Abstract
In this study, As-, Pb-, and Cu-contaminated soil was stabilized using calcined oyster shells (COS) and steel slag (SS). The As-contaminated soil was obtained from a timber mill site where chromate copper arsenate (CCA) was used as a preservative. On the other hand, Pb- and Cu-contaminated soil was obtained from a firing range. These two soils were thoroughly mixed to represent As-, Pb-, and Cu-contaminated soil. Calcined oyster shells were obtained by treating waste oyster shells at a high temperature using the calcination process. The effectiveness of stabilization was evaluated by 1-N HCl extraction for As and 0.1-N HCl extraction for Pb and Cu. The treatment results showed that As, Pb, and Cu leachability were significantly reduced upon the combination treatment of COS and SS. The sole treatment of SS (10 wt%) did not show effective stabilization. However, the combination treatment of COS and SS showed a significant reduction in As, Pb, and Cu leachability. The best stabilization results were obtained from the combination treatment of 15 wt% COS and 10 wt% SS. The SEM-EDX results suggested that the effective stabilization of As was most probably achieved by the formation of Ca-As and Fe-As precipitates. In the case of Pb and Cu, stabilization was most probably associated with the formation of pozzolanic reaction products such as CSHs and CAHs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is very toxic and carcinogenic to humans and hazardous to the environment. Arsenic occurs naturally in the environment, but it is also present in the environment as the result of industrial activities. The examples of various As sources can be mining, smelting, agriculture, accidents, preservation of wood, and illegal waste dumping. Among the sources of As contamination, chromate copper arsenate (CCA) is one of the most important anthropogenic As sources. CCA, which consists of CrO3, CuO, and As2O5 (Milton 1995), had been used worldwide to protect wood from rotting caused by insects and microbial agents (Warner and Solomon 1990; Chirenje et al. 2003; Saxe et al. 2007). However, CCA use was banned around the world due to its toxicity. In the USA, CCA has been used for the past 15 years, but its use was prohibited in January 2004 (Gezer et al. 2005). Also, the use of As2O5 for wood and the use of any mixture containing As2O5 at 0.1 wt% was banned in the Republic of Korea in October 2007 (Koo et al. 2008). Even though the use of CCA was forbidden, facilities where CCA had been used in the past still have problems with As leaching to the soil and groundwater. Therefore, remedial action is needed for As-contaminated soil adjacent to the facilities where CCA had been used.
On the other hand, lead (Pb) and copper (Cu) contamination in firing range soil is common and can cause serious problems to human beings due to their toxicity. There are 1700 small arms firing ranges in the Republic of Korea, and the ranges are significantly contaminated with Pb and Cu. The military-grade bullets are typically made of Pb alloy slugs enclosed in a Cu alloy jacket (Dermatas et al. 2004). Therefore, substantial amounts of Pb and Cu could build up in shooting range soils during continuous range operations, and remedial action will eventually be needed to prevent exposure to humans.
The stabilization/solidification (S/S) process which is widely used to immobilize heavy metals in contaminated soils (Ok et al. 2011a; Ok et al. 2011b; Ahmad et al. 2012) was applied in this study to stabilize As-, Pb-, and Cu-contaminated soil. Diverse S/S agents such as hydrated lime, quicklime, fly ash, Portland cement, and cement kiln dust have been widely used (Wang and Vipulanandan 1996; Li et al. 2001; Moon et al. 2004; Moon et al. 2006; Moon and Dermatas 2006; Moon and Dermatas 2007; Qiao et al. 2007; Moon et al. 2008; Moon et al. 2009; Moon et al. 2010; Yoon et al. 2010). However, the use of natural waste is very limited. Moreover, the use of natural waste over industrial waste has benefits in terms of cost effectiveness and minimal environmental impact.
