Abstract
To our knowledge, this is the first report on exploring the interactive effects of various biochars (BCs) and nanomaterials (NMs) on plant growth and bioavailability of trace elements in soil. This study evaluated the bioavailability and toxicity of arsenic (As), lead (Pb), and NMs to cabbage plants. The BCs were produced from rice husk (RB), sewage sludge, and bamboo wood (WB). The BCs at 2.5 and 5% (w w−1), NMs for removing As (NMs-As) and heavy metals (NMs-HM) at 3000 mg kg−1, and multi-walled carbon nanotubes (CNT) at 1000 mg kg−1 were applied in bioassay and incubation experiments (40 days), along with the unamended soil as the control. Results showed that the NMs-As and NMs-HM decreased seed germination at 3 days after sowing; however, their toxicity was eliminated by BCs. Growth parameters of cabbage revealed that the CNT was the most toxic NMs, as it was translocated in root and leaf cells, which was confirmed by transmission electron microscopic images. Bioavailable Pb was reduced by 1.2–3.8-folds in all amended rhizosphere and bulk soils. Amendments of 2.5% WB + NMs-As and 2.5% RB + NMs-As significantly decreased both bioavailable As and Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal and metalloid contamination of soils resulted from anthropogenic activities such as mining industry, waste incineration, and intensive use of sewage sludge, fertilizers and pesticides is threatening agricultural sustainability (Beesley et al. 2011; Rajapaksha et al. 2015; Seneviratne et al. 2017). For instance, trace elements (TEs) including arsenic (As) and lead (Pb) are very toxic to plant, animals, and humans and listed as the priority contaminants by the United States Environmental Protection Agency (USEPA) due to their high toxicity and bioavailability (Ahmad et al. 2012c, 2016; Almaroai et al. 2014a, b; Chaney et al. 2016). Many studies demonstrated that it is necessary to investigate the cost-effective technologies to meet specific remediation needs of the site contaminated with TEs (Beesley et al. 2011; Beiyuan et al. 2017). It is noteworthy that the amendments with high adsorption capacity for contaminants while promoting plant growth in contaminated soil have become essential for soil remediation and restoration strategies (Bernal et al. 2007; Vangronsveld et al. 2009). This risk-based approach is associated with the consequences of bioavailability of contaminant rather than mere reductions of the total concentration of toxic TEs in the soil (Beesley et al. 2011; Moon et al. 2015, 2016).
A great variety of engineered nanomaterials (NMs) has been used in various fields including remediation of heavy metal-contaminated soil (Zhang and Elliott 2006; Awad et al. 2010; Ma et al. 2010; Stefaniuk et al. 2016). The NMs have large surface area per unit mass, thereby increasing their adsorption capacity of in/organic contaminants (Klaine et al. 2008; Awad et al. 2010). Therefore, nano-zerovalent iron, zeolites, metal oxides, carbon materials, and metals have widely been used for soil remediation (Stefaniuk et al. 2016). Carbon nanotubes (CNTs) have demonstrated both positive and negative effects on plant growth, seed germination, and soil microbial community (Khodakovskaya et al. 2009, 2011, 2013). They can disturb soil/plant environmental balance by modifying the fate of TEs in soil or their translocation to plants by diffusing through the cell membrane (Wang et al. 2014; Oleszczuk et al. 2016).
The potential hazard assessment of NMs to plants and possible mechanisms are indeed important to be understood (Rizwan et al. 2017). Application of iron-rich NMs has reduced the ammonium acetate extractable As and Pb in contaminated agricultural soil (Almaroai et al. 2014b), but only few studies have been done (Liang et al. 2017). Hence, the comprehensive assessment of NMs in remediation of soil contaminated with TEs considering their toxicity to soil biological quality, plant growth, and environmental health should be necessary (Awad et al. 2010; Pan and Xing 2012).
Biochar (BC) is known as an optimal soil amendment for maintaining soil fertility and remediating organic/inorganic contaminants (McBeath et al. 2014; Ok et al. 2015; Rajapaksha et al. 2016). The BC typically immobilizes TEs and remediates the contaminated soils (Bandara et al. 2016). However, for plant growth, both negative and positive effects of BCs have been reported (Joseph and Lehmann 2009; Schimmelpfennig and Glaser 2012; Liu et al. 2013; Lehmann et al. 2015). Some of BCs may increase plant growth by improving soil physicochemical and biological properties depending on BC characteristics, soil properties, and plant requirements (Ahmad et al. 2012b; Rizwan et al. 2016; Seneviratne et al. 2017) while others may decrease crop growth and yield by altering acidity, salt contents, and short-term N limitation in soils (Joseph and Lehmann 2009; Van Zwieten et al. 2010; Rajkovich et al. 2012; Clough et al. 2013).
A study reported the minor effects of pecan shells BCs produced at 350 and 600 °C on the uptake of CeO2 NMs by corn, lettuce, soybean, and zucchini crops in the soils amended with 0.5 and 5% BCs, and 500–2000 mg kg−1 NMs (Servin et al. 2017), whereas Xu et al. (2016) found that BC-supported iron phosphate NMs suppressed Cd uptake by cabbage plants, possibly due to Cd phosphate formation. Therefore, it is necessary to evaluate different BCs versus NMs with different functional groups to understand their effects on improving soil quality, enhancing plant growth, and reducing the toxicity of TEs. The objectives of this study were to assess the toxicity of NMs and bioavailability of As and Pb to Chinese cabbage in a contaminated agricultural soil amended with/without BCs in a bioassay test lasting for 40 days. Additionally, the amended soils without cultivation were also incubated to determine the effects of cabbage root growth on the dissolution of As and Pb.
