Abstract
Previous researches have confirmed that modified nanoscale carbon black (MCB) can decrease the bioavailability of heavy metals in soil and accumulation in plant tissues, resulting in the increase of biomass of plant. However, as a nanoparticle, the effects of MCB on plant cell morphology and microbial communities in Cd-contaminated soil are poorly understood. This study, through greenhouse experiments, investigated the effects of MCB as an amendment for 5 mg·kg-1 Cd-contaminated soil on plant growth, plant cellular morphogenesis, and microbial communities. Two types of plants, metal-tolerant plant ryegrass (Lolium multiflorum), and hyperaccumulator plant chard (Beta vulgaris L. var. cicla) were selected. The results indicated that adding MCB to Cd-contaminated soil, the dry biomass of shoot ryegrass and chard increased by 1.07 and 1.05 times, respectively, comparing with control group (the treatment without MCB). Meanwhile, the physiological characteristics of plant root denoted that adding MCB reduced the damage caused by Cd to plants. The acid phosphatase activity of soils treated with MBC was higher and the dehydrogenase activity was lower than control group during whole 50 days of incubation, while the urease and catalase activity of soils treated with MBC were higher than control group after 25 days of incubation. When compared with the treatment without MCB, the abundances of nitrogen-functional bacteria (Rhodospirillum and Nitrospira) and phosphorus-functional bacteria (Bradyrhizobium and Flavobacterium) increased but that of nitrogen-functional bacteria, Nitrososphaera, declined. The presence of MCB resulted in increased microbial community abundance by reducing the bioavailability of heavy metals in soil, while increasing the abundance of plants by increasing the amount of available nitrogen in soil. The result of this study suggests that MCB could be applied to the in-situ immobilization of heavy metal in contaminated soils because of its beneficial effects on plants growth, root cellular morphogenesis, and microbial community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination of agricultural soils is becoming a serious environmental issue because of an increase of industrial activity, over-fertilization, and improper waste disposal (Rodriguez et al. 2015). According to the National Soil Pollution Investigation Bulletin issued by the Ministry of Environmental Protection and the Ministry of Land and Resources of China in 2014, 19.4% of farmland soil in China exceeded the allowed standard maximum amount of heavy metal contamination with heavy metal pollution by Cd, Ni, and Cu being the most prominent. In China, the vast majority of farmland soil contaminated by heavy metals is slightly polluted by Cd (Zhao and Luo 2015). Zhao et al. (2007) estimated that the area of farmland soil contaminated by heavy metals in China is about 2 × 107 hm2; as much as 1.2 × 107 t of grain was found to be contaminated every year, with economic losses of 2 × 1010 RMB. A nationwide survey revealed that cadmium (Cd) is the most frequently detected heavy metals in soil (Yang et al. 2018). The excessive accumulation of Cd in soils has led to phytotoxic metabolism and inevitably poses risks to human health via food chain (Hu et al. 2016). Long-term exposure to Cd not only contributed to mental and behavioral disorders but also increased the risk of cancer (Hartwig 2013). Therefore finding ways to remediate Cd pollution in farmland soils is very important to food safety in China.
Among many remediation technologies used for mild heavy metal contamination in soils, the technology of in-situ chemical immobilization of metals appears to be superior (Singh and Prasad 2015). The principle method involves applying chemical materials to soil to reduce the bioavailability of heavy metals by absorbing, chelating, and intercepting metals in soil, thus reducing the toxicity of metals in plants and organisms in the soil (Liu et al. 2008). In-situ chemical immobilization has recently been gaining prominence owing to its cost-effectiveness, elimination of toxic metallic ions, and environmental sustainability. A large number of amendments have been synthesized and tested for the in-situ remediation of heavy metal contaminated soils (Cao 2018). The effectiveness of remediation that employs in-situ chemical immobilization is greatly dependent on the physicochemical properties of the chemical immobilization materials. In other words, the materials are required not only to have a strong capacity to immobilize heavy metals in soil but also to have a few effects on plants and microorganisms in soil (Li et al. 2014).
Carbon black (CB) nanoparticles have been applied to the remediation of heavy metals contaminated soils due to their large surface areas, highly active reaction sites, as well as their strong adsorption and chelating abilities. Modified carbon black nanoparticles (MCB) that have been modified by the application of nitric acid have a relatively negative zeta potential, larger number of functional groups, and a relatively heterogeneous pore structure used for the exchange and complexation of cations when compared with CB (Zhou et al. 2010). The MCB can decrease the bioavailability and accumulation of heavy metals in plant tissues and increase plant biomass as reported by Cheng et al. (2015, 2019).
An evaluation of in-situ immobilization remediation technologies involves not only the analyses of the fractionations of heavy metals in soil, plant growth, and uptake of heavy metals but also the assessment of the restoration of soil habitat functioning by biological methods (Sun et al. 2016). An effective remediation using immobilization must maintain reasonably low solubility and bioavailability of heavy metal, healthy plant growth, and normal soil biological indices (Ruttens et al. 2010). Over the past 15 years, a number of patents and products that incorporate nanomaterials into agricultural practices have been developed (Servin et al. 2015), and negative and positive impacts of engineered nanomaterials (ENMs) on plant growth have been well reviewed (Kumar et al. 2018, 2019a, b). It is very important to evaluate the effects of nanoscale materials on plant cellular morphogenesis because of the very small size of the nanoscale materials (Jeon et al. 2015). The collective goal of all of these studies is to enhance the efficiency and sustainability of agriculture (Hao et al. 2017). When nanomaterials are used for remediation of heavy metal contaminated soils, the jointed biotoxicity of nanomaterials and heavy metals should be research, besides investigating the alleviation of heavy metals biotoxicity and the reducing of heavy metals uptake by plants (Ji et al. 2017).