In this study, oyster shells were used as the main stabilizing agent in their calcined state. Approximately 250,000 tons of waste oyster shells are produced annually in the Tong-young, Keoje, and Kosung areas in the Republic of Korea. About 50 and 10 % of these shells are used for seeding oyster beds and for fertilizer, respectively (Lee et al. 2005). However, the remaining 40 % of waste oyster shells are dumped in coastal areas, which can cause serious odor problems and environmental degradation (Lee et al. 2005). Therefore, the recycling of waste oyster shells has received great attention (Lee et al. 2008) for the potential of solving two environmental problems simultaneously. Moreover, steel slag (SS) was used as a secondary stabilizing agent. The SS is produced during the separation of the molten steel from impurities in furnaces. The molten slag is allowed to cool and then it is processed through a conventional aggregate crushing and screening operation. SS generation in the Republic of Korea increases annually by 3 %. Approximately 10 million tons of SS was generated in 2011. About 40 % of the SS is recycled as soil aggregate, and 26.3 % of SS is recycled as road construction aggregate (Choi 2012).
The purpose of this study was to evaluate the feasibility of the use of calcined oyster shells (COS) and SS for the immobilization of As, Pb, and Cu in contaminated soil. The calcination process of the waste oyster shells was conducted at a high temperature (900 °C for 2 h) to activate quicklime from the calcite. The effectiveness of the stabilization treatment was evaluated based on the 1 N HCl for As and 0.1-N HCl extraction method for Pb and Cu in accordance with the Korean Standard Test methods (MOE 2002). These test methods are used for the evaluation of disposal or reuse criteria for heavy-metal-contaminated soils in the Republic of Korea (MOE 2002). The mechanism responsible for As, Pb, and Cu immobilization was investigated by scanning electron microscopy (SEM)-energy dispersive X-ray spectroscopy (EDX) analyses.
Experimental methodology
Contaminated soil
The CCA-contaminated site and army firing range site are located in Busan Metropolitan City, Republic of Korea. The contaminated soil samples were obtained from both sites at a depth of 0 ∼ 30 cm from the soil surface. The collected soils were air-dried and passed through a #10 mesh (2 mm) to remove large particles such as cobbles and gravel. The composite soil sample was made by mixing the shooting range soil and CCA-contaminated soil at a ratio of 4:1 to represent As, Pb, and Cu contamination. The total As, Pb, and Cu concentrations in the soil were approximately 189, 8588, and 719 mg/kg, respectively, using aqua regia (1 ml of HNO3 (65 %, Merck) and 3 ml of HCl (37 %, J.T. Baker)). The As-, Pb-, and Cu-contaminated soil was classified as silty clay loam using a particle size analyzer (PSA) in accordance with the United States Department of Agriculture (USDA). The soil pH was approximately 7.64 in accordance with ASTM method D 4980–89. The physicochemical and mineralogical properties and total concentrations of heavy metals in the soil are presented in Table 1. The bulk chemistry of the As-, Pb- and Cu-contaminated soils that was measured by using X-ray fluorescence (XRF, ZSX100e, Rigaku, Japan) is presented in Table 2.
Stabilizing agents
The waste oyster shells (WOS) were obtained from Tong-young City in the Republic of Korea. WOS were first pulverized using a hammer mill, and the pulverized WOS were then powdered using a ball mill to obtain a fine and homogeneous powder that passes through the #20 sieve (0.85 mm). The WOS was then heated at 900 °C for 2 h in an electric furnace (J-FM3, JISICO, Republic of Korea) to activate the quicklime (CaO) in the WOS. The calcined WOS was designated calcined oyster shells (COS). The steel slag (SS) was collected from the POSCO company and sieved using a #4 sieve (opening size 4.76 mm). The major chemical compositions of the two stabilizing agents determined by X-ray fluorescence are presented in Table 2. The major element in the COS sample was calcium at 78.9 wt% as CaO, whereas the SS consisted mainly of calcium at 31.2 wt% as CaO and iron at 38.2 wt% as Fe2O3 (Table 2).
Stabilization treatments
The As-, Cu-, and Pb-contaminated composite soil sample was treated with a range of 10 to 15 wt% COS and SS with a range of 5 to 10 wt%. In order to enable full hydration of the samples, 20 % deionized water was added to the samples. All treated samples were cured for 28 days. The specific treatment conditions based on the percent binder/soil ratio (dry basis) are presented in Table 3. Following treatment, the effectiveness of the treatment was evaluated using 1-N HCl extraction fluid for As and 0.1-N HCl extraction fluid for Pb and Cu, respectively, for the SS fraction passing the #10 sieve (opening size 2 mm) in accordance with the Korean Standard Test methods (MOE 2002). The details regarding the extraction procedures in the KST methods are explained in previous publications (Yoon et al. 2010; Lee et al. 2013).