Materials and methods
Materials
The soil contaminated with the TEs (i.e., As and Pb) was collected from the top 30 cm of an agricultural field located in Gongju-si, Chungcheongnam-do, Korea (36°32′66′′N, 127°04′31′′E). The field was located near the Tancheon mine where the vegetable cultivation was banned a few years due to the high contamination with As and Pb (Igalavithana et al. 2017). The collected soil was air-dried and passed through a 2-mm sieve after removing debris. The soil was sandy loam in texture with 80, 9, and 11% of sand, silt, and clay contents, respectively (Table S2). The water-holding capacity of soil was 29.1% while the pH and EC were 4.9 and 0.1 dS m−1, respectively. Exchangeable Ca2+, Mg2+, K+, and Na+ were 28.11, 5.43, 14.9, and 1.31 mg kg−1, respectively.
Biochars produced from rice husk (RB) at 400 °C and sewage sludge (SB) at 500 °C were obtained from commercial Company (DAEWON GSI), Korea, while bamboo wood (WB) biochar pyrolyzed at 500 °C was purchased from Tachibana-banbuu Company, Japan. Characteristics of RB, SB, and WB are shown in Table S1 in Supporting Information (SI). Commercial nanomaterials for As (NMs-As) and heavy metals (NMs-HM) removal from the contaminated soil were obtained from AC Nano™ Nanotechnology Company (AC Environmental Co. Ltd., Canada). Multi-walled carbon nanotubes (CNTs) product was acquired from Hanwha Chemical, Korea (aligned with purity of ~90%). The crystalline compositions of NMs-As and NMs-HM were determined by scanning samples for 2θ ranging from 10 to 80° using a graphite monochromator and Cu Kα radiation (X-ray diffraction (XRD), X’pert PRO MPD, PANalytical, the Netherlands) as described by Ok et al. (2010). The XRD patterns of NMs were indexed using Jade 5.0 Software (Materials Data, Inc, Irvine, CA) (Jade 1999). Hybrid Chinese cabbage (Asia Alpine F1, Brassica rapa L. ssp. pekinensis) seeds were obtained from Jeil Seed & Agricultural Products Co., Ltd., Korea.
Bioassay and incubation experiments
The bioassay test consisted of fourteen amendments each with triplicates. Three different BCs at 2.5 and 5%, RB, SB, and WB, were mixed with soils while NMs-As and NMs-HM at 3000 mg kg−1, as a recommended dose by the AC Nano™ Nanotechnology Company for immobilization of heavy metals in soil, were dispersed by ultrasonication in ultrapure distilled water after 3-h shaking at 120 rpm.
The CNTs at 1000 mg kg−1 were dispersed in 0.5% Arabic gum powder (commercial food grade, Korea) solution by ultrasonication according to the method described by Bandyopadhyaya et al. (2002) to avoid the aggregation of CNTs in distilled water. The level of CNTs was selected according to a previous study by Cañas et al. (2008), who reported no toxicity of CNT at 900 mg L−1 to cabbage plants.
The combinations of the amendments mentioned above were also applied to soils, along with the unamended soil as a control. Specifically, the amended soils in 500-mL plastic beakers (200 g per each) were maintained at 70% water-holding capacity and then incubated at 25 °C for 1 week for equilibrium before planting cabbage seeds (Kim et al. 2015). The seeds of Chinese cabbage were sown in each beaker and germinated in As- and Pb-contaminated soil according to the method described by Miralles et al. (2012). Cabbage was grown in a growth chamber at 24 °C in the dark for 48 h, followed by exposure to light and dark for 16 and 8 h, respectively. In a similar way, an incubation experiment was conducted using the amended soils without cultivation to evaluate the interactive effects of NMs and BCs on As and Pb in a bulk soil (no growing roots). The modified USEPA method (EPA600/3-88-029) was used to evaluate the toxicity of NMs and heavy metals in the soils (Greene et al. 1988; Ahmad et al. 2012b).
Growth parameters
At 3 and 7 days after sowing, the number of germinated seeds counted (when the growing plumules became visible above the soil surface) and recorded for each replicate and then the percentage of germination rate was estimated on average for each treatment (n = 12). Growth parameters of cabbage plants at 40 days after sowing were measured. Specifically, the number of leaves, shoot length, root length, fresh weights of shoot and root, and fresh/dry weights of whole cabbage plants were measured.
Chemical analysis
The soil particle size distribution was determined by the pipette method (Shieldrick and Wang 1993), and water-holding capacity was also measured by the gravimetric method, according to the method described by Veihmeyer and Hendrickson (1931). Soil pH and electrical conductivity (EC) were measured in 1:5 soil-to-water mixture using a pH-EC meter (VERSA STAR Multiparameter, Orion 3 Star, Thermo, USA). Soil was previously characterized by the published study of Igalavithana et al. (2017).
Exchangeable cations were measured by using an inductively coupled plasma spectrometry (ICP-AES, Optima 3100XL, PerkinElmer, USA) after 1 M NH4OAc extraction (Sumner et al. 1996). The initially available form of Pb in soil was extracted with 0.1 M HCl while available As was extracted with 1 M HCl (Usman et al. 2005; Ahmad et al. 2012b). The total concentrations of As and Pb were measured by using an inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 7300 DV, PerkinElmer, USA) after digesting the samples in reverse aqua regia (9 mL 60% HNO3 and 3 mL 37% HCl) and a microwave oven-drying (Mars-X, HP-500 plus, CEM Corp.) at 175 ± 5 °C according to USEPA Method 3051a (USEPA 1995).
Major characteristics of soil are presented in Table S2. At harvest, soils were air-dried, and thereafter, 1.4-g soil was extracted with 20 mL 1 M NH4OAc at pH 7 for 2 h according to the method of Otte et al. (1993) for measuring the exchangeable/bioavailable As and Pb by an ICP-OES.
