Abstract
Many remediation options have been applied to the heavy metal-contaminated agricultural soils nearby abandoned mining sites mainly due to hazard effects of heavy metals to human through agricultural crop dietary. Hence, the current study was carried to examine the heavy metal immobilizing effect of biochar produced from rice hull and subsequent heavy metal uptake by lettuce. Rice hull biochar was incorporated into a heavy metal-contaminated upland soil at six application rates (0, 0.5, 1, 2, 5, and 10 % (v/v)) and soil biochar mixtures were examined using both incubation and pot trials for cultivation of lettuce. Incubation studies showed that biochar incorporation induced significant declines (>80 %) in the phytoavailable metal pool as assessed via 1 M NH4NO3 extraction, possibly due to increased heavy metal adsorption onto the applied biochar and increases in soil pH. Similar results were also observed in pot trials, where the uptake of heavy metals by lettuce was significantly reduced as biochar application rate increased. Despite the significant decline in soil phytoavailable metal pools, lettuce growth still declined as biochar application rate increased. This was attributed to the adsorption of available nitrogen on to the biochar resulting in nitrogen deficiency. Therefore, when the biochar is used for metal immobilization in agricultural soils, maintaining soil nutrient status should be also considered to ensure optimum growth of the crop plants besides metal immobilization rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils near mining sites are often contaminated by heavy metals due to deposition of airborne mining dust and inflow of aqueous runoff from acid mining drainage (Bech et al. 1997; Lee et al. 2001). This becomes a major human health concern when such soils are used in agriculture to produce food for human consumption (Lee et al. 2005; Liu et al. 2005). Therefore, many physical-, chemical-, and biological-based remediation technologies were developed (Mulligan et al. 2001) to immobilize heavy metals using various soil amendments such as compost, lime, and phosphate (Kumpiene et al. 2008; Bolan et al. 2014). Among the myriad of potential amendments proposed, liming materials are the most popular. These materials increase soil pH resulting in declines in available metal pools through three mechanisms, (1) deprotonation of the soil surface (Kim et al. 2012), (2) precipitation of metal ions as carbonates (Lee et al. 2009, 2012), and (3) changes in metal ion speciation, primarily to hydroxyl species which are preferentially adsorbed by soil components (Lombi et al. 2003). Other alkaline materials, such as fly ash and hydroxyapatite, also exhibit similar effects on metal immobilization through increases in soil pH when incorporated into soils (Kumpiene et al. 2008).
Biochar (BC) can be produced from a wide range of organic wastes via pyrolysis, where due to its versatility it has been widely applied in many different research areas, i.e., for carbon sequestration in soils (Lehmann et al. 2006; Lehmann 2007), as physicochemical soil amendments (Glaser et al. 2002; Liang et al. 2006) and to improve of soil productivity (Lehmann et al. 2003; Chan et al. 2007; Steiner et al. 2007; Major et al. 2010). In addition, like lime and fly ash, BC also increases soil pH (Chan and Xu 2009) and hence can act as an immobilizing agent for heavy metals, although relatively little research has been conducted in this respect.
Recently, hardwood-derived BC was successfully used to immobilize heavy metals in soils (Beesley et al. 2011). Xu et al. (2013) reported that the sorption capacities of the BC produced from dairy manure at 350 °C were 51.4, 54.4, and 32.8, mg g−1 for Cd, Cu, and Zn, respectively. In other studies, BC from orchard prunes reduced DTPA-extractable Cd, Pb, and Zn by 90, 38, and 24 %, respectively, when incorporated with mine tailings at 10 % (w/w) compared to the control (no BC treated) (Fellet et al. 2011). The extent to which BC increases heavy metal immobilization in soils varies depending on the feed materials used for BC production (Park et al. 2011a). For example, chicken manure-derived BC exhibited a relatively high immobilizing efficiency, showing declines in 1 M NH4NO3-extractable Cd and Pb of 89 and 94 % when incorporated with contaminated soil at 5 % (w/w), while green waste-derived BC showed only a 30 and 37 % decline in 1 M NH4NO3-extractable Cd and Pb (Park et al. 2011a). Chicken manure-derived BC actually increased 1 M NH4NO3-extractable Cu by 45 %, while simultaneously decreasing the extractabilities of all other metal ions (Cd and Pb). In contrast, 1 M NH4NO3-extractable Cu was decreased by green waste-derived BC even though the decrease (23 %) was limited and was concomitant with decreases in other examined metals (Cd and Pb). Such studies have demonstrated that appropriate feed materials for BC production need to be selected prior to practical application of BC as a metal immobilizing agent. Another aspect to consider is whether feed materials are easy to collect and whether bulk production of BC is sustainable, as substantial amounts of BC would be required for application to wide areas of contaminated soils in order to obtain an acceptable immobilizing effect. Taking such factors into consideration, a particularly good candidate for BC production are by-products derived from agriculture such as rice hull in Asia. For instance, 11,600,000 tons of biomass by-products were generated in 2009 in Korea among which biomass derived from rice cultivation accounted for 75 % (Park et al. 2011b). Furthermore, rice is a typical silicon accumulating plant and rice hull contains 15–20 % silica (Sun and Gong 2001; Ma and Yamaji 2006). Silicon (Si) has enhanced the growth and development of several crops and Si improved disease resistance in various plants including horticultural crops (Ma and Takahashi 1990; Savant et al. 1999; Matichekov and Bocharnikova 2004). Therefore, rice hull biochar would be better soil amendment than other materials for cultivation of crops in heavy metal-contaminated soil.
