion, eel is considered as a resistant species but recent studies indicate the contrary. Its characteristic life cycle makes the eel very sensitive to bioaccumulating contaminants, although effects at population level are difficult to measure in the continental immature phase. Many studies, recently been undertaken to study the degree and the effects of pollution on the eel and international concern about the stock decline resulted in an increasing quantity of information that demonstrates the negative impact of pollution on eel. A variety of contaminants have been found to affect the eel and effects were reported on several levels of biological organization, from subcellular, organ, individual up to even population level.
The toxic effects can occur at different moments in eel’s life cycle: during growing, silvering, migration, the development of reproductive cells, and larval stage. Most reports deal with the yellow eel stage and a wide range of effects have been demonstrated. However, in the yellow eel phase, the effects are apparently less harmful, because contaminants are stored in lipid tissue while growing. They also can be bio-concentrated in parasites (Zimmerman et al. 1999; Eira et al. 2009). It is assumed that most toxic effects start to harm during the silvering phase, when morphological and physiological changes take place initiated by hormonal changes. Meanwhile, fat is being metabolized, resulting in a remobilization of the live-long accumulated contaminants. Silver eels migrating to the Sargasso Sea, stop feeding and live on their fat stores. Thus, the energy stores must be sufficient to cover the 6,000 km long journey and to produce enough good quality eggs. Recent observations of decreasing fat stores in yellow eel questioned the ability for eels to succeed in fulfilling this migration and spawning (Belpaire et al. 2009). Some chemicals such as PCBs and Cd have been demonstrated to disturb the fat metabolism. A continuous fat burning during migration means an increasing continuous availability of contaminants and a high level of toxicity in the eel. This toxicity causes disturbance of the immune system, the reproduction system, the nervous system and the endocrine system. So the toxification leads to physiological disturbance, diminished resistance to infections of viruses and parasites, leading to a disturbed reproduction and finally even death of the eel. Both, the reduction of the lipid energy as a consequence of (specific) contaminants, and the mobilization of high loads of reprotoxic chemicals during migration, seem to be key elements decreasing the probability of a successful migration and normal reproduction. Hence, contaminants are believed to be an important issue in understanding the reasons of the decline of the species.
Eels are more vulnerable than other fish as they accumulate contaminants to a much higher degree than other species. In many fish species in western Europe, pollution has been reported to hamper normal reproduction and larval development (endocrine disruption). Considering the high levels of contamination in eels for many areas, endocrine disruption in mature silver eels can be expected, jeopardizing normal reproduction (Belpaire 2008). Many contaminants are widespread and measured concentrations are at a level which more than likely is causing ecotoxicological effects in eel. The review also shows that effects of contamination not only are measurable under laboratory conditions but also have been described in wild eel under natural circumstances in several countries (e.g. Maes et al. 2005; Maria et al. 2006; Geeraerts et al. 2007; Belpaire et al. 2009). It also has been demonstrated that the presence of parasites may synergetically reinforce the effects of some contaminants in the eel (e.g. Sures and Knopf 2004). Evidencing direct impact of pollution on the population dynamics is extremely difficult due to their complex life cycle. Therefore, the process of understanding the role of pollution is only advancing in dribs and drabs. Despite the enormous gaps of knowledge, this review demonstrated the increasing amount of evidence illustrating the detrimental impact of chemicals on diverse biological processes in eel, including physiology, migration and reproduction.
According to the data of eel landings the decline of the eel stock started as early as the mid-1960s (Dekker 2002b) while the recruitment started to come down early 1980s (EIFAC 1985). Many artificial chemicals have been developed and put on the market during the previous century, and production increased simultaneous with the post World War II intensification of agricultural and industrial activities. The more or less simultaneous decreases in recruitment in the Northern-hemisphere Anguilla species (A. Anguilla, A. rostrata and A. japonica) during the last 30 years, is an additional argument endorsing the idea that some contaminants which quickly spread over the industrialized world could be considered as key elements in the eel decline.
Recommendations
It is clear that more extensive research is necessary in order to evaluate how pollutants are detrimental to eel populations. Older substances such as PCBs, heavy metals and some pesticides are relatively well studied, however the impact on the eel of newer chemicals (e.g. BFRs, bisphenol A, VOCs, PFOS, alkylphenols, phthalates,…) which are known to pose serious and increasing problems in aquatic ecosystems, is poorly understood. The complex life cycle of the eel hampers studies of the toxic effects of individual chemicals on the reproduction of the eel. But, as controlled reproduction of A. japonica on experimental scale is now possible, this gives new opportunities for experimental work on the reprotoxic effect of (individual or combined) contaminants on the eel.
The development of adequate biomarkers indicating biological effects (see e.g. Aubry et al. 2007a, b; Maes and Volkaert 2007) with a great sensitivity for both the concentration and length of exposure will be another challenge and would allow detection of the genetic variation underlying environmentally dependent fitness traits in eels.
For the EU eel recovery plan (European Commission 2007) which is concentrating its efforts on the increase of the quantity of silver eels leaving continental waters, it is recommended to include empathically the quality aspect in the stock wide recovery plan. Quality targets should include contamination levels, biomarker responses, lipid content and condition. Getting a comprehensive overview of the quality of the silver eel population all over Europe seems to be an essential and urgent objective for the European eel management.