Transmission electron microscopy of CNT in root and leaf
After 15 days of sowing, the cabbage plants grown in the CNT-amended soil were collected and carefully washed with distilled water. The roots and cotyledonary leaf (vein and midrib areas) were cut into a piece of 1-mm2 area/length using a stainless steel scissor followed by fixing in 4% glutaraldehyde plus 1% paraformaldehyde solution in 0.1 M cacodylate buffer at pH 7.4 for 4 h (Larue et al. 2012). Samples were also dehydrated in series of 50–100% ethanol, embedded in Spurr’s resin and prepared ultrathin sections (80 nm). Finally, ultra-sections were deposited on coated copper grids and observed by an energy-filtering transmission electronic microscope (EF-TEM, LEO912AB, Carl Zeiss, Germany).
Statistics
Variable means were compared by a factorial design with two-way analysis of variance and Tukey’s honestly significant differences test at p < 0.05 (SAS 2004).
Results and discussion
Characterization of soils and NMs
Total As and Pb concentrations in soil were 1940 and 1445 mg kg−1, respectively (Table S2). The available forms of As and Pb extracted by 0.1 M HCl were 10.4 and 105.5 mg kg−1, respectively, while the concentrations of As and Pb extracted by 1 M HCl were 81 and 377.9 mg kg−1, respectively. Based on XRD analysis, the peak characteristics of titanium oxide (anatase: TiO2) and calcium sulfate (Gypsum) were recognized as main components of NMs-As, while NMs-HM consisted of calcium phosphate (fluorapatite) (Fig. S1). The XRD spectrum of NMs-As was similar to calcium titanium oxide NPs (CaTiO3) reported by Purwanto et al. (2008). Specifically, strong diffraction peaks indicating TiO2 and CaSO4.2H2O in NMs-As (Fig. S1a) and Ca4.895(PO4)2.995Cl.23F77(OH).35 in NMs-HM (Fig. S1b) were exhibited from XRD patterns.
Changes in soil pH and EC
The amendments of NMs-HM and BCs increased soil pH by up to 0.1 and 0.4–1.5 units compared to the unamended soils, respectively (Fig. 1). In contrast, the NMs-As decreased soil pH significantly by 6.9% compared to the unamended soil. It is evident that the amendments of RB, SB, and WB at 2.5 or 5% increased soil EC by averages of 1.5-, 1.7-, and 2.0-folds higher than the unamended soils. The amendments of NMs increased soil EC by 2.90-, 1.10-, and 1.14-times for NMs-As, NMs-HM, and CNT compared to the unamended soil, respectively.
Values of a pH and b electrical conductivity (EC) of soils amended with biochars at 2.5 and 5% (RB rice husk biochar, SB sewage sludge biochar, WB bamboo wood biochar), 3000-mg kg−1 nanomaterials for arsenic removal (NMs-As), 3000-mg kg−1 nanomaterials for heavy metals removal (NMs-HM), and 1000-mg kg−1 carbon nanotubes (CNTs) compared to the unamended soil at 40 days after sowing. Different letters above each bar indicate a significant difference at p ≤ 0.05
Results indicated that 5% SB or 5% WB and NMs-As induced salinity stress on cabbage as shown by the highest soil EC values and lowest seed germination rate at 3 days after sowing. The high values of pH and EC of BCs are most likely the main reasons for increasing pH and EC of the amended soils (Table S2). Specifically, the WB had the higher values of pH (10.2) and EC (5.14 dS m−1) than those of RB and SB, thus contributing to higher values of pH and EC in the amended soils.
Seed germination and plant growth
At 3 days after sowing, the germination rates of Chinese cabbage were increased by 57.1, 14.3, and 14.3% in the soils amended with 2.5% RB, 5% RB, and 2.5% WB, respectively, compared to the unamended soil (Table 1). The BCs maintain moisture and improve soil structure (Liu et al. 2013), and this might be the possible reason for enhancing the germination of cabbage due to the improved physicochemical properties of the amended soil. In recent studies, BC as a horticultural growing substrate increased crop growth through maintaining favorable moisture and aeration around the plant root systems (Awad et al. 2017; Kim et al. 2017). On the contrary, the amendments of 2.5% SB, 5% SB, and 5% WB decreased the germination rates by 42.9, 57.1, and 28.6% compared to the unamended soil, respectively.
At the same time, the NMs-As and NMs-HM decreased the cabbage germination rates by 71.4 and 42.9%, respectively, compared to the control. Notably, SB, NMs-As, and NMs-HM posed a short-term toxicity to seeds of Chinese cabbage, and led to delay in the germination rate at 3 days after sowing. At 7 days after sowing, no significant differences in cabbage germination rate were found in the soil amended with BCs or NMs compared to the unamended soil (p > 0.05; Table 1).
The interactive effects of different types of BCs and NPs on germination rate of cabbage are given in Table 2. It is revealed that each BC, applied with NMs-As or NMs-HM, led to eliminating the toxicity of NMs to cabbage, as evident by the no significant differences in germination rate at 3 and 7 days after sowing.
At 40 days after sowing, the mean values of fresh weight and dry weight were 1.5–2.2, 1.4–1.7, and 1.1–1.2 times higher in the soils amended with 2.5% SB, 5% WB, and 5% SB than those of the unamended soil, respectively (Table 1). However, there were no significant differences (p > 0.05) in the number of leaves and root length among the amendments of BCs, NMs, and their combinations compared to the unamended soil.
The amendments of 2.5% SB, 5% WB, and 5% SB led to the significant increases of mean shoot length and shoot fresh weight (p < 0.05) by 1.0–1.5 times compared to the unamended soil. In contrast, the amendments of 2.5% RB and 5% RB significantly decreased shoot length, shoot fresh weight, and whole fresh and dry weights of cabbage seedlings by 11.7–36.8 and 3.2–43.7%, respectively, in comparison with the unamended soil (p < 0.05). The highest mean values of growth parameters were recorded for the soil amended with 2.5% SB. This might be because of soil quality improvement following the addition of SB. Wu et al. (2016) reported that the release of soluble elements from BC could enhance plant growth in the amended soil. This was explained by Bandara et al. (2016) who found that increasing BC application rate decreases the enzyme activities in the serpentine soil.