The major objective of this study was to investigate the effects of MCB used as an amendment for Cd-contaminated soil on plant growth and cellular morphogenesis, as well as on the microbial community. The effectiveness of MCB remediation in different plant-soil systems was investigated by using two types of plants: a hyper-accumulator plant-chard (Beta vulgaris L. var. cicla) (Lyv et al. 2018) and a metal-tolerant plant-ryegrass (Lolium multiflorum) (Chu et al. 2018). The goal is to provide confirmation that the application of the MCB for in-situ immobilization remediation of Cd-contaminated soils is effective.
Materials and methods
MCB, soil, and plant seeds
Carbon black with a particle size of 20–70 nm was purchased from the Jinan Tyrone Rubber Company (Jinan, China). Modified Carbon black (MCB) was synthesized by modifying it with nitric acid and potassium permanganate. The surface area of MCB was 1114.23 m2·g-1, determined by the Brunauer–Emmer–Teller method. The details of the synthesis of MCB amendments have been published in our previous study (Cheng et al. 2015), and the basic properties of CB and MCB are shown in Table S1.
Cinnamon soil samples (0–20 cm) were collected from the prefecture-level city of Liaocheng in Shandong Province. The soil samples were air-dried and sieved using a 2 mm mesh. The general properties of the soil were pH 7.68, conductivity 0.703 us·cm-1, cation exchange capacity 8.29 cmol·kg-1, organic matter content 39.7 g·kg-1, clay content < 24% (< 0.002 mm), total Cu 25.13 mg·kg-1, Zn 48.25 mg·kg-1, Pb 26.42 mg·kg-1, and Cd 0.278 mg·kg-1. Before use, the soils were spiked with 5.0 mg·kg-1 Cd to mimic Cd-contaminated soils. Then, the soils were incubated for 50 days to age the soil and to make sure the Cd had time to pre-equilibrate in the soil before plant growth began.
Chard (Beta vulgaris L. var. cicla) seeds used in this study were obtained from the Academy of Agricultural Sciences Research in Jinan, Shandong Province, P. R. China, and ryegrass (Lolium multiflorum) seeds were purchased from the Valley Plant Company in China. The surfaces of the seeds were sterilized by NaClO and then the seeds were placed on moist gauze and germinated for about one week in a dark environment prior to the greenhouse experiments.
Pot experimental designs and procedures
Two percent MCB was added into the soil (5 mg·kg-1Cd) based on previous research(Cheng et al. 2015, 2019; Lyv et al. 2018), mixed thoroughly and placed in pots (1.5 kg soil per pot). For each pot, 0.33 g urea and 0.35 g K2HPO4 were added as fertilizer and mixed thoroughly. A total of four planting treatments in Cd contaminated soil were set up: ryegrass with or without MCB and chard with or without MCB. There were three replicates for each treatment. All pots were adjusted regularly to 70% of field water capacity using deionized water. Five well-germinated chard seeds and ten ryegrass seeds were sown into each pot. The plants were harvested after a growth period of 7 weeks. At the end of the experiment, the shoot and root tissues were separated, washed, and weighed after adhering water was removed with filter paper. The dry weight of root and shoot samples were determined after drying at 70 °C overnight before grinding to pass through a 0.25 mm sieve. Soil subsamples were air-dried and ground to pass through a 1-mm sieve.
Analytical methods
Physical and chemical properties of the soil
The air-dried soil samples were tested for pH (using a DZS-706 pH meter, INESA Scientific Instrument Co., Ltd., Shanghai, 10 g soil in 10 ml 0.01 M CaCl2), organic matter (wet combustion method, using H2SO4-K2Cr2O7), and total Zn, Cu, Pb, as well as Cd (by HNO3-HClO4-HF extraction) and available Cd (by DTPA [pH = 7.3] extraction). The heavy metal concentrations were analyzed using TAS-990 atomic adsorption spectroscopy.
Soil enzyme activities
The catalase activity was analyzed by titration with 0.1 mol·L-1 KMnO4, expressed as mL·g-1. Urease activity was determined by a colorimetric method, expressed as NH4-N mg·g-1. Acid phosphatase activity was determined by phenylenedisodium phosphate colorimetry method, expressed as P2O5 mg·g-1. Dehydrogenase activity was determined by using a sucrose solution at 37 °C for 24 h and measuring the glucose production with a colorimetric method, expressed as μg·g-1·h-1.
Heavy metal content in plants
Each dry-milled plant sample (0.5–1.0 g) was placed in a conical beaker, soaked in concentrated nitric acid (25 mL), and held overnight (18 h). Next, each beaker was placed on a hot plate and heated at 100 °C for 0.5 h. After cooling, added 5 mL perchloric acid into each beaker; then, each beaker was heated again until the solution was colorless, transparent, and evaporated to a final volume of 2 ml. Finally, two drops of concentrated nitric acid were added to each solution. The heavy metal concentrations were analyzed using TAS-990 atomic adsorption spectroscopy (Purkinje General, Beijing, China).
SEM and TEM and analysis
At the end of exposure period, fresh plant samples were washed with deionized water to remove any impurities adhering to the roots. The plant roots were fractured at the tips with a razor blade by applying a slight pressure to the top 1–2 cm of the root. The samples were steeped in deionized water at 4 °C, and then were fixed and dehydrated in serial concentration gradients of ethanol from 50% to 100%. The samples were further dried by the CO2 supercritical fluid drying method known as HCP-2. Then the samples were sputter-coated with gold before Scanning Electron Microscope (SEM, Inspect S50, FEI, USA) observation.