The current study used rice hull-derived BC as an alternative amendment for heavy metal immobilization to mitigate plant uptake. Lettuce, a common vegetable crop was chosen as a suitable test species to assess plant performance when grown in a BC-amended contaminated soil.
Methods and materials
Soil and rice hull-derived biochar
Soil sampling: The soil used for both incubation and pot studies was collected from a cultivated upland near an abandoned mining area where previous studies (Kim et al. 2012) had shown that the soils exceeded local environment guidelines for heavy metal (Cd, Cu, Pb, and Zn). Bulk soil (0–30 cm) collected from the upland was air-dried and sieved <2 mm, characterized, and stored in a plastic container until used for incubation and pot studies.
Soil characteristics: Soil pH and EC were measured in a 1:5 soil:distilled water suspension using a pH meter (MP220, Mettler Toledo, UK) and EC meter (MC226, Mettler Toledo, UK) after 1 h shaking. Soil organic matter was determined by the Walkley–Black method (Nelson and Sommers 1996) and clay content was determined using a micro pipette method (Miller and Miller 1987). For analysis of total nitrogen, soil was pretreated using the Kjeldal digestion method (Bremner 1996) and the amount of NH4 + was determined following distillation of the digested supernatant (Kjeltec 2300, Foss, Sweden). Available phosphorus was determined by the ascorbic acid method (Kuo 1996). Exchangeable cations (Ca, K, Mg, and Na) and cation exchange capacity (CEC), were both determined following a 1 N ammonium acetate (pH 7.0) soil extract and subsequent determination of cations in the extract, by atomic absorption spectrometer (AAS, AAnalyst 400, Perkin Elmer, USA) or NH4 + distillation (Sumner and Miller 1996) for CEC. The pseudo total heavy metal concentration in soil was determined by ICP-OES (iCAP 6,000 series, Thermo Scientific, U.K.) following aqua regia digestion of the soil in a commercial trace metal digestion system (SMA 20A, Gerhardt, UK). Hereafter, the aqua regia extractable concentration of heavy metals will be simply referred to as the total metal concentration (Kim et al. 2012).
Biochar characteristics: Rice hull-derived BC (pyrolyzed at 500 °C) was obtained commercially (DAEWON GSI, Korea). The supplied BC was finely milled in a mortar and pestle and sieved <0.5 mm, prior to use and characterization. The surface characteristics of the BC were observed using scanning electron microscopy (SEM, SUPRA 55VP, Germany). Cation content, pH, and EC of the BC were determined following extraction of the BC (60 mL) with distilled water (300 mL) as described by the European Committee for Standardization (CEN (Committee for European Normalization) 2001, 2011a, b). Total carbon and nitrogen contents were determined using a C/N auto-analyzer (Vario Max CN, Elementar Analysen system GmbH, Germany). Cation exchange capacity and total metal concentrations were determined using the methods described above for soils.
Incubation study
Soil (2 kg) was incorporated with BC at six different soil:BC (SBC) ratios; 0 (control), 0.5 (BC0.5), 1 (BC1), 2 (BC2), 5 (BC5), and 10 (BC10) % (w/w) and stored in opaque plastic containers (9 L) at a moisture content of 70 % of the mixture’s maximum water holding capacity (Kim et al. 2010b). Each treatment consisted of four replicates. The moist soil mixtures were lidded and incubated at 25 ± 2 °C for 8 weeks, while maintaining the moisture content through periodic weighing and occasional supplement of moisture as necessary. During incubation, sub-samples (100 g) were periodically collected from each container at 2 weeks intervals (0, 2, 4, and 8 weeks), air-dried and assessed for the effect of incorporated BC on heavy metal immobilization.