References
Ahmad I, Oliveira M, Pacheco M, Santos MA (2005) Anguilla anguilla L. oxidative stress biomarkers responses to copper exposure with or without b-naphthoflavone pre-exposure. Chemosphere 61:275–297
Ahmad I, Maria VL, Oliveira M, Pacheco M, Santos MA (2006) Oxidative stress and genotoxic effects in gill and kidney of Anguilla anguilla L. exposed to chromium with or without pre-exposure to b-naphthoflavone. Mutat Res 608:16–28
Akaishi FM, de Assis HCS, Jakobi SCG, Eiras-Stofella DR, St-Jean SD, Courtenay SC, Lima EF, Wagener ALR, Scofield AL, Ribeiro CAO (2004) Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to water soluble fraction (WSF) of crude oil. Arch Environ Contam Toxicol 46(2):244–253
Alcaide E, Esteve C (2007) Relationship among heavy metals accumulation, growth stage, and infectious diseases in wild eels from lake Albufera (Valencia, Spain) 13th international EAFP conference on fish and shellfish diseases, Grado, Italy (oral presentation)
Ankley GT, Blazer VS, Reinert RE, Agosin M (1986) Effects of aroclor-1254 on cytochrome-P-450-dependent monooxygenase, glutathione-S-transferase, and Udp-glucuronosyltransferase activities in channel catfish liver. Aquat Toxicol 9(2–3):91–103
Anonymous (1987) (French) Rapport du comité d’experts sur la pollution transfrontière du Rhin. La Gazette Officielle de la Pêche 920–921, p 27, 925–926, p 55
Arnold H, Braunbeck T (1994) Disulfoton as a major toxicant in the Rhine chemical spill at Basle in 1986: acute and chronic studies with eel and rainbow trout. In: Muller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Fishing News Books, London, pp 75–87
Aubry E, Cagnon C, Lalanne Y (2007a) Quantification of increase in hepatic CYP1A1 mRNA by real-time RT–PCR after exposure of the European eel (Anguilla anguilla) to a diesel oil water soluble fraction. Environ Bioindic 2(1):47–51
Aubry E, Cagnon C, Lalanne Y, Mouchès C (2007b) Assessment of young yellow European eel Anguilla anguilla L. exposure to a CYP1A1 inducer by the quantification of increase in hepatic CYP1A1 mRNA using real-time RT–PCR. J Fish Biol 71(C):470–477
Azzalis LA, Junqueira VBC, Simon K, Giavarotti L, Silva MA, Kogake M, Simizu K, Barros SBM, Fraga C, Porta EA (1995) Prooxidant and antioxidant hepatic factors in rats chronically fed an ethanol regimen and treated with an acute dose of lindane. Free Radic Biol Med 19(2):147–159
Balint T, Ferenczy J, Katai F, Kiss I, Kraczer L, Kufcsak O, Lang G, Polyhos C, Szabo I, Szegletes T, Nemcsok J (1997) Similarities, differences between the massive eel (Anguilla anguilla L.) devastations that occurred in Lake Balaton in 1991 and 1995. Ecotoxicol Environ Saf 37(1):17–23
Barron MG, Galbraith H, Beltman D (1995) Comparative reproductive and developmental toxicology of PCBs in birds. Comp Biochem Physiol C 112(1):1–14
Belpaire C (2008) Pollution in eel. A cause of their decline? Ph.D. thesis Catholic University Leuven. Research Institute for Nature and Forest, Brussels, pp 485, III annexes
Belpaire C, Goemans G (2007) Eels: contaminant cocktails pinpointing environmental contamination. ICES J Mar Sci 67(4):1423–1436
Belpaire C, Goemans G (2008) The European eel Anguilla anguilla, a rapporteur of the chemical status for the water framework directive? Vie Milieu 57(4):1–19
Belpaire C, Goemans G, De Boer J, Van Hooste H (2003) (Dutch) Verspreiding van gebromeerde vlamvertragers. In: Van Steertegem M (ed) Milieu- en Natuurrapport Vlaanderen 2003: MIRA-T., 2003 VMM, Erembodegem, pp 387–395
Belpaire C, Goemans G, Geeraerts C, Quataert P, Parmentier K (2008) Pollution fingerprints in eels as models for the chemical status of rivers. ICES J Mar Sci 65:1–9
Belpaire CGJ, Goemans G, Geeraerts C, Quataert P, Parmentier K (2009) Decreasing eel stocks: survival of the fattest? Ecol Freshw Fish 18(2):197–214
Bergemann M, Gaumert T (2005) (German) Gewässergütebericht der Elbe 2005. Wassergütestelle Elbe, Hamburg, p 6
Bilau L, Sioen I, Matthys C, De Vocht A, Goemans G, Belpaire C, Willems JL, De Henauw S (2007) Probabilistic approach to polychlorinated biphenyl (PCB) exposure through eel consumption in recreational fishermen vs. the general population. Food Addit Contam 24(12):1–8
Boëtius J, Boëtius I (1980) Experimental maturation of female silver eels, Anguilla anguilla. Estimates of fecundity and energy reserves for migration and spawning. Dana 1:1–28
Bordajandi LR, Gomez G, Fernandez MA, Abad E, Rivera J, Gonzalez MJ (2003) Study on PCBs, PCDD/Fs, organochlorine pesticides, heavy metals and arsenic content in freshwater fish species from the River Turia (Spain). Chemosphere 53:163–171
Brouwer A, Morse DC, Lans MC, Schuur AG, Murk A, Klasson-Wehler E, Bergman A, Visser TJ (1998) Interactions of persistent environmental organhalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol Ind Health 14(1–2):59–84
Brown JS, Steinert SA (2004) DNA damage and biliary PAH metabolites in flatfish from Southern California bays and harbors, and the Channel Islands. Ecol Indic 3(4):263–274
Bruslé J (1990) Effects of heavy metals on eels, Anguilla sp. J Aquat Living Resour 3:131–141
Bruslé J (1991) The eel (Anguilla sp) and organic chemical pollutants. Sci Total Environ 102:1–19
Buchmann K, Bjerregaard J (1990a) Mebendazole treatment of pseudodactylogyrosis in an intensive eel-culture system. Aquaculture 86(2–3):139–153
Buchmann K, Bjerregaard P (1990b) Effect of ivermectin, pyrantel and morantel on the European eel and its monogenean parasites. Bull Eur Assoc Fish Pathol 10(5):146–148
Buchmann K, Székely C, Bjerregaard P (1990a) Treatment of pseudoactylogyrus in restating of Anguilla anguilla 2. Trials with bunamidine, praziquantel and levamizole. Bull Eur Assoc Fish Pathol 10(1):18–20
Buchmann K, Székely C, Bjerregaard P (1990b) Treatment of pseudodactylogyrus infestations of Anguilla anguilla 1. Trials with niclosamide, toltrazuril, phenolsulfonphthalein and rafoxanide. Bull Eur Assoc Fish Pathol 10(1):14–17
Buchmann K, Felsing A, Slotved HC (1992) Effects of metrifonate, sodium chloride and bithionol on an European population of the gill parasitic Monogeneans pseudodactylogyrus spp. and the host. Bull Eur Assoc Fish Pathol 12(2):57–60
Bureau Du Colombier S, Bareille G, Bolliet V, Lambert P, Bardonnet A (2007) Micro-pollutant content in Anguilla anguilla glass eels and relationship with migratory behaviour. Vie Milieu 57(4):223–227