Fresh root samples were fixed with 4% osmic acid and 2.5% glutaraldehyde in 0.2 mol·L-1 phosphate buffer at pH 7.3. They were then dehydrated in a graded acetone series and embedded in Spurr’s resin, cut into ultrathin sections (70 nm), and collected on Ni grids, which was described as the process of Transmission Electron Microscope (TEM, H-800, Hitachi, Japan).
Results
Effects of MCB on plant growth
Plant growth rate
Figure 1 shows the growth rate of shoot of ryegrass and chard during 50 days cultivation. On 21 days after planting, the biomass of ryegrass in Cd-contaminated soil treated with MCB was significantly higher than that treated without MCB. On 41 days, the biomass of chard in Cd-contaminated soil treated with MCB was significantly higher than that treated without MCB. This result indicates that adding MCB to Cd-contaminated soil can promote plant growth; the beneficial effect of MCB on the growth of a heavy metal tolerant plant-ryegrass was greater than that on a heavy metal hyper-accumulating plant-chard.
Plant growth rate. The growth rate was calculated using \( r=\frac{G_2-{G}_1}{t_2-{t}_1} \), where G1and G2 represent the dry biomass of the plant shoot tissue at times t1 and t2 (g), t1 and t2 are the plant growth time (d), and r is the average growth rate (g/d). The comparison uses “Fully Factorial(M)ANOVA”(SYSTAT).In a column, means with the same letter are not significantly different at p< 0.05 in different treatments
Biomass of plant roots and shoots
The dry biomass of ryegrass and chard shoot and root from the treatment with MCB in Cd-contaminated soil were significantly higher than those without MCB after 50 days planting (Fig. S1). Compared with the biomass in treatments without MCB, the dry biomass of shoot and root of ryegrass increased by 1.07 and 0.70 times, and the dry biomass of chard shoot and root increased by 1.05 and 0.67 times, respectively.
Effects of MCB on root physiological characteristics
Root morphological characteristics
As shown in SEM images, morphology of the plant roots varied among different treatments. The surfaces of ryegrass and chard roots in non-polluted soil were smooth and flat. The root tip cells were detected with regular shapes and arranged closely (Fig. 2 a-1, b-1). While the surfaces of roots in Cd-contaminated soil became rough, shrunken, and partly fractured. The root tip cells were heterogeneous in size and shape, exhibiting cell shrinkage, irregular shapes, and disordered arrangements (Fig. 2 a-2, b-2). The results suggested that these changes were caused by the cadmium. The plants in Cd-contaminated soils with MCB (Fig.2 a-3, b-3), the surfaces of the plant roots were either smooth or slightly concave with no structural damage. These results suggested that MCB reduced the damage caused by Cd2+ to plants.
Root cellular morphogenesis
The TEM images of the plant root cells for different treatments are shown in Fig. 3. The normal ryegrass root cells were detected with regular cellular structure and rich organelles (Fig. 3 a-1). The cellular ultrastructure of the ryegrass root cells in Cd-contaminated soil was clearly different when compared with those of the control plants. The cell walls were irregularly thickened, and plasmolysis had occurred. Specifically, some of the organelles showed irregularities, including vacuolation and other cytopathic effects (Fig. 3 a-2). However, when the Cd-contaminated soils were remediated with MCB (Fig. 3 a-3), the cell walls became thinner, while the protoplasts and organelles recovered gradually. These results suggested that Cd2+ can enter and damage plant root cells. The cellular ultrastructure of the chard root cells polluted with Cd2+ had similar abnormal changes of ryegrass (Fig.3 b-2). The ultrastructural features of the chard root cells polluted with Cd2+ are shown in Fig. 3 b-2. Significantly, organelle damage, disintegration, malformation, and cellular vacuolation were observed in plant root cells. Meanwhile, the cellular structure also improved when the Cd2+ polluted soils were remediated with MCB (Fig. 3 b-3), which indicated that MCB nanomaterials adsorb Cd2+ from soils and protect plants.
Effects of MCB on soil enzymatic activity
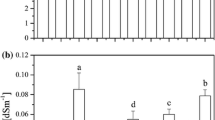
Soils were characterized by their levels of urease, acid phosphatase, dehydrogenase, and catalase activity (Fig. 4). After 50 days of incubation in greenhouse, the urease, acid phosphatase, and catalase activity of soils treated with MCB were higher than those without MCB while the dehydrogenase activity was lower. The urease and catalase activity of soils treated with MBC were higher or lower, respectively, than without MCB treatment during 50 days of incubation. After 25 days, the urease and catalase activities of soils treated with MCB were higher than those without MCB. During the entire 50-day incubation period, the acid phosphatase activity in soils treated with MCB was always higher than those without MCB treatment, which denoted that MCB is advantageous to the activation of phosphatase. Meanwhile, the dehydrogenase activity of soils treated with MCB was always lower than those without MCB treatment; the present results suggested that MCB materials restricted the dehydrogenase activity.
Effects of MCB on soil microbial community
Soil microbial community
High-throughput sequencing technology was used to analyze the soil microbial community of five sample groups: CK, ryegrass without MCB, ryegrass with MCB, chard without MBC, and chard with MCB. The species and relative abundance of bacteria in different sample groups are shown in Fig. 5. Thirty-nine genera were found in five sample groups (Fig. 5), and there were the same number of genera and different relative abundances. The predominant bacteria of the five treatment groups were unclassified_c_Betaproteobacteria, which are associated with nitrogen transformation in soil. The abundance values of CK, ryegrass without MCB, ryegrass with MCB, chard without MBC, and chard with MCB were 3.36%, 4.24%, 6.86%, 4.63%, and 6.03%, respectively. It can be seen that growing plants can increase the abundance of unclassified_c_Betaproteobacteria, and MCB can further increase the abundance of these bacteria.