Plant growth study
As described for the incubation studies, SBC mixtures were prepared at six treatment levels of BC [0, 0.5, 1, 2, 5, and 10 % (w/w)]. Prior to use in the plant growth study, the SBC mixtures were moistened at 70 % of their maximum water holding capacity and incubated at 25 ± 2 °C for 1 week. A portion of each treatment soil (400 g) was distributed to five plastic pots (diameter 10 cm × height 10 cm) and 28-days-old lettuce seedlings of uniform size were transplanted into each pot. Lettuce (Lactuca sativa L.) was subsequently cultivated for 30 days in a growth chamber (day time, 16 h; night time 8 h; temperature, 25 °C; light, 500 mol m−2 s−1) and then harvested for assessment of the effect of BC on plant metal uptake and growth. During cultivation nutrient solution was supplied once every 3 days in the early stage (<2 weeks) and then every other day in the later stage (>2 weeks) through the bottom of each pot by adding the nutrient solution to the saucer of each pot. The nutrient solution contained, major nutrients (6, 0.5, 1.5, 4, 2, 1 mg L−1 for NO3-N, NH4-N, P, K, Ca, and SO4-S, respectively), and minor nutrients (2, 0.01, 0.2, 0.2, 0.2, and 0.5 mg L−1 for Fe, Cu, B, Mn, Zn, and Mo, respectively).
Analysis of soil and plant
Incubated soils: Soil pH and total metal contents were determined as described above (Sect. 2.1). Phytoavailable metal contents (DIN (Deutches Institute für Normung) 1995) were determined using 1 M NH4NO3 extraction (Kim et al. 2010a). Briefly, soil (10 g) was extracted with 1 M NH4NO3 (25 mL) and shaking on an end-over-end shaker for 2 h and the metal content in the extracted solution determined by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, iCAP 6,000 series, Thermo Scientific, UK). The surface area of the SBC mixtures was measured by the Brunauer-Emmett-Teller (BET) method using a surface area analyzer (NOVA-1200, Quantachrome Corp., USA). All analysis was conducted in triplicate.
Pot trial soils: Soils used in the pot trial were analyzed before and after lettuce cultivation. Soil pH and the phytoavailable metal content were determined using the methods described above for incubated soils. Inorganic nitrogen content, as NH4–N and NO3–N, was determined following extraction with 2 M KCl. The NH4–N in the extract was initially determined, via nitrogen distillation and titration after adding MgO, while the NO3–N contents in the extract were determined by nitrogen distillation and titration after adding Devarda’s alloy (Mulvaney 1996). Dissolved organic carbon (DOC) was determined following extraction of soil (5 g) with distilled water (25 mL) and shaking for 2 h. The extract solution was filtered (<0.45 µm) prior to DOC determination using a TOC analyzer (2100S, Analytik Jena, Germany).
Plant material: At harvest, lettuce leaves were washed once with tap and twice with distilled water to remove any adhering soil and dried in a fan-forced oven at 65 °C for 48 h. The dried plant tissues were weighed and then powdered using a commercial blender prior to determination of metal content following acid digestion. As previously described (Kim et al. 2010a), dried plant tissue (0.5 g) was digested with concentrated HNO3 (5 mL) using a digester (Tecator 2020, Foss, Sweden), diluted 50 mL using distilled water and filtered through Whatman No. 42 filter papers prior to determination of total metal content by ICP-OES.
Data treatment and statistics
In order to assess the relative effect of BC on metal immobilization, the percentage of immobilized metal was calculated using Eq. 1 (Park et al. 2011a).
For ease of visualization, all data presented in tables and figures correspond to mean values with errors corresponding to one standard deviation of the mean. Any significant differences among treatments were determined by ANOVA using SAS 9.3 software (SAS for Windows v. 9.3, SAS Institute Inc., Cary, NC).
Results and discussion
Properties of soil and biochar
Soil: The selected physiochemical properties of the upland soil are presented in Table 1. The soil was slightly acidic (pH 5.7) and highly contaminated with heavy metals, with levels of Cd, Pb, and Zn exceeding the Korean standard guideline values of 4, 200, and 300 mg kg−1 for Cd, Pb, and Zn, respectively (MoE (Ministry of Environment) 2010). Other soils properties commonly associated with heavy metal availability, such as organic content (8.4 g kg−1) and clay content (8.4 %), were relatively low compared to the average values reported for Korean cultivated upland soils (Rim et al. 1997; Jo and Koh 2004). Nutrient status, as indicated by available P (23 mg kg−1) and total N (1.3 g kg−1), indicated that the soil was nutrient poor and that additional nutrient supplements were required for optimal plant growth.
Biochar: Heavy metal concentrations in the BC were very low compared to those in the soil and hence the amount of each metal added to the soil through BC application was negligible (Table 1). The pH was 10.2 implying potential for an increase in soil pH when incorporated into soil. The BC surface exhibited many porous areas (Supplementary Fig. 1) inducing elevated surface area where more heavy metal ions could potentially be adsorbed (Beesley and Marmiroli 2011). The potential for higher metal adsorption to the BC surface was also evidenced by the high CEC (50 cmolc kg−1).