Burzawa-Gerard E, Dumas-Vidal A (1991) Effects of 17β-estradiol and carp gonadotropin on vitellogenesis in normal and hypophysectomized European silver female eel (Anguilla anguilla L.) employing a homologous radioimmunoassay for vitellogenin. Gen Comp Endocrinol 84:264–276
Bushnell PG, Nikinmaa M, Oikari A (1985) Metabolic effects of dehydroabietic acid on rainbow-trout erythrocytes. Comp Biochem Physiol C 81(2):391–394
Cajaraville MP, Hauser L, Carvalho G, Hylland K, Olabarietta I, Lawrence AJ, Lowe D, Goksoyr A (2003) Genetic damage and the molecular/cellular response to pollution. In: Lawrence A, Hemingway K (eds) Effects of pollution on fish: molecular effects and population responses. Blackwell, Oxford, pp 14–82
Canyurt MA (1983) Toxic effects of lindane and parathion methyl on three fresh water fish species. Bull Cent Rech Sci Biarritz 14(3–4):257–262
Carlson E, Zelikoff JT (2008) The immune system of fish: a target organ of toxicity. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press, USA, pp 489–529
Ceron JJ, Ferrando MD, Sancho E, Gutierrez-Panizo C, AndreuMoliner E (1996) Effects of diazinon exposure on cholinesterase activity in different tissues of European eel (Anguilla anguilla). Ecotoxicol Environ Saf 35(3):222–225
Christou MD (2000) Substances dangerous to the environment in the context of Council Directive 96/82/EC. Report by Technical Working Group 7 EUR 19651 EN, p 43
Corsi I, Mariottini M, Badesso A, Caruso T, Borghesi N, Bonacci S, Iacocca A, Focardi S (2005) Contamination and sub-lethal toxicological effects of persistent organic pollutants in the European eel (Anguilla anguilla) in the Orbetello lagoon (Tuscany, Italy). Hydrobiologia 550:237–249
Daverat F, Limburg KE, Thibault I, Shiao JC, Dodson JJ, Caron FO, Tzeng WN, Iizuka Y, Wickstrom H (2006) Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar Ecol Prog Ser 308:231–241
De Boer J, Hagel P (1994) Spatial differences and temporal trends of chlorobiphenyls in yellow eel (Anguilla anguilla) from inland waters of the Netherlands. Sci Total Environ 141(1/3):155–174
De Boer J, van der Valk F, Kerkhoff MAT, Hagel P (1994) 8-Year study on the elimination of PCBs and other organochlorine compounds from eel (Anguilla anguilla) under natural conditions. Environ Sci Technol 28(13):2242–2248
Dekker W (2002a) Monitoring of glass eel recruitment: volume 1 thematic overview. Volume 2A: country reports northern part. Volume 2B: country reports southern part. C007/02-WD. IJmuiden, pp 256
Dekker W (2002b) Status of the European eel stock and fisheries. In: Aida K, Tsukamoto K, Yamauchi K (eds) Advances in eel biology. Springer, Tokyo, pp 237–250
Di Giulio RT, Hinton DE (2008) The toxicology of fishes. CRC Press, Florida
Durrieu G, Maury-Brachet G, Girardin ML, Rochard E, Boudou A (2005) Contamination by heavy metals (Cd, Zn, Cu, and Hg) of eight fish species in the Gironde Estuary (France). Estuaries 28(4):581–591
Dutta HM, Meijer H (2003) Sublethal effects of diazinon on the structure of the testis of bluegill, Lepomis macrochirus: a microscopic analysis. Environ Pollut 125(3):355–360
EIFAC (1985) Report of the 1985 meeting of the working party on eel and of the workshop on eel aquaculture. September 1985, Perpignan. EIFAC/XIV/86/3. European Inland Fisheries Advisory Commission of the Food and Agriculture Organization of the United Nation, Rome, pp 23
Eira C, Torres JPM, Miquel J, Vaqueiro MC, Soares AMVM, Vingada J (2009) Trace element concentrations in Proteocephalus macrocephalus (Cestoda) and Anguillicola crassus (Nematoda) in comparison to their fish host, Anguilla anguilla in Ria de Aveiro, Portugal. Sci Total Environ 407(2):991–998
Eisler R (1997) Copper hazards to fish, wildlife and invertebrates: a synoptic review. USGS/BRD/BSR-1997-0002. Contaminant Hazard Reviews Report 33. USGS/BRD Patuxent Wildlife Research Center, Laurel, pp 98
Erichsen PChr et al (2000) (Danish) Miljøfremmede stoffer i sediment, vandløbsvand, fisk og jord. Miljø og Teknologi/Vand og Landskab 14:7–16
European Commission (2007) Council regulation (EC) No 1100/2007 of 18 September 2007 establishing measures for the recovery of the stock of European eel. No 1100/20071-7
Fabbri E, Caselli F, Piano A, Sartor G, Capuzzo A (2003) Cd2+ and Hg2+ affect glucose release and cAMP-dependent transduction pathway in isolated eel hepatocytes. Aquat Toxicol 62(1):55–65
Fernandez-Vega C, Sancho E, Ferrando MD, Andreu-Moliner E (1999) Thiobencarb toxicity and plasma AChE inhibition in the European eel. J Environ Sci Health B 34(1):61–73
Ferrando MD, Andreu-Moliner E (1989) Effects of temperature, exposure time and other water parameters on the acute toxicity of endosulfan to European eel, Anguilla anguilla. J Environ Sci Health B 24(3):219–224
Ferrando MD, Andreu-Moliner E (1991a) Effects of lindane on fish carbohydrate-metabolism. Ecotoxicol Environ Saf 22(1):17–23
Ferrando MD, Andreu-Moliner E (1991b) The effect of time on physiological-changes in eel Anguilla anguilla, induced by lindane. Comp Biochem Physiol C 100(1–2):95–98
Ferrando MD, Andreu-Moliner E, Almar MM, Cebrian C, Nunez A (1987) Acute toxicity of organochlorined pesticides to the European eel, Anguilla anguilla: the dependency on exposure time and temperature. Bull Environ Contam Toxicol 39(3):365–369
Ferrando MD, Almar MM, Andreu E (1988) Lethal toxicity of lindane on a teleost fish, Anguilla anguilla from Albufera Lake (Spain). Hardness and temperature effects. J Environ Sci Health B 23(1):45–52
Ferrando MD, Sancho E, Andreu-Moliner E (1991) Comparative acute toxicities of selected pesticides to Anguilla anguilla. J Environ Sci Health B 26(5–6):491–498
Foster J (2005) The Sussex eel report. F001EEL05-6. Environment Agency, Sussex, pp 16
Foulquier L, Reynier B, Grauby A, Bovard P (1971) Toxicity of lindane to eels (Anguilla anguilla L.). CR Seances Acad Agric Fr 57(12):1060–1068
Gaumert T, Löffler J, Bergemann M, Reincke HR (2001) (German) Sude. Aland und Havel. Fischbestandskundliche Untersuchungen sowie Schadstoffbelastung von Brassen. Aal und Zander in den Unterläufen der Elbenebenflüsse. Wassergütestelle Elbe, Hamburg, p 123