Among the five treatment groups, three nitrogen-functional bacterial groups were detected, including Rhodospirillum, Nitrospira, and a Nitrososphaera (Fig. 6). The abundance values of Rhodospirilla and Nitrospira were the highest in the treatments of plants grown with MCB and were the lowest in the control. Meanwhile, the Nitrososphaera was the lowest in the treatments of growing plants with MCB, and the highest in the control. No significant difference was observed between the abundance values of the heavy metal tolerant plant, ryegrass, and hyper-accumulative plant, chard. This indicated that MCB promotes Rhodospirilla and Nitrospira and inhibits Nitrososphaera.
Among the five treatment groups, two phosphorus-functional bacteria were detected, including Bradyrhizobium and Flavobacterium (Fig. 6). The abundances values of both Bradyrhizobium and Flavobacterium were the highest in the treatments of plants growing with MCB and the lowest in the control. The abundance of Bradyrhizobium was much higher than that of Flavobacterium. No significant difference was observed in the abundance of the heavy metal tolerant plant, ryegrass, and hyper-accumulative plant, chard.
Principal component analysis
Figure S4 demonstrates the results of principal component analysis (PCA) of the soil microbial community in different treatments after 50 days of cultivation. The contributions of the four principal components (PCs), DTPA-Cd (bioavailable Cd), available N, available P, and available K in soil, to the variance in the soil microbial community were 0.7053, 0.2657, 0.0154, and 0.0136, respectively. Based on the results of PCA, principal components 1 (PC1) and 2 (PC2) explained 97.10% of the variation in the data. In addition, PC1 and PC2 mainly reflected the influence of DTPA-Cd and available N content in soil on the microbial community, respectively. The microbial community changed significantly in different treatments and was separated into three groups (CK, ryegrass and chard growth without MCB, and ryegrass and chard growth with MCB). A positive correlation was observed between the microbial genome composition abundance and available N content in the treatments of CK and growing plant, and the abundance did not exhibit a positive correlation with them in the treatments of MCB-growing plants. A negative correlation was observed between the microbial genome composition abundance and DTPA-Cd content in the treatments of ryegrass and chard growth with MCB. The result suggests that the application of MCB resulted in increased microbial community abundance by mainly reducing the bioavailability of heavy metals in soil, because PC1 and PC2 accounted for 70.53% and 26.57% of the variation in the data.
Discussion
Effects of MCB on the plants growth
When metal-tolerant plant-ryegrass and hyperaccumulator plant-chard were stressed by cadmium, the morphology of the plant roots varied which can be observed by SEM (Fig. 2 a-2, b-2). The plant roots showed an increase in surface roughness, apparently the result of cadmium absorption (Rodrigo et al. 2013). However, the level of damage caused to the roots of chard was more serious than that caused to the roots of ryegrass, suggesting that their detoxification mechanisms were different (Qin et al. 2018). The damage of ultrastructure of plant root cells by Cd2+ observed using TEM was mainly characterized by the cell walls irregularly thickened, plasmolysis and the organelles vacuolation, etc. (Fig. 3 a-2, b-2). The effect of cadmium on plant growth has been well documented (Rizwan et al. 2017). Bouzon et al. (2012) found the cell wall of Hypnea musciformis thickening treated with different concentrations of Cd2+. This is due to the fact that the sulfated polysaccharide contained in the cell wall forms small vesicles with Cd and then merges into the cell wall. Rodrigo et al. (2013) reported that cadmium also caused changes in the ultrastructure of cortical and subcortical cells, including increased cell wall thickness and vacuole volume, as well as the destruction of chloroplast internal organization and increased number of plastoglobuli. Costa et al. (2017) observed that major alterations of Sargassum cymosum exposed to cadmium were disorganization of cell wall fibrils. The surfaces of the plant roots were either smooth or slightly concave with no structural damage in Cd-contaminated soils and the cellular structure also improved when the Cd polluted soils were remediated with MCB (Fig. 2 a-3, b-3). The result suggested that MCB could tremendously reduce the Cd toxicity.
The effects of carbon nanomaterial on plant growth have been found in previous experiments (Kumar et al. 2019b). Hao et al. (2018) found that after 30 days of exposure in 50 or 500 mg/kg doses, fullerene(C60), reduced graphene oxide (rGO), and multi-walled carbon nanotubes (MWCNTs), negatively affected the shoot height and root length of rice, significantly decreased root cortical cells diameter and resulted in shrinkage and deformation of cells. Most studies found an increase in plant growth and yield at lower concentration of carbon nanomaterials, but a decrease in these observes (Kumar et al. 2019b). The present experiment showed that 2% MCB addition resulted in enhanced growth rate of ryegrass and chard (Fig. 1) and increased biomass of plant root and shoot in Cd-contaminated soil (Fig. S1). This result can be attributed to the reduction of Cd bioavailability caused by adding MCB to soil, the addition of MCB alleviated the toxicity of Cd to plants, thus promoting plant growth (Mohamed et al. 2017). Ji et al. (2017) also found that Cd had significant toxicity to the plant growth by observing the plant height, biomass, and root length, but nanoparticles TiO2 exhibited the potential ability to alleviate the Cd toxicity. Konate et al. (2017) reported that addition of nanoparticles Fe3O4 (2000 mg/L) in Cu or Cd solution (1 mM) significantly decreased the growth inhibition induced by heavy metals in the wheat seedlings. The beneficial effect of MCB on a heavy metal tolerant plant-ryegrass was greater than that on a heavy metal hyper-accumulating plant-chard. This result may be explained by the different tolerance and enrichment mechanisms of Cd2+ in these two plant species (Ma et al. 2005; Mohtadi et al. 2012). It may also explained that chard as a typical hyper-accumulator plant can accumulate a large quantity of Cd in its shoot while causing very weak physiological influences including biomass change (Lyv et al. 2018).