SEM-EDX analyses
Prior to SEM analyses, air-dried subsamples of treated samples were prepared using double-sided carbon tape and coated with platinum (Pt). SEM analyses were performed using a Hitachi S-4800 SEM instrument equipped with an (ISIS 310 EDX) system.
Results and discussion
Effectiveness of the stabilization treatment
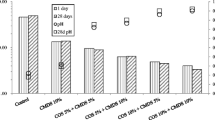
The stabilization treatment results are presented in Figs. 1, 2 and 3. As, Pb, and Cu concentrations decreased with increasing COS and SS dosages. The sole treatment of SS was not effective in reducing As, Pb, and Cu leachability, whereas the COS treatment was only partially successful. The inclusion of the SS in the treatment is expected to provide the iron necessary for the immobilization of As (Drahota and Filippi 2009). The use of SS was also reported to enhance the cementitious reactions responsible for the encapsulation of many contaminants (Zhu et al. 2013). The combination treatment was attempted here because SS and COS could complement each other. Taylor (1997) reported that when slag is placed in water alone, it dissolves to small extent, but a protective film deficient in Ca quickly forms and inhibits further reaction. But, the reaction continues if pH is kept sufficiently high. Hence, it is expected that COS would provide the high pH environment, with its rich CaO content, whereas the SS would provide the iron necessary to immobilize As, in addition to ameliorating the cementitious reactions responsible for the encapsulation of all contaminants.
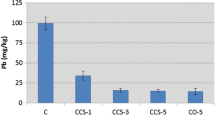
The lowest As, Pb, and Cu concentrations were attained in the sample treated with 15 % COS and 10 % SS. Specifically, an approximate 71 % reduction in As leachability was obtained upon 15 % COS and 10 % SS treatment. In the case of Pb and Cu leachability, a reduction of more than 99 and 98 % in Pb and Cu leachability, respectively, was obtained from the 15 % COS and 10 % SS treatment. The smaller reduction rate observed in As leachability as compared to the level of Pb and Cu leachability could be partly due to the strong acidity of the 1-N HCl extraction fluid as compared to that of the 0.1-N HCl extraction fluid.
It appears that the acidity of the 0.1-N HCl solution was exhausted for the 10 % COS and 5 % SS treatment and resulted in a high pH value of 12.04 where a significant reduction in Pb and Cu leachability was attained. This shows that the combination treatment of 10 % COS and 5 % SS was sufficient enough to significantly decrease Pb (99 % reduction) and Cu leachability (96 % reduction). In the case of As immobilization, the combination treatment of 15 % COS and 5 % SS was needed in order to achieve a significant reduction of As leachability (68.5 % reduction) upon 1-N HCl extraction. As compared to Pb and Cu immobilization, an additional of 5 % COS was needed in order to achieve effective immobilization, most likely due to the strong acidity of 1-N HCl extraction fluid. The addition of 10 % SS to 10 % COS was more effective in immobilizing As, when compared to the 10 % COS and 5 % SS sample. This may indicate that the additional 5 % SS played a role in reducing As leachability. However, the role of an additional 5 % SS was limited in the presence of 15 % COS, which indicates that As leachability was reduced to the maximum extent possible with the 15 % COS and 5 % SS treatment.
It is quite apparent that neither sorption of As at low pH nor precipitation of Cu and Pb as oxides at high pH alone are responsible for the immobilization of As, Cu, and Pb. Even though sorption of As (V) anion is favorable at low pH, however, the oxides responsible for the As uptake are not expected to be stable at the extreme low pH environment. Similarly, Pb is not highly insoluble under hyper alkaline conditions because of the formation of the anionic Pb hydroxide complexes at high pH. Therefore, As, Pb, and most likely Cu are expected to be partially encapsulated in pozzolanic products or incorporated into stable phases at low pH. Moreover, Pb is reported to be sequestered into Pb-Si-O compounds at high alkaline pH values (Rose et al. 2000).