Gaumert T, Löffler J, Bergemann M, Reincke HR (2002) (German) Stör. Fischereibiologische Untersuchungen sowie Schadstoffbelastung von Brassen, Aal und Zander im Marschbereich dieses Elbenebenflusses. Wassergütestelle Elbe, Hamburg, pp 70
Gaumert T, Bergemann M, Löffler J, Reincke HR (2003) (German) Elster, Mulde und Saale. Fischereibiologische Untersuchungen sowie Schadstoffbelastung von Brassen. Aal und Zander in den Unterläufen der Elbenebenflüsse. Wassergütestelle Elbe, Hamburg, p 122
Geeraerts C, Goemans G, Belpaire C (2007) (Dutch) Ecologische en ecotoxicologische betekenis van verontreinigende stoffen gemeten in paling. MIRA/2007/05; INBO/R/2007/40. INBO, Groenendaal-Hoeilaart, pp 241
Geets A, Liewes EW, Ollevier F (1992) Efficacy of some anthelmintics against the swimbladder nematode Anguillicola crassus of eel Anguilla anguilla under saltwater conditions. Dis Aquat Org 13(2):123–128
Gimeno L, Ferrando MD, Sanchez S, Andreu E (1994) Endosulfan effects on liver and blood of the eel, Anguilla anguilla. Comp Biochem Physiol C 108(3):343–348
Goemans G, Belpaire C, Raemaekers M, Guns M (2003) (Dutch) Het Vlaamse palingpolluentenmeetnet, 1994–2001: gehalten aan polychloorbifenylen, organochloorpesticiden en zware metalen in paling. IBW.Wb.V.R.2003.99. Instituut voor Bosbouw en Wildbeheer, Groenendaal-Hoeilaart, pp 169
Golovanova IL (2008) Effects of heavy metals on the physiological and biochemical status of fishes and aquatic invertebrates. Inland Water Biol 1(1):93–101
Gony S (1990) Short note on the effects of cadmium on the gill of the glass eel (Anguilla anguilla). Int Rev Gen Hydrobiol 75(6):835–836
Grauby A, Foulquier C, Descamps B, Jaulent Y (1973) (French) Résultats relatifs à la toxicité d’effluents industriels de la région de Fos et de l’étang de Berre sur les anguilles, les daphnies, les loups et les muges. CEA, Labo. Radio écologie continentale, contrat no VEN-0368. Agence de Bassin Rhône Méditerranée-Corse, pp 135
Gravato C, Teles M, Oliveira M, Santos MA (2006) Oxidative stress, liver biotransformation and genotoxic effects induced by copper in Anguilla anguilla L.—the influence of pre-exposure to b-naphthoflavone. Chemosphere 65(10):1821–1830
Grosell M, Hogstrand C, Wood CM, Hansen HJM (2000) A nose-to-nose comparison of the physiological effects of exposure to ionic silver versus silver chloride in the European eel (Anguilla anguilla) and the rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 48(2–3):327–342
Hahn ME, Stegemann JJ (1994) Regulation of cytochrome P4501A1 in teleosts: sustained induction of CYP1A1 mRNA, protein, and catalytic activity by 2,3,7,8-tetrachlorodibenzofuran in the marine fish Stenotomus chrysops. Toxicol Appl Pharmcol 127(2):187–198
Hajslova J, Pulkrabova J, Poustka J, Cajka T, Randak T (2007) Brominated flame retardants and related chlorinated persistent organic pollutants in fish from river Elbe and its main tributary Vltava. Chemosphere 67:1195–1203
Harrad S, Smith D (1999) Eel consumption as a pathway of human exposure to PCBs. Int J Environ Health Res 9(1):31–37
Hasselberg L, Meier S, Svardal A (2004) Effects of alkylphenols on redox status in first spawning Atlantic cod (Gadus morhua). Aquat Toxicol 69(1):95–105
Henderson RJ, Tocher DR (1987) The lipid-composition and biochemistry of fresh-water fish. Prog Lipid Res 26(4):281–347
Hibiya T (1992) An atlas of fish histology—normal and pathological features. Kodansha, Tokyo
Hoek-van Nieuwenhuizen M, Kotterman KJJ (2007) (Dutch) Biologische Monitoring Zoete Rijkswateren: Microverontreinigingen in rode aal—2006. Imares report C001/07. IMARES, pp 45
Hoff PT, Van Dongen W, Esmans EL, Blust R, De Coen WM (2003) Evaluation of the toxicological effects of perfluorooctane sulfonic acid in the common carp (Cyprinus carpio). Aquat Toxicol 62:349–359
Hoff PT, Van Campenhout K, Van de Vijver K, Covaci A, Bervoets L, Moens L, Huyskens G, Goemans G, Belpaire C, Blust R, De Coen W (2005) Perfluorooctane sulfonic acid and organohalogen pollutants in liver of three freshwater fish species in Flanders (Belgium): relationships with biochemical and organismal effects. Environ Pollut 137(2):324–333
Holmberg B, Jensen S, Larsson A, Lewander K, Olsson M (1972) Metabolic effects of technical pentachlorophenol (PCP) on the eel Anguilla anguilla L. Comp Biochem Physiol B 43(1):171–183
Hoogenboom LAP, Kotterman KJJ, Hoek-van Nieuwenhuizen M, van der Lee MK, Traag WA (2007) (Dutch) Onderzoek naar dioxines, dioxineachtige PCB’s en indicator-PCB’s in paling uit Nederlandse binnenwateren. 2007.003. RIKILT-Instituut voor Voedselveiligheid, Wageningen, pp 34
Howard TE, McLeay DJ, Walden CC (1971) Sublethal effects of bleached kraft mill effluents to fish. CPAR Project Report 9-2. Pulp and Paper Pollution Abatement, Ottawa, Canada, pp 63
Hu WY, Jones PD, DeCoen W, King L, Fraker P, Newsted J, Giesy JP (2003) Alterations in cell membrane properties caused by perfluorinated compounds. Comp Biochem Physiol C 135(1):77–88
Ibuki Y, Goto R (2002) Phototoxicity of benzo[a]pyrene by ultraviolet a irradiation: induction of apoptosis in Jurkat cells. Environ Toxicol Pharmacol 11(2):101–109
ICES (2002) Report of the ICES/EIFAC Working Group on Eels—ICES Headquarters—28–31 August 2001. ICES CM 2002/ACFM:03. ICES Advisory Committee on Fisheries Management, pp 58
ICES (2006) Report of the 18th session of the Joint EIFAC/ICES Working Group on Eels (WGEEL), Rome (Italy), 23–27 January 2006. ICES CM 2006/ACFM:16; EIFAC Occasional Paper No. 38. ICES Advisory Committee on Fisheries Management, Rome, Italy, pp 122
ICES (2007) Report of the 2007 Session of the Joint EIFAC/ICES Working Group on Eels (WGEEL), Bordeaux (France), 3–7 September 2007. ICES CM 2007/ACFM:23; EIFAC Occasional Paper No. 39. ICES Advisory Committee on Ecosystems, pp 163
ICES (2008) Report of the 2008 session of the Joint EIFAC/ICES Working Group on Eels (WGEEL), Leuven (Belgium), 3–9 September 2008. ICES CM 2008/ACOME:15; EIFAC Occasional Paper No. 43. ICES Advisory Committee on Ecosystems, pp 352
Jancovic M, Mann H (1969) Untersuchungen Uber Die Akute Toxische Wirkung Von Nitrilotriessigsaure (NTA). Arch Fischereiwiss 20(2–3):178–181
Janicki RH, Kinter WB (1971) DDT: disrupted osmoregulatory events in the intestine of the eel Anguilla rostrata adapted to seawater. Science 173(4002):1146–1148