It is notable that addition of 2% MBC in Cd-contaminated soil (5 mg kg-1), the protoplasts of root cells were filled with many nano-sized particles (black spots in TEM graph). These particles may be MBC, because previous studies reported that nanoscale particles enter the root relatively easily via transpiration and other metabolic functions (Corredor et al. 2009; Wang et al. 2012). Thus, MCB themselves may have negative effects on plant, but it is not clear at present and need to be study in the future.
Effects of MCB on soil enzymatic activity
Soil enzymatic activity was studied as an indicator of the effectiveness of soil rehabilitation treatments as well as the functioning of soil ecosystems (Gucwa-Przepiora et al. 2016). The activities of urease, acid phosphatase, dehydrogenase, and catalase of soil significantly decreased while heavy metal concentration increased (Angelvicova et al. 2014; Minnikova et al. 2017). Recent studies showed that carbon nanomaterials themselves had also influence on the enzyme activity. Jin et al. (2013) found that high concentrations of single-walled carbon nanotubes reduced the enzyme activity of cellobiohydrolase. Hao et al. (2018) reported that at the high-exposure dose of MWCNTs and C60, activities of the antioxidant enzymes in roots increased significantly. In our experiment, the acid phosphatase activity of soils treated with MBC was higher (Fig. 4c) and the dehydrogenase activity lower (Fig. 4b) than without MCB treatment during whole 50 days of incubation, while the urease and catalase activity of soils treated with MBC were higher than the control after 25 days of incubation (Fig. 4a and d). Generally, significant reductions in enzymatic activities occur in soils contaminated with heavy metals and metals are more toxic to intra-cellular enzyme activities (e.g., dehydrogenase) than extra-cellular activities (e.g., phosphatase). Hu et al. (2014) indicated that the activities of dehydrogenase, urease, catalase and acid phosphatase were 25.2%, 49.3%, 52.4%, and 94.7% of the controls in paddy soils heavily polluted by Cu, Zn, and Cd. The result of Hu et al. (2014) suggested that heavy metal had the greatest impact on dehydrogenase and minimal impact on acid phosphatase. In this work, the dehydrogenase activity in the treatments with MCB averaged approximately 71.31% of the control and the acid phosphatase activity 126.9% of the control (Fig. 4), and it confirmed that MCB had a beneficial effect on acid phosphatase activity and inhibitory effect on dehydrogenase activity in soil.
This result, which the urease and catalase activity of soils treated with MBC were higher than the control group after 25 days of incubation, might be explained in two aspects. On the one hand, the urease and catalase was very sensitive to the excessive levels of Cd in soil (Yang et al. 2006). With an increase of incubation time, Cd in soil was gradually bound on MBC, and thereby the biological toxicity of Cd in soil reduced and the activity of urease and catalase increased. It was reported by Minnikova et al. (2017) that inverse correlations were observed between the content of loosely bound metals and the activity of urease in alluvial and meadow-alluvial. However, it was need to takes time that the loosely bound heavy metals in soil move to the MCB surface and that ion exchange reaction, chelation reaction, etc. occur on the surface of MCB (Cheng et al. 2014). Konate et al. (2017) suggested that addition of Fe3O4 nanoparticles reduced the toxicity of heavy metals and increased the activity of superoxide dismutase and peroxidases, which was related to the adsorption of heavy metals in the soil by Fe3O4 nanoparticles. On the other hand, MCB as sources of C for the soil microbial community contributed to recover enzyme activity to some extent with an increase of incubation time (Lejon et al. 2010).
Effects of MCB on soil microbial community
In soil ecosystem, heavy metals exhibit toxicological effects on soil microorganism that may lead to the decrease of their numbers and activities (Khan et al. 2010). The pollution of heavy metal in soil significantly decreased abundance of bacteria and fungi and also changed their community structure (Deng et al. 2015). The impact of engineering nanomaterials on soil microbial communities was poorly understood. Existing research showed that the carbon nanomatericals (natural nanostructured material-biochar, industrial carbon black, three types of multiwalled carbon nanotubes-MWCNTs, and graphene) only moderately affected dry soil microbial communities, even after 1-year exposure (Ge et al. 2016). ZnO and CeO2 nanoparticles hindered thermogenic metabolism, reduced numbers of soil Azotobacter, P-solubilizing, and K-solubilizing bacteria; TiO2 nanoparticles decreased the abundance of functional bacteria; and SiO2 nanoparticles slightly boosted the soil microbial activity (Chai et al. 2015).
In this work, the same thirty-nine genera and different relative abundances were found in five sample groups (CK, ryegrass without MCB, ryegrass with MCB, chard without MBC, and chard with MCB) (Fig. 5), and the predominant bacteria of the five treatment groups were unclassified_c_Betaproteobacteria. The abundances value of predominant bacteria was higher in the treatments of plant with MCB than that without MCB. In particular, three nitrogen-functional bacterial, Rhodospirilla and Nitrospira and inhibits Nitrososphaera, were detected among the five treatment groups (Fig. 6). The higher the abundance values of Nitrospira associated with nitrification (Daims et al. 2015) and Rhodospirilla associated with nitrogen fixation (Madigan et al. 1984) are, the more beneficial it is to nitrogen conversion and plant growth in soil. On the contrary, the higher the abundance values of the Nitrososphaera associated with nitrosation (Liang et al. 2014) is, the worse it is for plant growth. In our experiments, the addition of MCB to Cd-contaminated soil promoted nitrification and fixation of nitrogen and inhibited the nitrosation of nitrogen, and apparently promoted plant growth (Fig. 1). Similarly, the addition of MCB increased the abundances value of two phosphorus-functional bacteria, Bradyrhizobium and Flavobacterium (Fig. 6), and Bradyrhizobium played an important role in phosphorus transformation in soil, because the abundance of Bradyrhizobium was much higher than that of Flavobacterium. The results of PCA also indicated that the application of MCB resulted in increased microbial community abundance by mainly reducing the bioavailability of heavy metals in soil (Fig. S4).