The application of 2.5% SB, 5% SB, 5% WB, and 5% RB led to a short-term reduction in cabbage seed germination rate and plant growth at 3 days after sowing, which might be due to the presence of toxic phenolic compounds in BCs (Kern et al. 2015). However, the toxicity of these compounds was obviously eliminated at the end of experiment as indicated by the increased growth parameters of cabbage at harvest (Table 1). Kern et al. (2015) reported that the toxic substances in RB (i.e., phenols and furfural) reduced root length of Lepidium sativum. Furthermore, the germination and growth of plants were increased following the removal of these toxic substances by washing RB with acetone/water (Kern et al. 2015). This may explain the presence of short-term toxicity of biochars to root systems of cabbage during the first 3 days after germination.
The characteristics of BC such as pyrolytic temperature and feedstock type are critical factors affecting pH, adsorption capacity, porous structure, surface area, labile C, and ash content, thereby contributing to improved soil physiochemical and biological properties (Ahmad et al. 2014; Frohne et al. 2014; Rinklebe et al. 2016) and the enhanced cabbage growth in current study. A possible mechanism of BC on improving germination and growth of cabbage is assumed to be the improvement of soil physiochemical and biological properties, i.e., increasing water-holding capacity, CEC, plant nutrient availability, and soil aggregation (Joseph and Lehmann 2009; Lee et al. 2015; Ok et al. 2015). Moreover, Atkinson et al. (2010) revealed that BC may enhance the plant growth by increasing the microbial biomass and activity in the amended soil, followed by increasing cations, anions, and plant available nutrient.
Likewise, the amendment of NMs-HM increased the mean values of shoot length, shoot fresh weight, and whole fresh and dry weights significantly by an average of 110% compared to the unamended soil. Conversely, shoot fresh weight, and whole fresh and dry weights were decreased by 9.8–30.5% in the soils amended with NMs-As (Table 1).
Application of SB at 2.5 or 5% reduced phytotoxicity of NMs to cabbage, as indicated by no significant difference in growth parameters of cabbage except 5% SB and NMs (Table 2). The applications of 5% SB + NMs-As and 5% SB + NMs-HM increased whole dry weights by of 126 and 112% higher than the unamended soil, respectively. The 5% WB + NMs-As had the highest mean shoot fresh weight and whole fresh and dry weights compared to the unamended soil. However, the application of RB with NMs had an adverse impact on cabbage growth, as indicated by a significant decrease in shoot fresh weight and both whole fresh and dry weights of cabbage. With the exception of 5% WB + NMs-As, the application of WB at 2.5 or 5% with NMs decreased the shoot fresh weight and whole fresh and dry weights compared to the unamended soil.
The NMs-As is composed of calcium sulfate, which caused a lower soil pH (4.9) than the soil without that (Fig. 1). Besides, the calcium may replace H+ on surfaces of clay minerals and organic matter in the soil amended with NMs-As, causing a decrease in soil pH (Shainberg et al. 1989). It might be possible that the low pH and high EC in the soil amended with NPs-As (containing calcium sulfate) are not favorable for root growth of Chinese cabbage and delayed the germination (Table 1) (Wang et al. 2011). Similarly, the ash content in SB could be a possible reason to induce salinity stress to cabbage roots at the beginning of germination test. For instance, the SB induced salinity by increasing EC in the amended soil compared to the control (Paz-Ferreiro et al. 2012).
Uptake of carbon nanotubes
The TEM images of CNTs in cabbage leaf and root confirmed translocated CNT from the soil to plants by diffusing through the cell membrane (Fig. 2). The CNTs were observed in cell walls of parenchyma cells of cabbage root (Fig. 2a, c) and leaf (Fig. 2e), owing to a gradual cell wall increase. Furthermore, CNTs were appeared in vacuoles and led to a poor cell structure and irregular distribution of chloroplasts in the cytoplasm (Fig. 2b–e). Similar to our findings, a high rate of CNT application may cause the deleterious effects on plants by disruption of membranes or oxidation of proteins (Larue et al. 2012). In this study, the CNT was the most toxic NMs because of their translocation in root and leave, and afterward down to single particles and induced stress-related genes regarding water channel (Wang et al. 2014).
Similar to our findings in Fig. 2, the translocation of CNT was confirmed by Raman spectroscopy and TEM observations through the presence of elongated structures in leaves and roots of wheat and rapeseed plants (Larue et al. 2012). The exposure of CNTs such as single-walled (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) decreased biomass and diversity of soil microorganisms, especially ammonium-oxidizing bacteria (Wang et al. 2014). Besides, the translocated CNTs binding with Pb or As in cabbage plants may be led to a higher toxicity than other NMs, as indicated by the lowest shoot length and fresh/dry weights of cabbage (Table 2).
Bioavailability of As and Pb
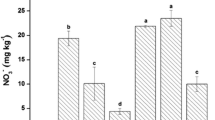
Rhizosphere soils amended with CNT, NMs-As, and RB at 2.5 or 5% had no significant differences in bioavailable As content compared to the unamended soil (p > 0.05; Fig. 3). Except for NMs-As synergistic application with 2.5% RB or 2.5% WB, the bioavailable As in the soil amended with NMs-HM, 5% WB, 2.5% SB, 5% SB, 2.5% SB + NMs-As and 5% SB + NMs-As was increased significantly by 1.5–2.2 times compared to the unamended soil (Fig. 3). Amendments of NMs-As + 2.5% WB and NMs-As + 2.5% RB decreased bioavailable As concentrations significantly by an averaged 140% higher than the unamended soils (p < 0.05). The application of NMs-As decreased the soil pH significantly by 6.9% compared to the unamended soil and decreased dramatically the bioavailable As due to low water-soluble As as reported by Beesley and Marmiroli (2011). The adsorption of Pb and As on surfaces of NMs might be one of the main reasons for a reduction of its toxicity to cabbage in the soil treated with NMs-As. This is confirmed by the high capacity of TiO2 NMs to adsorb Pb from aqueous solution as reported by Deedar and Aslam (2009) and Giammar et al. (2007).