SEM-EDX analyses
In order to investigate the mechanism responsible for effective As, Pb, and Cu immobilization, the sample treated with 10 wt% COS and 5 % SS was analyzed by SEM-EDX because this sample resulted in the significant reduction as compared to the sole treatment of SS in As, Pb, and Cu leachability tested by the 1-N HCl and 0.1-N extraction fluids.
The SEM-EDX results are presented in Fig. 4. Figure 4a shows the specific particles in the sample where As was clearly observed. Elemental dot map results indicated that effective As immobilization was strongly associated with Ca, Fe, and O (Fig. 4a). This suggests that Ca-As and Fe-As precipitates are the most probable compounds responsible for effective As immobilization, which is in line with the discussion above. It has been reported that various Ca-As precipitates were found to be the main compounds responsible for As immobilization by numerous researchers upon lime treatment (Dutré and Vandecasteele 1996; Dutré and Vandecasteele 1998; Moon et al. 2004; Moon et al. 2011b). Moreover, Fe-As precipitates which could be responsible for effective As immobilization have been widely reported (Chung et al. 2001; Kim et al. 2003; Lee 2006; Kumpiene et al. 2008; Wilopo et al. 2008; Drahota and Filippi 2009).
In the case of the Pb immobilization mechanism, Pb immobilization was most probably associated with Ca, Al, Si, and O (Fig. 4b). This may be due to the formation of pozzolanic reaction products such as calcium silicate hydrates (CSHs) and calcium aluminum hydrates (CAHs). Moreover, lead silicates are also possible compounds responsible for Pb immobilization. Rose et al. (2000) reported that Pb could be incorporated within the CSH structure through linkages to Si–O chains upon hydration of tricalcium silicate, which is the main compound in Portland cement. Lead silicate (Pb3SiO5) has been reported as the compound responsible for Pb immobilization when the fly ash was used on Pb contamination at 3.125 %. A different type of lead silicate (Pb2SiO4) was also reported as the most probable phase responsible for Pb immobilization in quicklime–fly ash-treated slurries (Moon and Dermatas 2006). Moreover, specific types of CSHs such as CaH4Si2O7 and Ca5Si6O16 (OH)2 were identified in the presence of montmorillonite as the phases responsible for Pb immobilization upon quicklime treatment (Moon et al. 2006).
In the case of Cu immobilization, even though the SEM-EDX results were not presented herein, effective Cu immobilization by CSH based on incorporation rather than substitution has already been reported (Johnson 2002). Moreover, Cu immobilization can be achieved by the formation of Cu6Al2O8CO3·12H2O when the Cu source is blended with Portland cement (Qiao et al. 2007). Even though the aforementioned hypothesis needs further investigation, recent research also showed that Cu immobilization was strongly associated with both pozzolanic reaction products and ettringite (Moon et al. 2011a). Therefore, Cu immobilization could have similar removal mechanism responsible for Pb immobilization.
Conclusions
As-, Pb-, and Cu-contaminated soil obtained from a timber mill site and a firing range was stabilized using calcined oyster shells and steel slag. The dosage of calcined oyster shells ranged from 0 to 15 wt%, and either 5 or 10 % steel slag was used. Following the stabilization treatment, the samples were cured for 1 month. The effectiveness of As immobilization was evaluated using 1-N HCl extraction, while Pb and Cu immobilization was evaluated using 0.1-N HCl extraction. The stabilization results showed that an approximate 71 % reduction in As leachability was obtained from the sample treated with 15 % COS and 10 % SS. Moreover, leachability reductions of more than 99 and 98 % in Pb and Cu, respectively, were attained from the same sample. The SEM results showed that As immobilization upon the combination treatment of COS and SS was strongly associated with Ca, Fe, and O, indicating that effective As immobilization could be mainly achieved by the formation of Ca-As and Fe-As precipitates. On the other hand, the pozzolanic reaction products such as CSHs and CAHs could be the most probable compounds responsible for effective Pb and Cu immobilization. Moreover, lead silicates could be the possible compounds responsible for Pb immobilization.
Overall, the combination treatment of COS and SS rather than the treatment of only SS was effective in simultaneously stabilizing As-, Cu-, and Pb-contaminated soil based on severe leaching conditions.