Jha AN (2004) Genotoxicological studies in aquatic organisms: an overview. Mutat Res Fund Mol Mech 552(1–2):1–17
Jimenez BD, Burtis LS (1989) Influence of environmental variables on the hepatic mixed function oxidase system in the bluegill sunfish (Lepomis macrochirus). Comp Biochem Physiol C 93(1):11–21
Jimenez BD, Burtis LS, Ezell GH, Egan BZ, Lee NE, Beauchamp JJ, Mccarthy JF (1988) The mixed-function oxidase system of bluegill sunfish, Lepomis macrochirus—correlation of activities in experimental and wild fish. Environ Toxicol Chem 7(8):623–634
Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP (1998) Widespread sexual disruption in wild fish. Environ Sci Technol 32(17):2498–2506
Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty GC, Sumpter JP, Tyler CR (2002a) Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol Reprod 66:272–281
Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJW, MCAllister BG, Beresford N, Henslaw AC, Brighty GC, Tyler CR, Sumpter JP (2002b) Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol Reprod 67:515–524
Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, Tyler CR, van Aerle R, Santos E, Brighty GC (2006) Predicted exposures to steroid estrogens in UK rivers correlate with widespread sexual disruption in wild fish populations. Environ Health Perspect 114(1):32–39
Kafafi SA, Afeefy HY, Ali AH, Said HK, Kafafi G (1993) Binding of polychlorinated biphenyls to the aryl hydrocarbon receptor. Environ Health Perspect 101:422–428
Kegley SE, Hill BR, Orme S, Choi AH (2008) PAN Pesticide Database Action Network. http://www.pesticideinfo.org. Assessed June 2009
Kinter WB, Merkens LS, Janicki RH, Guarino AM (1972) Studies on the mechanism of toxicity of DDT and polychiorinated biphenyls (PCBs): disruption of osmoregulation in marine fish. Environ Health Perspect 1:169–173
Kleinow KM, Melancon MJ, Lech JJ (1987) Biotransformation and induction—implications for toxicity, bioaccumulation and monitoring of environmental xenobiotics in fish. Environ Health Perspect 71:105–119
Knights B (1997) Risk assessment and management of contamination of eels (Anguilla ssp.) by persistent xenobiotic organochlorine compounds. Chem Ecol 13(3):171–212
Knutzen J, Brevik E, Green N, Biseth A, Egaas E, Schlabach M, Skare JU (1998) (Norwegian) Overvåking av miljøgifter i fisk og skalldyr fra Grenlandsfjordene 1996. (Monitoring of micropollutants in fish and shellfish from the Grenland fjords (S.Norway 1996). OR-3834. Norsk institutt for vannforskning (NIVA), pp 150
Knutzen J, Becher G, Biseth A, Bjerkeng B, Brevik E, Green N, Schlabach M, Skare JU (1999) (Norwegian) Overvåking av miljøgifter i fisk og skalldyr fra Grenlandsfjordene 1997. (Monitoring of micropollutants in fish and shellfish from the Grenlandsfjords (S. Norway) 1997. OR-4065. Norsk institutt for vannforskning (NIVA), pp 195
Knutzen J, Bjerkeng B, Green NW, Kringstad A, Schlabach M, Skare JU (2001) (Norwegian) Overvåking av miljøgifter i fisk og skalldyr fra Grenlandsfjordene (Monitoring of micropollutants in fish and shellfish from Grenlandfjordene) 2000. OR-4452. Norsk institutt for vannforskning (NIVA), pp 230
Krinitz J, Stachel B, Reincke HR (2002) (German) Chlorierte Ether in Wasser- und Fischgewebeproben der Elbe und ihrer Nebenflüsse (1992–2000). Wassergütestelle Elbe, Hamburg, pp 133
Lahnsteiner F, Berger B, Kletzl M, Weismann T (2005) Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat Toxicol 75(3):213–224
Langston WJ, Chesman BS, Burt GR, Pope ND, McEvoy J (2002) Metallothionein in liver of eels Anguilla anguilla from the Thames Estuary: an indicator of environmental quality? Mar Environ Res 53:263–293
Larsson P, Hamrin S, Okla L (1990) Fat content as a factor inducing migratory behaviour in the eel (Anguilla anguilla L.) to the Sargasso Sea. Naturwissenschaften 77:488–490
Larsson P, Hamrin S, Okla L (1991) Factors determining the uptake of persistent pollutants in an eel population (Anguilla anguilla L.). Environ Pollut 69(199):39–50
Lawrence AJ, Elliott M (2003) Introduction and conceptual model. In: Lawrence AJ, Hemingway K (eds) Effects of pollution on fish: molecular effects and population responses. Blackwell, Oxford, pp 1–13
Lindell MJ, Bremie G, Broberg O, Larsson P (2001) Monitoring of persistent organic pollutants (POPs): examples from Lake Vättern, Sweden. Ambio 30(8):545–551
Lionetto MG, Maffia M, Cappello MS, Giordano ME, Storelli C, Schettino T (1998) Effect of cadmium on carbonic anhydrase and Na+ -K+-ATPase in eel, Anguilla anguilla, intestine and gills. Comp Biochem Phys A 120(1):89–91
Livingstone DR, Mitchelmore CL, Peters LD, O’Hara SCM, Shaw JP, Chesman BS, Doyotte A, McEvoy J, Ronisz D, Larsson DGJ, Förlin L (2000) Development of hepatic CYP1A and blood vitellogenin in eel (Anguilla anguilla) for use as biomarkers in the Thames Estuary, UK. Mar Environ Res 50:367–371
Luizi F, Korsgaard B, Petersen IM (1997) Plasma lipoproteins in European eels (Anguilla anguilla): effects of estradiol. Fish Physiol Biochem 16(4):273–280
Maes GE, Volkaert FAM (2007) Challenges for genetic research in European eel management. ICES J Mar Sci 64:1463–1471
Maes GE, Raeymaekers JAM, Pampoulie C, Seynaeve A, Goemans G, Volckaert FAM (2005) The catadromous European eel Anguilla anguilla (L.) as a model for freshwater evolutionary ecotoxicology: relationship between heavy metal bioaccumulation, condition and genetic variability. Aquat Toxicol 72:93–115
Maes J, Goemans G, Belpaire C (2008) Spatial variation and temporal pollution profiles of polychlorinated biphenyls, organochlorine pesticides and heavy metals in European yellow eel (Anguilla anguilla L.) (Flanders, Belgium). Environ Pollut 153:223–237
Maggi P, Cossa D (1973) Relative harmfulness of five anionic detergents in the sea. I. Acute toxicity with regard to fifteen organisms. Rev Trav Inst Peches Marit 37(3):411–417
Mancini L, Caimi S, Ciardullo S, Zeiner M, Bottoni P, Pancioni L, Cataudella S, Caroli S (2005) A pilot study on the contents of selected pollutants in fish from the Tiber River (Rome). Microchem J 79:171–175
Mann H (1973) Effect of borates on fishes and some other organisms of fresh- and brackish water. Arch Fischereiwiss 23(1–3):171–175
Maria VL, Correia AC, Santos MA (2002) Anguilla anguilla L. biochemical and genotoxic responses to benzo[a]pyrene. Ecotoxicol Environ Saf 53(1):86–92