Bioavailable As in the soils amended with biochars at 2.5 and 5% (RB rice husk biochar, SB sewage sludge biochar, WB bamboo wood biochar), 3000-mg kg−1 nanomaterials for arsenic removal (NMs-As), and 3000-mg kg−1 nanomaterials for heavy metals removal (NMs-HM), and 1000-mg kg−1 carbon nanotubes (CNT) compared to the unamended soil at 40 days after sowing. Different letters above each bar indicate a significant difference at p ≤ 0.05
Most of the amendments had no significant changes in bioavailable As concentration compared to the unamended soils (p > 0.05) except for 2.5% WB + NMs-As and 2.5% RB + NMs-As amendments. Bioavailable Pb concentration decreased significantly in all amendments by 1.2–3.8-folds compared to the unamended rhizosphere and bulk soils (p < 0.05; Fig. 4). For instance, the release of phosphate from NMs-HM at higher pH could replace the sorbed As due to the chemical similarity between arsenate and phosphate, contributing to increasing bioavailable As concentration in the amended soil as reported by Hartley et al. (2009). Consequently, the mobility of As in enrich phosphate soil may be explained probably by competitive anion exchange/sorption sites (arsenates and phosphates) besides the formation of soluble organo-As complexes with metal(loid) such as Fe or Mn (Hartley et al. 2009). On the contrary, a high level of phosphate in -HM facilitates insoluble Pb precipitation (e.g., the formation of hydroxypyromorphite) (Cao et al. 2011).
Bioavailable Pb in the soils amended with biochars at 2.5 and 5% (RB rice husk biochar, SB sewage sludge biochar, WB bamboo wood biochar), 3000-mg kg−1 nanomaterials for arsenic removal (NMs-As), and 3000-mg kg−1 nanomaterials for heavy metals removal (NMs-HM), and 1000-mg kg−1 carbon nanotubes (CNTs) compared to the unamended soil at 40 days after sowing. Different letters above each bar indicate a significant difference at p ≤ 0.05
Application of BC protects the plant root system in the presence of toxic compounds by sorption of these compounds onto its surface (Lehmann et al. 2011). A high surface area of BCs derived from wood and sewage sludge may adsorb Pb and mitigate its toxicity (Kim et al. 2015). The formation of stable complexes with Pb on BC surface might be a possible mechanism to reduce the bioavailable Pb in a soil through ligand exchange with hydroxyl functional groups on its surface (Ahmad et al. 2014; Frohne et al. 2014; Rinklebe et al. 2016). An increasing soil pH facilitates the sorption of Pb onto BCs due to the enhanced negative surface charge (Ahmad et al. 2012a, 2014). The relatively high values of pH in the BC-amended soil were associated with the increase in bioavailable As by increasing the net negative charge of soil constituents (Karami et al. 2011; Ahmad et al. 2014; Abid et al. 2016; Rinklebe et al. 2016). In addition, a high soil pH facilitates insoluble Pb precipitation (e.g., the formation of hydroxypyromorphite) (Cao et al. 2011).
The interaction between RB or WB and CNTs decreased the availability of Pb in the soil and increased the availability of As affecting the growth of cabbage adversely. It is well known that a soil pH is one of the key factors influencing concentrations of soil bioavailable/extractable As and Pb after the addition of BCs or/and NMs.
Moon et al. (2016) suggested that formation of Ca–As precipitates and Ca–Pb silicate hydrate (CSHs) as the possible mechanism of As and Pb immobilization in a soil. This may be explained by the immobilization of Pb or As in the soils amended with NMs/BCs or NMs-As (CaTiO2 and CaSO4; possibly CaTiO3 NPs), respectively, in combination with 2.5% RB or 2.5% WB in this study. The applications of BCs and NMs could change the speciation of the TEs in the soil and immobilize Pb in the form of chloropyromorphite as reported in a recent study using X-ray absorption fine structure spectroscopy (Rajapaksha et al. 2015).
The cabbage roots altered As and Pb solubility through the modification of physicochemical and biological soil properties at root interfaces as indicated from higher available metals in rhizosphere soils than the no cultivated soil. Rhizodeposition of protons and organic acids may also decrease soil pH and induce the dissolution of As and immobilization of Pb in the soil (Figs. 3, 4). The interactive effects of BCs and NMs on the bioavailability of Pb and As were highly different between bulk and rhizosphere soils because the cabbage roots create their microenvironment.
Conclusions
Application of NMs-As or NMs-HM posed a short-term toxicity to cabbage and delayed seed germination at 3 days after sowing. The CNTs were the most toxic nanomaterials and translocated in root and leaf cells. The application of NMs-As with 2.5% RB or 2.5% WB decreased bioavailable As and Pb in the soil compared to the unamended soil. In both rhizosphere and bulk soils, the bioavailable Pb was reduced significantly in all amended soils. The adsorption of Pb on surfaces of NMs and BCs was probably the main reason for reducing its toxicity to cabbage. The application of NMs-As led to a low water-soluble As in the amended soils through decreasing pH as reducing As toxicity to cabbage. Amendments of 2.5% WB + NMs-As and 2.5% RB + NMs-As can be recommended to enhance plant growth and immobilize As and Pb in contaminated soils.
Change history
27 December 2017
Unfortunately, in the original publication of the article, Prof. Yong Sik Ok’s affiliation was incorrectly published. The author’s affiliation is as follows.