Overall, the effect mechanisms of adding MCB to Cd-contaminated soil on plant growth mainly includes the application of MCB resulted in increased microbial community abundance by reducing the bioavailability of heavy metals in soil, while increasing the biomass of plants by increasing the available nitrogen in soil (Fig. 7). Therefore, we confirmed this assertion that the addition of MCB reduced the bioavailability of Cd in soil (Fig. S2) and modified the microbial community (Fig. 6) and enzymatic activity (Fig. 4), and thereby promoted plant growth (Fig. 1) in soil.
But according to our experiment, the influence of MCB alone on plant growth was poorly understood.
Conclusions
This study clearly shows that the application of nanoscale carbon black modified by HNO3-KMnO4 (MCB) can result in the increase of plant biomass in two species planted in Cd-contaminated soil, and the effect of MCB on the growth rate of a heavy metal-tolerant plant-ryegrass was greater than the effect of MCB on that of heavy metal hyper-accumulating-chard. Meanwhile, the physiological characteristics of plant roots demonstrate that the application of MCB reduced the damage caused by Cd to plants. Addition of MCB to Cd-contaminated soil can improve the activity of the urease, acid phosphatase, and catalase enzymes and restrict the dehydrogenase activity. When compared with the CK treatment, the abundances of the nitrogen-functional bacteria (Rhodospirillum and Nitrospira) and the phosphorus-functional bacteria (Bradyrhizobium and Flavobacterium) increased, while the abundance of the nitrogen-functional bacteria (a Nitrososphaera cluster) reduced in the treatments with MCB. The application of MCB resulted in increased microbial community abundance by reducing the bioavailability of heavy metals in soil, while the biomass of plants increased because of the increase of available nitrogen in soil. This result is attributed to the reduction of Cd bioavailability caused by adding MCB to soil. Adding MCB alleviates the toxicity of Cd to plants and microorganisms, thus promotes their growth and reproduction. Modified nanoscale carbon black might be applied for the in-situ immobilization of heavy metal and remediation of contaminated soils because of its beneficial effects on plant growth, root cellular morphogenesis, and the microbial community in Cd-contaminated soil in the future. However, before MCB can be used for remediation of heavy metal contaminated soil, in addition to analyzing the feasibility of economic cost and engineering technology, it is very necessary to understand MCB itself biological toxicity because we have observed a fact that MCB entered root cells of ryegrass and chard.
References
Angelvicova L, Lodenius M, Tulisalo E, Fazekasova D (2014) Effect of heavy metals on soil enzyme activity at different field conditions in middle Spis mining area (Slovakia). Bull Environ Contam Toxicol 93:670–675. https://doi.org/10.1007/s00128-014-1397-0
Bouzon ZL, Ferreira EC, Santos R, Scherner F, Horta PA, Maraschin M, Schmidt EC (2012) Influences of cadmium on fine structure and metabolism of Hypnea musciformis (Rhodophyta, Gigartinales) cultivated in vitro. Protoplasma 249:637–650. https://doi.org/10.1007/s00709-011-0301-6
Cao X (2018) Immobilization of heavy metals in contaminated soils amended by phosphate-, carbonate-, and silicate-based materials: from lab to field. In: Luo Y, Tu C (eds) Twenty years of research and development on soil pollution and remediation in China, pp 535–543. https://doi.org/10.1007/978-981-10-6029-8_32
Chai H, Yao J, Sun J, Chang C, Liu W, Zhu M, Ceccanti B (2015) The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull Environ Contam Toxicol 94:490–495. https://doi.org/10.1007/s00128-015-1485-9
Cheng J, Liu Y, Wang H (2014) Effects of surface-modified Nano-scale carbon black on Cu and Zn fractionations in contaminated soil. Int J Phytoremediation 16:86–94. https://doi.org/10.1080/15226514.2012.759530
Cheng J, Yu L, Li T, Liu Y, Lu C, Li TT, Wang H (2015) Effects of nano-scale carbon black modified by HNO3 on immobilization and phytoavailability of Ni in contaminated soil. J Chem 2:1–7. https://doi.org/10.1155/2015/839069
Cheng J, Sun Z, Yu Y, Li X, Li T (2019) Effects of modified carbon black nanoparticles on plant-microbe remediation of petroleum and heavy metal co-contaminated soils. Int J Phytoremediation 21(7):634–642. https://doi.org/10.1080/15226514.2018.1556581
Chu Z, Wang X, Wang Y, Liu G, Dong Z, Lu X, Chen G, Zha F (2018) Effects of coal spoil amendment on heavy metal accumulation and physiological aspects of ryegarss (Lolium perenne L.) growing in copper mine tailings. Environ Monit Assess 190:1–12. https://doi.org/10.1007/s10661-017-6400-x
Corredor E, Testillano PS, Coronado MJ, González-Melendi P, Fernández-Pacheco R, Marquina C, Ibarra MR, Fuente JMDL, Rubiales D, Pérez-De-Luque A (2009) Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol 9:45–55. https://doi.org/10.1186/1471-2229-9-45