Maria VL, Pacheco M, Santos MA (2006) Anguilla anguilla L. genotoxic responses after in situ exposure to freshwater wetland (Pateira de Fermentelos, Portugal). Environ Int 32(4):510–515
Martel PH, Kovacs TG, O’Connor BI, Voss RH (1994) A survey of pulp and paper-mill effluents for their potential to induce mixed-function oxidase enzyme-activity in fish. Water Res 28(8):1835–1844
Marty GD, Hoffmann A, Okihiro MS, Hepler K, Hanes D (2003) Retrospective analysis: bile hydrocarbons and histopathology of demersal rockfish in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Mar Environ Res 56(5):569–584
Mason CF, Barak NAE (1990) A catchment survey for heavy metals using the eel (Anguilla anguilla). Chemosphere 21(4–5):695–699
McKinney JD, Waller CL (1994) Polychlorinated biphenyls as hormonally active structural analogues. Environ Health Perspect 102(3):290–297
Melancon MJ, Elcombe CR, Vodicnik MJ, Lech JJ (1981) Induction of cytochromes P450 and mixed-function oxidase activity by polychlorinated-biphenyls and beta-naphthoflavone in carp (Cyprinus carpio). Comp Biochem Physiol C 69(2):219–226
Miura C, Takahashi N, Michino F, Miura T (2005) The effect of para-nonylphenol on Japanese eel (Anguilla japonica) spermatogenesis in vitro. Aquat Toxicol 71(2):133–141
Monod G, Devaux A, Riviere JL (1988) Effects of chemical pollution on the activities of hepatic xenobiotic metabolizing enzymes in fish from the River Rhone. Sci Total Environ 73(3):189–201
Mourad MH (1991) Cardiovascular and respiratory changes in eel, Anguilla anguilla L. during exposure to lethal doses of lindane at different water temperatures. Acta Ichthyol Piscat 21(1):73–79
Murk AJ, Van den Berg JHJ, Fellinger MRMJC, Swennen C, Duiven P, Boon JP, Brouwer A, Koeman JH (1994) Toxic and biochemical effects of 3,3′,4,4′-tetrachloroiphenyl (CB77) and Cophen A50 on eider ducklings (Somateria mollissima) in a semi-field experiment. Environ Pollut 86:21–30
Nassour I, Léger CL (1989) Deposition and mobilisation of body fat during sexual maturation in female trout (Salmo gairdneri Richardson). Aquat Living Resour 2:152–159
Neff JM (2009) Concentrations of phenols in tissues of marine organisms. In: Neff JM (ed) Bioaccumulation in marine organisms—effects of contaminants from oil well produced waters. Battelle, pp 209–212
Nevo E, Noy R, Lavie B, Beiles A, Muchtar S (1986) Genetic diversity and resistance to marine pollution. Biol J Linn Soc 29(2):139–144
Nigro M, Frenzilli G, Scarcelli V, Gorbi S, Regoli F (2002) Induction of DNA strand breakage and apoptosis in the eel Anguilla anguilla. Mar Environ Res 54(3–5):517–520
Nogueira PR, Laurenço J, Mendo S, Rotchell JM (2006) Mutation analysis of ras gene in the liver of European eel (Anguilla anguilla L.) exposed to benzo[a]pyrene. Mar Pollut Bull 52(12):5212–5216
Norman A, Rattfelt JJ, Andersson PL, Norrgren L (2007) Reproduction effects in zebrafish exposed to a mixture of structurally diverse brominated flame retardants. Abstract book BFR2007 1–4
Nowell LH, Capel PD, Dileanis PD (1999) Pesticides in stream sediment and aquatic biota: distribution, trends, and governing factors. Lewis, Boca Raton
Oliveira M, Santos MA, Gravato C, Pacheco M (2003) Chromium effects on Anguilla anguilla liver organ culture. Fresen Environ Bull 12(4):349–352
Oliveira M, Serafim A, Bebianno MJ, Pacheco M, Santos MA (2008) European eel (Anguilla anguilla L.) metallothionein, endocrine, metabolic and genotoxic responses to copper exposure. Ecotoxicol Environ Saf 70(1):20–26
Oliveiro Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat Toxicol 74(1):53–69
Orban E, Masci M, Di Lena G, Nevigato T, Casini I, Caproni R, Gabelli L (2004) Nutritional qualità and safety of ells (Anguilla anguilla) from the main freshwater basin of Latium (Italy) as related to the environment and to the season. Int Aliment Italy 438:760–767
Pacheco M, Santos MA (1998) Induction of liver EROD and erythrocytic nuclear abnormalities by cyclophosphamide and PAHs in Anguilla anguilla L. Ecotoxicol Environ Saf 40(1–2):71–76
Pacheco M, Santos MA (1999) Biochemical and genotoxic responses of adult eel (Anguilla anguilla L.) to resin acids and pulp mill effluent: laboratory and field experiments. Ecotoxicol Environ Saf 42(1):81–93
Pacheco M, Santos MA (2001) Tissue distribution and temperature-dependence of Anguilla anguilla L. EROD activity following exposure to model inducers and relationship with plasma cortisol, lactate and glucose levels. Environ Int 26(3):149–155
Pacheco M, Santos MA (2002a) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.). Ecotoxicol Environ Saf 53:331–347
Pacheco M, Santos MA (2002b) Naphthalene and beta-naphthoflavone effects on Anguilla anguilla L. hepatic metabolism and erythrocytic nuclear abnormalities. Environ Int 28(4):285–293
Palíková M, Barus V (2003) Mercury content in Anguillicola crassus (Nematoda) and its host Anguilla anguilla. Acta Vet Brno 72(2):289–294
Palstra AP, van Ginneken VJT, Murk AJ, van den Thillart GEEJM (2006) Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 93(3):145–148
Palstra AP, Curiel D, Fekkes M, de Bakker M, Székely C, van Ginneken V, van den Thillart GEEJM (2007) Swimming stimulates oocyte development in European eel. Aquaculture 270:321–332
Peters LD, Doyotte CL, Mitchelmore JM, Livingstone DR (2001) Seasonal variation and estradiol-dependent elevation of Thames estuary eel Anguilla anguilla plasma vitellogenin levels and comparisons with other United Kingdom estuaries. Sci Total Environ 279:137–150
Pierron F, Baudrimont M, Bossy A, Bourdinaud JP, Brèthes D, Elie P, Massabuau JC (2007a) Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquat Toxicol 81:304–311
Pierron F, Beaudrimont M, Gonzalez P, Bourdinaud JP, Elie P, Massabuau JC (2007b) Common pattern of gene expression in response to hypoxia or cadmium in the gills of the European glass eel (Anguilla anguilla). Environ Sci Technol 41:3003–3005
Pierron F, Beaudrimont M, Dufour S, Elie P, Bossy A, Baloche S, Mesmer-Dudons N, Gonzalez P, Bourdinaud JP, Massabuau JC (2008) How cadmium could compromise the completion of the European eel’s reproductive migration. Environ Sci Technol 42(12):4607–4612