References
Abid, M., Niazi, N. K., Bibi, I., Farooqi, A., Ok, Y. S., Kunhikrishnan, A., et al. (2016). Arsenic (V) biosorption by charred orange peel in aqueous environments. International Journal of Phytoremediation, 18(5), 442–449.
Ahmad, M., Hashimoto, Y., Moon, D. H., Lee, S. S., & Ok, Y. S. (2012a). Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated mechanistic approach. Journal of Hazardous Materials, 209, 392–401.
Ahmad, M., Lee, S. S., Yang, J. E., Ro, H.-M., Lee, Y. H., & Ok, Y. S. (2012b). Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicology and Environmental Safety, 79, 225–231.
Ahmad, M., Moon, D. H., Lim, K. J., Shope, C. L., Lee, S. S., Usman, A. R., et al. (2012c). An assessment of the utilization of waste resources for the immobilization of Pb and Cu in the soil from a Korean military shooting range. Environmental Earth Sciences, 67(4), 1023–1031.
Ahmad, M., Ok, Y. S., Rajapaksha, A. U., Lim, J. E., Kim, B.-Y., Ahn, J.-H., et al. (2016). Lead and copper immobilization in a shooting range soil using soybean stover-and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. Journal of Hazardous Materials, 301, 179–186.
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., et al. (2014). Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99, 19–33.
Almaroai, Y. A., Usman, A. R., Ahmad, M., Moon, D. H., Cho, J.-S., Joo, Y. K., et al. (2014a). Effects of biochar, cow bone, and eggshell on Pb availability to maize in contaminated soil irrigated with saline water. Environmental Earth Sciences, 71(3), 1289–1296.
Almaroai, Y. A., Vithanage, M., Rajapaksha, A. U., Lee, S. S., Dou, X., Lee, Y. H., et al. (2014b). Natural and synthesised iron-rich amendments for As and Pb immobilisation in agricultural soil. Chemistry and Ecology, 30(3), 267–279.
Atkinson, C. J., Fitzgerald, J. D., & Hipps, N. A. (2010). Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant and Soil, 337(1–2), 1–18.
Awad, Y. M., Abdelhafez, A. A., Ahmad, M., Lee, S.-S., Kim, R.-Y., Sung, J.-K., et al. (2010). Synthesis of nanoscale zerovalent iron particle and its application to Cr(VI) removal from aqueous solutions. Korean Journal of Environmental Agriculture, 29(4), 402–407.
Awad, Y. M., Lee, S. E., Ahmed, M. B. M., Vu, N. T., Farooq, M., Kim, I. S., et al. (2017). Biochar, a potential hydroponic growth substrate, enhances the nutritional status and growth of leafy vegetables. Journal of Cleaner Production, 156, 581–588.
Bandara, T., Herath, I., Kumarathilaka, P., Hseu, Z.-Y., Ok, Y. S., & Vithanage, M. (2016). Efficacy of woody biomass and biochar for alleviating heavy metal bioavailability in serpentine soil. Environmental Geochemistry and Health, 39(2), 391–401.
Bandyopadhyaya, R., Nativ-Roth, E., Regev, O., & Yerushalmi-Rozen, R. (2002). Stabilization of individual carbon nanotubes in aqueous solutions. Nano Letters, 2(1), 25–28.
Beesley, L., & Marmiroli, M. (2011). The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environmental Pollution, 159(2), 474–480.
Beesley, L., Moreno-Jiménez, E., Gomez-Eyles, J. L., Harris, E., Robinson, B., & Sizmur, T. (2011). A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environmental Pollution, 159(12), 3269–3282.
Beiyuan, J., Awad, Y. M., Beckers, F., Tsang, D. C., Ok, Y. S., & Rinklebe, J. (2017). Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere, 178, 110–118.
Bernal, M., Clemente, R., & Walker, D. (2007). The role of organic amendments in the bioremediation of heavy metal-polluted soils. In A. B. Gore (Ed.), Environmental research at the leading edge (pp. 1–57). New York: Nova Science Publisher, Inc.
Cañas, J. E., Long, M., Nations, S., Vadan, R., Dai, L., Luo, M., et al. (2008). Effects of functionalized and nonfunctionalized single‐walled carbon nanotubes on root elongation of select crop species. Environmental Toxicology and Chemistry, 27(9), 1922–1931.
Cao, X., Ma, L., Liang, Y., Gao, B., & Harris, W. (2011). Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environmental Science & Technology, 45(11), 4884–4889.
Chaney, R. L., Kim, W. I., Kunhikrishnan, A., Yang, J. E., & Ok, Y. S. (2016). Integrated management strategies for arsenic and cadmium in rice paddy environments. Geoderma, 270, 1–2.
Clough, T. J., Condron, L. M., Kammann, C., & Müller, C. (2013). A review of biochar and soil nitrogen dynamics. Agronomy, 3(2), 275–293.
Deedar, N., & Aslam, I. (2009). Evaluation of the adsorption potential of titanium dioxide nanoparticles for arsenic removal. Journal of Environmental Sciences, 21(3), 402–408.
Frohne, T., Rinklebe, J., & Diaz-Bone, R. A. (2014). Contamination of floodplain soils along the Wupper River, Germany, with As Co, Cu, Ni, Sb, and Zn and the impact of pre-definite redox variations on the mobility of these elements. Soil and Sediment Contamination: An International Journal, 23(7), 779–799.
Giammar, D. E., Maus, C. J., & Xie, L. (2007). Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environmental Engineering Science, 24(1), 85–95.
Greene, J., Bartels, C., Warren-Hicks, W., Parkhurst, B., & Linder, G. (1988). Protocols for short-term toxicity screening of hazardous-waste sites. Environmental Protection Agency, Corvallis, OR (USA). New York: Environmental Research Lab.
Hartley, W., Dickinson, N. M., Riby, P., & Lepp, N. W. (2009). Arsenic mobility in brownfield soils amended with green waste compost or biochar and planted with Miscanthus. Environmental Pollution, 157(10), 2654–2662.