Costa GB, Simioni C, Pereira DT, Ramlov F, Maraschin M, Chow F, Horta PA, Bouzon ZL, Schmidt EC (2017) The brown seaweed Sargassum cymosum: changes in metabolism and cellular organization after long-term exposure to cadmium. Protoplasma 254(2):817–831. https://doi.org/10.1007/s00709-016-0992-9
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, Bergen MV, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature 528:504–509. https://doi.org/10.1038/nature16461
Deng L, Zeng G, Fan C, Lu L, Chen X, Chen M, Wu H, He X, He Y (2015) Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Appl Microbiol Biotechnol 99:8259–8269. https://doi.org/10.1007/s00253-015-6662-6
Ge Y, Priester JH, Mortimer M, Chang CH, Ji Z, Schimel JP, Holden PA (2016) Long-term effects of multiwalled carbon nanotubes and graphene on microbial communities in dry soil. Environ Sci Technol 50(7):3965–3974. https://doi.org/10.1021/acs.est.5b05602
Gucwa-Przepiora E, Nadgorska-Socha A, Fojcik B, Chmura D (2016) Enzymatic activities and arbuscular mycorrhizal colonization of Plantago lanceolata and Plantago major in a soil root zone under heavy metal stress. Environ Sci Pollut Res 23:4742–4755. https://doi.org/10.1007/s11356-015-5695-9
Hao Y, Cao X, Ma C, Zhang Z, Zhao N, Ali A, Hou T, Xiang Z, Zhuang J, Wu S, Xing B, Zhang Z, Rui Y (2017) Potential applications and antifungal activities of engineered nanomaterials against gray mold disease agent Botrytis cinerea on rose petals. Front Plant Sci 8:1332. https://doi.org/10.3389/fpls.2017.01332
Hao Y, Ma C, Zhang Z, Song Y, Cao W, Guo J, Zhou G, Rui Y, Liu L, Xing B (2018) Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ Pollut 232:123–136. https://doi.org/10.1016/j.envpol.2017.09.024
Hartwig A (2013) Cadmium and Cancer. In: Sigel A, Sigel H, Sigel R (eds) Cadmium: from toxicity to essentiality. Met Ions Life Sci 11:491–507. https://doi.org/10.1007/978-94-007-5179-8-15
Hu XF, Jiang Y, Sun Y, Hu X, Liu L, Luo F (2014) Effects of mining wastewater discharges on heavy metal pollution and soil enzyme activity of the paddy fields. J Geochem Explor 147:130–150. https://doi.org/10.1016/j.gexplo.2014.08.001
Hu Y, Wang D, Li Y (2016) Environmental behaviors and potential ecological risks of heavy metals (Cd, Cr, Cu Pb and Zn) in multimedia in an oilfield in China. Environ Sci Pollut Res 23:13964–13972. https://doi.org/10.1007/s11356-016-6589-1
Jeon H, Carl G, Simon J, Kim G (2015) A mini-review: cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J Biomed Mater Res B Appl Biomater 102:1580–1594. https://doi.org/10.1002/jbm.b.33158
Ji Y, Zhou Y, Ma C, Feng Y, Hao Y, Rui Y, Wu W, Gui X, Le VN, Han Y, Wang Y, Xing B, Liu L, Cao W (2017) Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol Biochem 110:82–93. https://doi.org/10.1016/j.plaphy.2016.05.010
Jin L, Son Y, Yoon TK, Kang YJ, Kim W, Chung H (2013) High concentrations of single-walled carbon nanotubes lower soil enzyme activity and microbial biomass. Ecotoxicol Environ Saf 88:9–15. https://doi.org/10.1016/j.ecoenv.2012.10.031
Khan S, El-Latif Hesham A, Qiao M, Rehman S, He J (2010) Effects of Cd and Pb on soil microbial community structure and activities. Environ Sci Pollut Res 17:288–296. https://doi.org/10.1007/s11356-009-0134-4
Konate A, He X, Zhang Z, MaY ZP, Alugongo GM, Rui Y (2017) Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 9(5):790. https://doi.org/10.3390/su9050790
Kumar SV, Kumar AD, Kumar MP, Shah A, Kumar V, Gantait S (2018) Engineered nanomaterials for plant growth and development: a perspective analysis. Sci Total Environ 630:1413–1435. https://doi.org/10.1016/j.scitotenv.2018.02.313
Kumar A, Gupta K, Dixit S, Mishra K, Srivastava S (2019a) A review on positive and negative impacts of nanotechnology in agriculture. Int J Environ Sci Technol 16:2175–2184. https://doi.org/10.1007/s13762-018-2119-7
Kumar SV, Kumar AD, Gantait S, Kumar V, Gurel E (2019b) Applications of carbon nanomaterials in the plant system: a perspective view on the pros and cons. Sci Total Environ 667:485–499. https://doi.org/10.1016/j.scitotenv.2019.02.409
Lejon DPH, Pascault N, Ranjard L (2010) Differential copper impact on density, diversity and resistance of adapted culturable bacterial populations according to soil organic status. Eur J Soil Biol 46:168–174. https://doi.org/10.1016/j.ejsobi.2009.12.002
Li J, Xu Y, Ling X, Lin D, Sun Y, Wang L (2014) In situ immobilization remediation of heavy metals in contaminated soils: a review. Ecol Environ Sci 23(4):721–728. https://doi.org/10.16258/j.cnki.1674-5906.2014.04.013
Liang Y, He X, Liang S, Zhang W, Chen X, Feng S, Su Y (2014) Community structure analysis of soil ammonia oxidizers during vegetation restoration in southwest China. J Basic Microbiol 54(3):180–189. https://doi.org/10.1002/jobm.201300217