References
Ahmad M, Hashimoto Y, Moon DH, Lee SS, Ok YS (2012) Immobilization of lead in a Korean military shooting range soil using eggshell waste: an integrated mechanistic approach. J Hazard Mater 209–210:392–401
Ball DF (1964) Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soil. J Soil Sci 15:84–92
Chirenje T, Ma LQ, Clark C, Reeves M (2003) Cu, Cr and As distribution in soils adjacent to pressure-treated decks, fences and poles. Environ Pollut 124:407–417
Choi J-S (2012) The status and utilization prospect of steel making slag. Architect Res 569(8):18–21
Chung IJ, Choi YS, Hong SW, Park HM (2001) Immobilization of arsenic in tailing by using iron and hydrogen peroxide. Environ Technol 22(7):831–835
Dermatas D, Menouno N, Dutko P, Dadachov M, Arienti P, Tsaneva V (2004) Lead and copper contamination in small arms firing ranges. Global Nest J 6:141–148
Drahota P, Filippi M (2009) Secondary arsenic minerals in the environment: a review. Environ Int 35:1243–1255
Dutré V, Vandecasteele C (1998) Immobilization mechanism of arsenic in waste solidified using cement and lime. Environ Sci Technol 32:2782–2787
Dutré V, Vandecasteele C (1996) An evaluation of the solidification/stabilization of industrial arsenic containing waste using extraction and semi-dynamic leach tests. Waste Manage 16(7):625–631
FitzPatrick EA (1983) Soils: Their formation, classification and distribution. Longman Science & Technical, London, p 353
Gezer ED, Yildiz ÜC, Temiz A, Yildiz S, Dizman E (2005) Cu, Cr and As distributionin soils adjacent to CCA-treated utility poles in eastern blacksea region of turkey. Build Environ 40:1684–1688
Johnson CA (2002) Metal binding in the cement matrix: an overview of our current knowledge. Department of Water Resources and Drinking Water, Water-Rock Interaction Group, EAWAG, for Cemsuisse, Switzerland
Kim JY, Davis AP, Kim KW (2003) Stabilization of available arsenic in highly contaminated mine tailings using iron. Environ Sci Technol 37:189–195
Koo J, Song B, Kim H (2008) Characteristics of the release of chromium, copper, and arsenic from CCA-treated wood exposed to the natural environment. Anal Sci Technol 21(1):1–8
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manage 28:215–225
Lee KC (2006) Immobilization characteristics of arsenic contaminated soil using soluble phosphate and arsenic coagulant, Master thesis, Sangmyung University, Republic of Korea
Lee JY, Hong CO, Lee CH, Lee DK, Kim PJ (2005) Dynamics of heavy metals in soil amended with oyster shell meal. Korean J Environ Agr 24:358–363
Lee CH, Lee DK, MA A, Kim PJ (2008) Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming materials. Waste Manage 28:2702–2708
Lee KY, Moon DH, Lee SH, Kim KW, Cheong KH, Park JH, Ok YS, Chang YY (2013) Simultaneous stabilization of arsenic, lead, and copper in contaminated soil using mixed waste resources. Environ Earth Sci 69:1813–1820
Li XD, Poon CS, Sun H, Lo IMC, Kirk DW (2001) Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J Hazard Mater A 82:215–230
MDI (2005) Jade Version 7.1. Material’s Data Inc., Livermore, California, USA
Milton F (1995) The preservation of wood. A self-study manual for wood treaters, college of natural resources, University of Minnesota, p. 102
MOE (2002) The Korean Standard Test (KST) Methods for Soils (in Korean), Korean Ministry of Environment, Gwachun, Kyunggi
Moon DH, Cheong KH, Khim J, Grubb DG, Ko I (2011a) Stabilization of Cu-contaminated army firing range soils using waste oyster shells. Environ Geochem Health 33:159--166
Moon DH, Dermatas D (2006) An evaluation of lead leachability from stabilized/solidified soils under modified semi-dynamic leaching conditions. Eng Geol 85(1-2):67–74