Igalavithana, A. D., Lee, S.-E., Lee, Y. H., Tsang, D. C., Rinklebe, J., Kwon, E. E., et al. (2017). Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere, 174, 593–603.
Jade 5. (1999). XRD Pattern Processing. Irvine, CA: Materials Data Inc.
Joseph, S., & Lehmann, J. (2009). Biochar for environmental management: Science and technology. London: Earthscan.
Karami, N., Clemente, R., Moreno-Jiménez, E., Lepp, N. W., & Beesley, L. (2011). Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. Journal of Hazardous Materials, 191(1), 41–48.
Kern, J., Mukhina, I., Dicke, C., Lanza, G., & Kalderis, D. (2015). Effects of rice husks and their chars from hydrothermal carbonization on the germination rate and root length of Lepidium sativum. In EGU general assembly conference abstracts, 2015 (Vol. 17, p. 10002).
Khodakovskaya, M., Dervishi, E., Mahmood, M., Xu, Y., Li, Z., Watanabe, F., et al. (2009). Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano, 3(10), 3221–3227.
Khodakovskaya, M. V., de Silva, K., Nedosekin, D. A., Dervishi, E., Biris, A. S., Shashkov, E. V., et al. (2011). Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proceedings of the National Academy of Sciences, 108(3), 1028–1033.
Khodakovskaya, M. V., Kim, B. S., Kim, J. N., Alimohammadi, M., Dervishi, E., Mustafa, T., et al. (2013). Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small, 9(1), 115–123.
Kim, H.-S., Kim, K.-R., Kim, H.-J., Yoon, J.-H., Yang, J. E., Ok, Y. S., et al. (2015). Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environmental Earth Sciences, 74(2), 1249–1259.
Kim, H. S., Kim, K. R., Yang, J. E., Ok, Y. S., Kim, W. I., Kunhikrishnan, A., et al. (2017). Amelioration of horticultural growing media properties through rice hull biochar incorporation. Waste and Biomass Valorization, 8(2), 483–492.
Klaine, S. J., Alvarez, P. J., Batley, G. E., Fernandes, T. F., Handy, R. D., Lyon, D. Y., et al. (2008). Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environmental Toxicology and Chemistry, 27(9), 1825–1851.
Larue, C., Pinault, M., Czarny, B., Georgin, D., Jaillard, D., Bendiab, N., et al. (2012). Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. Journal of Hazardous Materials, 227, 155–163.
Lee, S. S., Shah, H. S., Awad, Y. M., Kumar, S., & Ok, Y. S. (2015). Synergy effects of biochar and polyacrylamide on plants growth and soil erosion control. Environmental Earth Sciences, 74(3), 2463–2473.
Lehmann, J., Kuzyakov, Y., Pan, G., & Ok, Y. S. (2015). Biochars and the plant-soil interface. Plant and Soil, 395, 1–5.
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., & Crowley, D. (2011). Biochar effects on soil biota—A review. Soil Biology and Biochemistry, 43(9), 1812–1836.
Liang, S.-X., Jin, Y., Liu, W., Li, X., Shen, S.-G., & Ding, L. (2017). Feasibility of Pb phytoextraction using nano-materials assisted ryegrass: Results of a one-year field-scale experiment. Journal of Environmental Management, 190, 170–175.
Liu, X., Zhang, A., Ji, C., Joseph, S., Bian, R., Li, L., et al. (2013). Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant and Soil, 373(1–2), 583–594.
Ma, X., Geiser-Lee, J., Deng, Y., & Kolmakov, A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Science of the Total Environment, 408(16), 3053–3061.
McBeath, A. V., Smernik, R. J., Krull, E. S., & Lehmann, J. (2014). The influence of feedstock and production temperature on biochar carbon chemistry: A solid-state 13C NMR study. Biomass and Bioenergy, 60, 121–129.
Miralles, P., Church, T. L., & Harris, A. T. (2012). Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environmental Science and Technology, 46(17), 9224–9239.
Moon, D. H., Cheong, K. H., Koutsospyros, A., Chang, Y.-Y., Hyun, S., Ok, Y. S., et al. (2016). Assessment of waste oyster shells and coal mine drainage sludge for the stabilization of As-, Pb-, and Cu-contaminated soil. Environmental Science and Pollution Research, 23(3), 2362–2370.
Moon, D. H., Wazne, M., Cheong, K. H., Chang, Y.-Y., Baek, K., Ok, Y. S., et al. (2015). Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environmental Science and Pollution Research, 22(14), 11162–11169.
Ok, Y. S., Chang, S. X., Gao, B., & Chung, H.-J. (2015). SMART biochar technology—A shifting paradigm towards advanced materials and healthcare research. Environmental Technology and Innovation, 4, 206–209.
Ok, Y. S., Oh, S. E., Ahmad, M., Hyun, S., Kim, K. R., Moon, D. H., et al. (2010). Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environmental Earth Sciences, 61(6), 1301–1308.
Oleszczuk, P., Ćwikła-Bundyra, W., Bogusz, A., Skwarek, E., & Ok, Y. S. (2016). Characterization of nanoparticles of biochars from different biomass. Journal of Analytical and Applied Pyrolysis, 121, 165–172.
Otte, M., Haarsma, M., Broekman, R., & Rozema, J. (1993). Relation between heavy metal concentrations in salt marsh plants and soil. Environmental Pollution, 82(1), 13–22.
Pan, B., & Xing, B. (2012). Applications and implications of manufactured nanoparticles in soils: A review. European Journal of Soil Science, 63(4), 437–456.
Paz-Ferreiro, J., Gascó, G., Gutiérrez, B., & Méndez, A. (2012). Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biology and Fertility of Soils, 48(5), 511–517.