Pieters H, Van Leeuwen SPG, De Boer J (2005) (Dutch) Verontreinigingen in aal en snoekbaars: monitoringprogramma ten behoeve van de Nederlandse sportvisserij 2004. C053/05. Nederlands Instituut voor Visserij Ondezoek (RIVO), IJmuiden, pp 21
Rattfelt JJ, Norman A, Norrgren L, Haglund P, Andersson PL (2008) Maternal transfer of brominated flame retardants in zebrafish (Danio rerio). Chemosphere 73(2):203–208
Regala RP, Rice CD, Schwedler TE, Dorociak IR (2001) The effects of tributyltin (TBT) and 3, 3k4, 4k5-pentachlorobiphenyl (PCB-126) mixtures on antibody responses and phagocyte oxidative burst activity in channel catfish, Ictalurus punctatus. Arch Environ Contam Toxicol 40(3):386–391
Robinet T, Feunteun E (2002) Sublethal effects of exposure to chemical compounds: a cause for the decline in Atlantic eels? Ecotoxicology 11(4):265–277
Roche H, Buet A, Ramade F (2002) Accumulation of lipophilic microcontaminants and biochemical responses in eels from the camargue biosphere reserve. Ecotoxicology 11(3):155–164
Rødsæther MC, Olafsen J, Raa J, Myhre K, Steen JB (1977) Copper as an initiating factor of vibriosis (Vibrio anguillarum) in eel (Anguilla anguilla). J Fish Biol 10(1):17–21
Roosens L, Dirtu A, Goemans G, Belpaire C, Gheorghe A, Neels H, Blust R, Covaci A (2008) Brominated flame retardants and organochlorine contaminants in fish from the Scheldt River, Belgium. Environ Int 37:976–983
Sanchez-Galan S, Linde AR, Ayllon F, Garcia-Vazquez E (2001) Induction of micronuclei in eel (Anguilla anguilla) by heavy metals. Ecotoxicol Environ Saf 49(Environmental research Section B):139–143
Sancho E, Ferrando MD, Andreu E, Gamon M (1992a) Acute toxicity, uptake and clearance of diazinon by the European eel, Anguilla anguilla (L.). J Environ Sci Health B 27(2):209–221
Sancho E, Ferrando MD, Gamon M, AndreuMoliner E (1992b) Organophosphorus diazinon induced toxicity in the fish Anguilla anguilla L. Comp Biochem Physiol C 103(2):351–356
Sancho E, Ferrando MD, Andreu E, Gamon M (1993a) Bioconcentration and excretion of diazinon by eel. Environ Contam Toxicol 50(4):578–585
Sancho E, Ferrando MD, Gamon M, Andreu-Moliner E (1993b) An approach to the diazinon toxicity in the European eel: bioaccumulation studies. Sci Total Environ Suppl 1: 461–468
Sancho E, Ferrando MD, Gamon M, Andreu-Moliner E (1994) Uptake and clearance of diazinon in different tissues of the European eel (Anguilla anguilla L.). Biomed Environ Sci 7(1):41–49
Sancho E, Ferrando MD, Andreu E (1997) Sublethal effects of an organophosphate insecticide on the European eel, Anguilla anguilla. Ecotoxicol Environ Saf 36(1):57–65
Sancho E, Ferrando MD, Andreu E (1998a) Effects of sublethal exposure to a pesticide on levels of energetic compounds in Anguilla anguilla. J Environ Sci Health B 33(4):411–424
Sancho E, Ferrando MD, Fernandez C, Andreu E (1998b) Liver energy metabolism of Anguilla anguilla after exposure to fenitrothion. Ecotoxicol Environ Saf 41(2):168–175
Santillo D, Johnston P, Labunska I, Brigden K (2005) Swimming in Chemicals: Widespread presence of brominated flame retardants and PCBs in eels (Anguilla anguilla) from rivers and lakes in 10 European countries. Technical Note 12/2005/October 2005. Greenpeace, Exeter, pp 56
Santos MA, Hall A (1990) Influence of inorganic lead on the biochemical blood composition of the eel, Anguilla anguilla L. Ecotoxicol Environ Saf 20:7–9
Santos MA, Pires F, Hall A (1990) Metabolic effects of kraft mill effluents on the eel Anguilla anguilla L. Ecotoxicol Environ Saf 20(1):10–19
Schwaiger J, Mallow U, Ferling H, Knoerr S, Braunbeck T, Kalbfus W, Negele RD (2002) How estrogenic is nonylphenol? A transgenerational study using rainbow trout (Oncorhynchus mykiss) as a test organism. Aquat Toxicol 59(3–4):177–189
Shallaja MS, D’Silva C (2003) Evaluation of impact of PAH on a tropical fish, Oreochromis mossambicus using multiple biomarkers. Chemosphere 53(8):835–841
Sivarajah K, Franklin CS, Williams WP (1978) The effects of polychlorinated biphenyls on plasma steroid levels and hepatic microsomal enzymes in fish. J Fish Biol 13(4):401–409
Soontornchat C, Li MH, Cooke PS, Hansen LG (1994) Toxicokinetic and toxicodynamic influences on endocrine disruption by polychlorinated biphenyls. Environ Health Perspect 102(6–7):568–571
Spies RB, Rice DW (1988) Effects of organic contaminants on reproduction of the starry flounder platichthys stellatus in San-Francisco Bay 2. Reproductive success of fish captured in San-Francisco Bay and spawned in the laboratory. Mar Biol 98(2):191–200
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal-ions. Free Radic Biol Med 18(2):321–336
Storelli MM, Barone G, Garofalo R, Marcotrigiano GO (2007) Metals and organochlorine compounds in eel (Anguilla anguilla) from the Lesina lagoon, Adriatic Sea (Italy). Food Chem 100(4):1337–1341
Sures B (2006) How parasitism and pollution affect the physiological homeostasis of aquatic hosts. J Helminthol 80:151–157
Sures B, Knopf K (2004) Individual and combined effects of cadmium and 3,3k,4,4k,5-pentachlorobiphenyl (PCB 126) on the humoral immune response in European eel (Anguilla anguilla) experimentally infected with larvae of Anguillicola crassus (Nematoda). Parasitology 128:445–454
Sures B, Taraschewski H, Jackwerth E (1994) Lead content of Paratenuisentis ambiguus (Acanthocephala), Anguillicola crassus (Nematodes) and their host Anguilla anguilla. Dis Aquat Org 19(2):105–107
Svobodova Z, Vykusova B, Machova J, Hrbkova M, Groch L (1994) The long-term effects of PCBs on fish. In: Müller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Fishing New Books, Blackwell, Oxford, UK, pp 88–89
Tapie N, Budzinski H, Elie P, Gonthier P (2006) (French) Contamination en polychlorobiphenyls (PCB) des anguilles du système fluvio estuarien de la Gironde. UMR 5472 CNRS. Laboratoire de Physico- Toxico-Chimie des Systèmes Naturels (LPTC)—Unité “Ecosystèmes estuariens et poissons migrateurs amphihalins”, CEMAGREF, CESTAS Cedex, TALENCE Cedex, pp 58
Teles M, Pacheco M, Santos MA (2003) Anguilla anguilla L. liver ethoxyresorufin O-deethylation, glutathione S-tranferase, erythrocytic nuclear abnormalities, and endocrine responses to naphthalene and beta-naphthoflavone. Ecotoxicol Environ Saf 55(1):98–107
Teles M, Pacheco M, Santos MA (2005) Physiological and genetic responses of European eel (Anguilla anguilla L.) to short-term chromium or copper exposure—influence of preexposure to a PAH-like compound. Environ Toxicol 20(1):92–99