Liu Z, Qian G, Zhou J, Li C, Xu Y, Qin Z (2008) Improvement of ground granulated blast furnace slag on stabilization/solidification of simulated mercury-doped wastes in chemically bonded phosphate ceramics. J Hazard Mater 157(1):146–153. https://doi.org/10.1016/j.jhazmat.2007.12.110
Lyv Y, Yu Y, Li T, Cheng J (2018) Rhizosphere effects of Loliumperenne L. and Betavulgaris var. cicla L. on the immobilization of Cd by modified nanoscale black carbon in contaminated soil. J Soils Sediments 18(1):1–11. https://doi.org/10.1007/s11368-017-1724-2
Ma JF, Ueno D, Zhao FJ, McGrath SP (2005) Subcellular localisation of Cd and Zn in the leaves of a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Planta 220(5):731–736. https://doi.org/10.1007/s00425-004-1392-5
Madigan M, Cox SS, Stegeman RA (1984) Nitrogen fixation and nitrogenase about activities in members of the family Rhodospirilla. J Bacteriol 157(1):73–81
Minnikova TV, Denisova TV, Mandzhieva SS, Kolesnikov SI, Minkian TM, Chaplygin VA, Burachevskaya MV, Sushkova SN, Bauer TV (2017) Assessing the effect of heavy metals from the Novocherkassk power station emissions on the biological activity of soils in the adjacent areas. J Geochem Explor 174:70–78. https://doi.org/10.1016/gexplo.2016.06.007
Mohamed BA, Ellis N, Kim CS, Bi X (2017) The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ Pollut 230:320–338. https://doi.org/10.1016/j.envpol.2017.06.075
Mohtadi A, Ghaderian SM, Schat H (2012) A comparison of lead accumulation and tolerance among heavy metal hyperaccumulating and non-hyperaccumulating metallophytes. Plant Soil 352:267–276. https://doi.org/10.1007/s11104-011-0994-5
Qin Y, Li X, Xu W, Chai Y, Chi S, Wang W, Li T, Huang C, Zheng Y (2018) Effects of exogenous cadmium on activity of antioxidant enzyme, Cd uptake and chemical forms of ryegrass. Appl Ecol Environ Res 16(2):1019–1035. https://doi.org/10.15666/aeer/1602_10191035
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Zia-ur-Rehman M, Farid M, Abbas F (2017) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16. https://doi.org/10.1016/j.jhazmat.2016.05.061
Rodrigo WDS, Schmidt ÉC, Bouzon ZL (2013) Changes in ultrastructure and cytochemistry of the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales) treated with cadmium. Protoplasma 250(1):297–305. https://doi.org/10.1007/s00709-012-0412-8
Rodriguez MJA, Arana DC, Ramos-Miras JJ, Gil C, Boluda R (2015) Impact of 70 years urban growth associated with heavy metal pollution. Environ Pollut 196:156–163. https://doi.org/10.1016/j.envpol.2014.10.014
Ruttens A, Adriaensen K, Meers E, De Vocht A, Geebelen W, Carleer R, Mench M, Vangronsveld J (2010) Long-term sustainability of metal immobilization by soil amendments: cyclonic ashes versus lime addition. Environ Pollut 158:1428–1434. https://doi.org/10.1016/j.envpol.2009.12.037
Servin A, Elmer W, Mukherjee A, Torre-Roch R, Handi H, White JC, Bindarban P, Dimkpa C (2015) A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J Nanopart Res 17:92. https://doi.org/10.1007/s11051-015-2907-7
Singh A, Prasad SM (2015) Remediation of heavy metal contaminated ecosystem: an overview on technology advancement. Int J Environ Sci Technol 12:353–366. https://doi.org/10.1007/s13762-014-0542-y
Sun Y, Sun G, Xu Y, Liu W, Liang X, Wang L (2016) Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J Environ Manag 166:204–210. https://doi.org/10.1016/j.jenvman.2015.10.017
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46:4434–4441. https://doi.org/10.1021/es204212z
Yang Z, Liu S, Zhang D, Feng S (2006) Effects of cadium, zinc and lead on soil enzyme activities. J Environ Sci 18(6):1135–1147. https://doi.org/10.1016/S1001-0742(06)60051-X
Yang Q, Li Z, Lu X, Duan Q, Huang L, Bi J (2018) A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ 642:690–700. https://doi.org/10.1016/j.scitotenv.2018.06.068
Zhao Q, Luo Y (2015) The macro strategy of soil protection in China. Bull Chin Acad Sci 30(4):452–458. https://doi.org/10.16418/j.issn.1000-3045.2015.04.003
Zhao Q, Huang G, Qan H (2007) Ecological agriculture and food safety. Acta Pedol Sin 44(6):1127–1134. https://doi.org/10.11766/trxb200606200624
Zhou D, Wang Y, Wang H, Wang S, Cheng J (2010) Surface-modified nanoscale carbon black used as sorbents for Cu(II) and Cd(II), J Hazard Mater 174:34–39. https://doi.org/10.1016/j.jhazmat.2009.09.012
Funding
This work was supported by the National Natural Science Fund Committee, China (Grant Numbers. 41877119 and 41471255) and the National Key Research and Development Program of China (Grant Numbers. 2018YFD0800306-04 and 2018YFF0213404).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Kitae Baek
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Cheng, J., Sun, Z., Li, X. et al. Effects of modified nanoscale carbon black on plant growth, root cellular morphogenesis, and microbial community in cadmium-contaminated soil. Environ Sci Pollut Res 27, 18423–18433 (2020). https://doi.org/10.1007/s11356-020-08081-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08081-z