Purwanto, A., Hidayat, D., Terashi, Y., & Okuyama, K. (2008). Synthesis of monophasic Ca x Ba (1 − x) TiO3 nanoparticles with high Ca content (x > 23%) and their photoluminescence properties. Chemistry of Materials, 20(24), 7440–7446.
Rajapaksha, A. U., Ahmad, M., Vithanage, M., Kim, K.-R., Chang, J. Y., Lee, S. S., et al. (2015). The role of biochar, natural iron oxides, and nanomaterials as soil amendments for immobilizing metals in shooting range soil. Environmental Geochemistry and Health, 37(6), 931–942.
Rajapaksha, A. U., Chen, S. S., Tsang, D. C., Zhang, M., Vithanage, M., Mandal, S., et al. (2016). Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere, 148, 276–291.
Rajkovich, S., Enders, A., Hanley, K., Hyland, C., Zimmerman, A. R., & Lehmann, J. (2012). Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biology and Fertility of Soils, 48(3), 271–284.
Rinklebe, J., Shaheen, S. M., & Frohne, T. (2016). Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere, 142, 41–47.
Rizwan, M., Ali, S., Qayyum, M. F., Ibrahim, M., Zia-ur-Rehman, M., Abbas, T., et al. (2016). Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environmental Science and Pollution Research, 23(3), 2230–2248.
Rizwan, M., Ali, S., Qayyum, M. F., Ok, Y. S., Adrees, M., Ibrahim, M., et al. (2017). Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. Journal of Hazardous Materials, 322, 2–16.
SAS. (2004). SAS/STAT user’s guide, version 9.1. Cary, NC: SAS Institute Inc.
Schimmelpfennig, S., & Glaser, B. (2012). One step forward toward characterization: Some important material properties to distinguish biochars. Journal of Environmental Quality, 41(4), 1001–1013.
Seneviratne, M., Weerasundara, L., Ok, Y. S., Rinklebe, J., & Vithanage, M. (2017). Phytotoxicity attenuation in Vigna radiata under heavy metal stress at the presence of biochar and N fixing bacteria. Journal of Environmental Management, 186, 293–300.
Servin, A. D., De la Torre-Roche, R., Castillo-Michel, H., Pagano, L., Hawthorne, J., Musante, C., et al. (2017). Exposure of agricultural crops to nanoparticle CeO2 in biochar-amended soil. Plant Physiology and Biochemistry, 110, 147–157.
Shainberg, I., Sumner, M., Miller, W., Farina, M., Pavan, M., & Fey, M. (1989). Use of gypsum on soils: A review. In B. A. Stewart (Ed.), Advances in soil science (pp. 1–111). Berlin: Springer.
Shieldrick, B., & Wang, C. (1993). Particle size distribution. Soil sampling and methods of analysis. In M. R. Carter (Ed.), Canadian society of soil science (pp. 499–513). Boca Raton, FL: Lewis Publishers.
Stefaniuk, M., Oleszczuk, P., & Ok, Y. S. (2016). Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chemical Engineering Journal, 287, 618–632.
Sumner, M., Miller, W., Sparks, D., Page, A., Helmke, P., Loeppert, R., et al. (1996). Cation exchange capacity and exchange coefficients. In R. S. Swift & D. L. Sparks (Eds.), Methods of soil analysis. Part 3-chemical methods. Volume 5 of Soil Science Society of America books series (pp. 1201–1229). Madison, WI: Soil Science Society of America, American Society of Agronomy.
USEPA. (1995). Method 3051: Microwave assisted acid digestion of siliceous and organically based matrices. In: SW-846 (3rd Eds.), Test methods for evaluating solid waste: Physical/chemical methods, update III, 1995 (pp. 122–142). Washington, DC: United States Environmental Protection Agency.
Usman, A., Kuzyakov, Y., & Stahr, K. (2005). Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. Journal of Soils and Sediments, 5(4), 245–252.
Van Zwieten, L., Kimber, S., Morris, S., Chan, K., Downie, A., Rust, J., et al. (2010). Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant and Soil, 327(1–2), 235–246.
Vangronsveld, J., Herzig, R., Weyens, N., Boulet, J., Adriaensen, K., Ruttens, A., et al. (2009). Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environmental Science and Pollution Research, 16(7), 765–794.
Veihmeyer, F., & Hendrickson, A. (1931). The moisture equivalent as a measure of the field capacity of soils. Soil Science, 32(3), 181–194.
Wang, C., Liu, H., Chen, J., Tian, Y., Shi, J., Li, D., et al. (2014). Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. Journal of Hazardous Materials, 274, 404–412.
Wang, W.-J., He, H.-S., Zu, Y.-G., Guan, Y., Liu, Z.-G., Zhang, Z.-H., et al. (2011). Addition of HPMA affects seed germination, plant growth and properties of heavy saline-alkali soil in northeastern China: Comparison with other agents and determination of the mechanism. Plant and Soil, 339(1–2), 177–191.
Wu, H., Che, X., Ding, Z., Hu, X., Creamer, A. E., Chen, H., et al. (2016). Release of soluble elements from biochars derived from various biomass feedstocks. Environmental Science and Pollution Research, 23(2), 1905–1915.
Xu, Y., Fang, Z., & Tsang, E. P. (2016). In situ immobilization of cadmium in soil by stabilized biochar-supported iron phosphate nanoparticles. Environmental Science and Pollution Research, 23(19), 19164–19172.
Zhang, W. X., & Elliott, D. W. (2006). Applications of iron nanoparticles for groundwater remediation. Remediation Journal, 16(2), 7–21.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (NRF-2015R1A2A2A11001432).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Awad, Y.M., Vithanage, M., Niazi, N.K. et al. Potential toxicity of trace elements and nanomaterials to Chinese cabbage in arsenic- and lead-contaminated soil amended with biochars. Environ Geochem Health 41, 1777–1791 (2019). https://doi.org/10.1007/s10653-017-9989-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-9989-3