Teles M, Pacheco M, Santos M (2007) Endocrine of metabolic responses of Anguilla anguilla L. caged in a freshwater wetland (Pateira de Fermentelos, Portugal). Sci Total Environ 372(2–3):562–570
Thomsen JP (1980) Study on the possible association of copper pollution with vibriosis in eel. Nord Vet Med 32(2):90–95
Thurow F (1959) Uber Fängerfrage und Wachstum des Aales in der westlichen Ostsee. Zeit f Fisch 8:597–626
Toivola DM, Isomaa B (1991) Effects of dehydroabietic acid on the erythrocyte-membrane. Chem Biol Interact 79(1):65–78
Tulonen J, Vuorinen PJ (1996) Concentrations of PCB’s and other organochlorine compounds in eels (Anguilla anguilla L.) of the Vanajavesi watercourse in southern Finland, 1990–1993. Sci Total Environ 187(1):11–18
Turner KJ, Sharpe RM (1997) Environmental oestrogens—present understanding. Rev Reprod 2:69–73
Tuurala H, Soivio A (1982) Structural and circulatory changes in the secondary lamellae of Salmo gairdneri gills after sublethal exposures to dehydroabietic acid and zinc. Aquat Toxicol 2(1):21–29
Usero J, Izquierdo D, Morillo J, Gracia I (2003) Heavy metals in fish (Solea vulgaris, Anguilla anguilla and Liza aurata) from salt marshes on the southern Atlantic coast of Spain. Environ Int 29:949–956
Van Campenhout K, Goenaga Infante H, Bervoets L, Goemans G, Belpaire C (2008) A field survey of metal binding to metallothionein and other cytosolic ligands in liver of eels using an on-line isotope dilution method in combination with size exclusion (SE) high pressure liquid chromatography (HPLC) coupled to inductively coupled plasma time-of-flight mass spectrometry (ICP-TOFMS). Sci Total Environ 394(2/3):379–389
van den Thillart GEEJM, Dufour S, Elie P, Volkaert FAM, Sebert P, Rankin JC, Székely C, van Rijsingen J (2005) Estimation of the reproduction capacity of European eel: EELREP. Q5RS-2001-01836, pp 272
van den Thillart GEEJM, van Ginneken V, Korner F, Heijmans R, Van der Linden R, Gluvers A (2004) Endurance swimming of European eel. J Fish Biol 65(2):312–318
van Ginneken VJT, van den Thillart GEEJM (2000) Eel fat stores are enough to reach the Sargasso. Nature 403:156–157
van Ginneken VJT, Balm P, Sommandas V, Onderwater M, Van den Thillart G (2002) Acute stress syndrome of the yellow European eel (Anguilla anguilla Linnaeus) when exposed to a graded swimming-load. Neth J Zool 52(1):29–42
van Ginneken VJT, Ballieux TB, Willemze R, Coldenhoff K, Lentjes E, Antonissen E, Haenen O, van den Thillart GEEJM (2005) Hematology patterns of migrating European eels and the role of EVEX virus. Comp Biochem Physiol C 140:97–102
van Ginneken V, Dufour S, Sbaihi M, Balm P, Noorlander K, de Bakker M, Doornbos J, Palstra AP, Antonissen E (2007) Does a 5500-km swim trial stimulate early sexual maturation in the European eel (Anguilla anguilla L.)? Comp Biochem Physiol A 147(4):1095–1103
van Ginneken VJT, Bruijs M, Murk T, Palstra AP, van den Thillart GEEJM (2009) The effect of PCBs on the spawning migration of European silver eel (Anguilla anguilla L.). In: van den Thillart GEEJM, Dufour S, Rankin JC (eds) Spawning migration of the European eel. Reproduction index, a useful tool for conservation management. Springer, New York, pp 363–384
van Leeuwen SPJ, Traag WA, Hoogenboorn LAP, Booij G, Lohman M, Dao QT, De Boer J (2002) (Dutch) Dioxines, furanen en PCB’s in aal: onderzoek naar wilde aal, gekweekte aal, geïmporteerde en gerookte aal. RVO Rapport nummer C034/02 Project nummer 998-760005-17. Nederlands Instituut voor Visserij Onderzoek (RIVO) BV, pp 34
Van Veld PA, Nacci DE (2008) Toxicity resistance. In: Di Giulio RT, Hinton DE (eds) The toxicology of fishes. CRC Press, Florida, pp 597–641
Versonnen BJ, Goemans G, Belpaire C, Janssen CR (2004) Vitellogenin content in European eel (Anguilla anguilla) in Flanders, Belgium. Environ Pollut 128:363–371
Vollestad LA (1992) Geographic variation in age and length at metamorphosis of maturing European eel: environmental effects and phentotypic plasticity. J Anim Ecol 61(1):42–81
Walton DG, Fancey LL, Green JM, Kiceniuk JW, Penrose WR (1983) Seasonal-changes in aryl-hydrocarbon hydroxylase-activity of a marine fish Tautogolabrus adspersus (Walbaum) with and without petroleum exposure. Comp Biochem Physiol C 76(2):247–253
Whale G, Sheahan D, Matthiessen P (1988) The toxicity of tecnazene, a potato sprouting inhibitor, to fresh-water fauna. Chemosphere 17(6):1205–1217
Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE (2000) Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol 30(4):347–570
Wildish DJ (1974) Lethal response by Atlantic salmon parr to some polyoxyethylated cationic and nonionic surfactants. Water Res 8(7):433–437
Wolkers J, Jorgensen EH, Nijmeijer SM, Witkamp RF (1996) Time-dependent induction of two distinct hepatic cytochrome P4501A catalytic activities at low temperatures in Arctic charr (Salvelinus alpinus) after oral exposure to benzo(a)pyrene. Aquat Toxicol 35(2):127–138
Zhu J-Y, Huang H-Q, Bao XD, Lin Q-M, Cai Z (2006) Acute toxicity profile of cadmium revealed by proteomics in brain tissue of Paralichthys olivaceus: potential role of transferrin in cadmium toxicity. Aquat Toxicol 78:127–135
Zimmerman S, Sures B, Taraschewski H (1999) Experimental studies on lead accumulation in the eel-specific endoparasites Anguillicola crassus (Nematoda) and Paratenuisentis ambiguus (Acanthocephala) as compared with their host, Anguilla anguilla. Arch Environ Contam Toxicol 37:190–195
Acknowledgments
This research was supported by the Flemish Environment Agency and the authors gratefully acknowledge the support of B. Peeters, L. Bervoets, G. Maes and K. Vlietinck. They also want to thank F. Ollevier, F. Volckaert and G. van den Thillart for their comments on the manuscript. We are grateful to I. Lambeens and L. Galle for her assistance with the draft of the tables. We also would like to thank M. Hoffman and three anonymous reviewers for reviewing and providing constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geeraerts, C., Belpaire, C. The effects of contaminants in European eel: a review. Ecotoxicology 19, 239–266 (2010). https://doi.org/10.1007/s10646-009-0424-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0424-0