Moon DH, Dermatas D (2007) Arsenic and lead release from fly ash stabilized/solidified soils under modified semi-dynamic leaching conditions. J Hazard Mater 141:388–394
Moon DH, Dermatas D, Menounou N (2004) Arsenic immobilization by calcium-arsenic precipitates in lime treated soils. Sci Total Environ 330:171–185
Moon DH, Dermatas D, Grubb DG (2006) The effectiveness of quicklime-based stabilization/solidification on lead (Pb) contaminated soils, In: Environmental Geotechnics (5th ICEG), Thomas H.R. (ed.), Thomas Telford Publishing, London, 1, 221–228
Moon DH, Wazne M, Yoon I-H, Grubb DG (2008) Assessment of cement kiln dust (CKD) for stabilization/solidification (S/S) of arsenic contaminated soils. J Hazard Mater 159:512–518
Moon DH, Grubb DG, Reilly TL (2009) Stabilization/solidification of selenium-impacted soils using Portland cement and cement kiln dust. J Hazard Mater 168:944–951
Moon DH, Lee JR, Grubb DG, Park JH (2010) An assessment of Portland cement, cement kiln dust and class C fly ash for the immobilization of Zn in contaminated soils. Environ Earth Sci 61:1745–1750
Moon DH, Kim KW, Yoon IH, Grubb DG, Shi DY, Cheong KH, Choi HI, Ok YS, Park JH (2011b) Stabilization of arsenic-contaminated mine tailings using natural and calcined oyster shells. Environ Earth Sci 64:597–605
Ok YS, Lee SS, Jeon WT, Oh SE, Usman ARA, Moon DH (2011a) Application of eggshell waste for the immobilization of cadmium and lead in a contaminated soil. Environ Geochem Health 33:31–39
Ok YS, Lim JE, Moon DH (2011b) Stabilization of Pb and Cd contaminated soils and soil quality improvements using waste oyster shells. Environ Geochem Health 33:83–91
Qiao XC, Poon CS, Cheeseman CR (2007) Investigation into the stabilization/solidification performance of Portland cement through cement clinker phases. J Hazard Mater B139:238–243
Rose J, Moulin I, Hazemann J-L, Masion A, Bertsch PM, Bottero J-Y, Mosnier F, Haehnel C (2000) X-ray absorption spectroscopy study of immobilization processes for heavy metals in calcium silicate hydrates: 1. case of lead. Langmuir 16:9900–9906
Saxe JK, Wannamaker EJ, Conklin SW, Shupe TF, Beck BD (2007) Evaluating landfill disposal of chromated copper arsenate (CCA) treated wood and potential effects on groundwater: evidence from Florida. Chemosphere 66:496–504
Taylor HFW (1997) Cement Chemistry. Academic Press, 2nd Edition
Wang SY, Vipulanandan C (1996) Leachability of lead from solidified cement–fly ash binders. Cement Concrete Res 26(6):895–905
Warner JE, Solomon KR (1990) Acidity as a factor in leaching of copper, chromium and arsenic from CCA-treated dimension lumber. Environ Toxicol Chem 9:1331–1337
Wilopo W, Sasaki K, Hirajima T, Yamanaka T (2008) Immobilization of arsenic and manganese in contaminated groundwater by permeable reactive barriers using zero valent iron and sheep manure. Mater Trans 49(10):2265–2274
Yoon IH, Moon DH, Kim KW, Lee KY, Lee JH, Kim MG (2010) Mechanism for the stabilization/solidification of arsenic-contaminated soils with Portland cement and cement kiln dust. J Environ Manage 91:2322–2328
Zhu G, Hao Y, Xia C, Zhang Y, Hu T, Sun S (2013) Study on cementitious properties of steel slag. J Min Metall Sect B-Metall 49(2):217–224
Acknowledgments
This study was supported by the Korean Ministry of Environment as the GAIA (Geo-Advanced Innovative Action) project (No. 2014000540011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Moon, D.H., Wazne, M., Cheong, K.H. et al. Stabilization of As-, Pb-, and Cu-contaminated soil using calcined oyster shells and steel slag. Environ Sci Pollut Res 22, 11162–11169 (2015). https://doi.org/10.1007/s11356-015-4612-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4612-6