Incubation study
Soil incorporation of BC resulted in a decrease in total metal concentration proportional to the amount of BC applied indicating a simple dilution effect. For instance, for BC10, the total concentrations of Cd, Cu, Pb, and Zn were 4, 103, 2,421, and 610 mg kg−1, respectively, which was 84, 82, 87, and 89 % of the corresponding metal content in the control soil.
Biochar soil incorporation increased soil pH immediately after BC application proportional to the amount applied. The highest increase of 1.4 pH units, relative to the pH of the control soil (pH 5.7), was observed in BC10 (Fig. 1). Following BC incorporation, a gradual increase in soil pH was observed for all soil treatments as incubation time increased. For example, for BC10, soil pH increased from 7.1 to 7.7 over 8 weeks. During the production of BC, pyrolysis of organic substances at elevated temperatures results in an increase in carbonate contents and generation of a wide range of additional functional groups, such as −COO¯ and O¯, on the BC surface. Thus, as a direct result of pyrolysis, BC becomes an alkaline material (Yuan et al. 2011), which induces soil pH increases when applied to soils and provides a negatively charged surface where cations can be absorbed. Application of various biochars to an ultisol soil (initial soil pH 4.0) at a rate of 10 g kg−1 saw soil pH increase by 0.32–1.28 units, which was attributed to the high pH and alkalinity of the BC exhibiting a liming effect (Yuan and Xu 2011). In agreement with that study, the increased soil pH observed here after BC application was most likely directly due to the high pH and alkalinity of the rice hull-derived BC. It was consequently expected that the observed increases in soil pH would be a key factor influencing metal phytoavailability.
Phytoavailable (1 M NH4NO3 extractable) metal pools decreased with the initial amount of BC application and continued to decrease during the 8 week incubation (Fig. 2). In order to evaluate the effect of BC on the metal immobilization, the immobilization rate was calculated using the phytoavailable metal pools determined in both control and BC10 soils after 8 weeks incubation. At 10 % (w/w) BC application, high immobilization rates were observed for all metals tested; 97 % for Cd, 90 % for Cu, and 100 % for Pb and Zn.
The high levels of metal immobilization observed following BC application which was primarily attributed to BC-induced increases in soil pH, which has previously been shown to be the most important soil environmental factor influencing metal solubility (Walker et al. 2004; Kumpiene et al. 2008; Kim et al. 2010a). Many previous studies, have also reported that a wide diversity of alkaline materials including limestone, beringite, red mud, and furnace slag have increased soil pH and subsequently increased heavy metals immobilization when incorporated into soils (Lombi et al. 2003; Gray et al. 2006; Lee et al. 2009). Increases in soil pH result in deprotonation of the soil surface resulting in an increase in soil surface negative charge, thus facilitating increases metal ion absorption. Also hydroxyl species of metal cations, which have higher affinity for the soil surface, are generated under alkaline conditions (Naidu et al. 1994; Bolan et al. 2003). BC-induced pH increases resulting in increased metal immobilization in soil seem to be a common occurrence independent of the source of BC feed materials. Beesley et al. (2010) reported an increase in soil pH and decreases in both Cd and Zn soil pore water concentrations following application of hardwood BC to a multi-element polluted soil. Jiang et al. (2012) also reported decreases in acid soluble Cu and Pb following application of rice straw BC to an artificially contaminated soil concomitant with an increase in soil pH. In close agreement, with these two previous studies, here, the application of rice hull-derived BC to a contaminated soil increased soil pH proportional to the amount of BC applied and subsequently decreased phytoavailable metal pools (Fig. 3). In addition to pH-induced immobilization of metals to soil surfaces; BC itself provides an additional high surface area with a wide range of functional groups that can directly decrease phytoavailable metal pools by absorption onto the BC surface. This interpretation is supported by examination of the data circled in Fig. 3. The BC5 soil when incubated for <2 weeks showed a similar soil pH to those of the BC1 and BC2 soils which had incubated for >4 weeks. However, phytoavailable Cd, Pb, and Zn pools were much lower in the BC5 soil than those in the BC1 and BC2 soil despite similar soil pH. This implied that, for the same pH, the larger amount of BC in the BC5 soil compared to either BC1 or BC2 soils provided more sorption sites for Cd, Pb, and Zn cations, and consequently decreased metal phytoavailability. This is also supported by the observed increases in BET surface area with increased BC incorporation. For instance, after 4 weeks incubation, the BET of SBC mixtures increased from 15 m2 g−1 in the control to 16 and 23 m2 g−1 in BC2 and BC10, respectively.
Pot trial
Soil pH and phytoavailable metal pools
As was shown in the incubation study, BC incorporation with soil 1 week before transplantation of lettuce increased soil pH proportional to the BC application rate and this increase was maintained throughout growth until harvest. For instance, the soil pH of BC10 was 7.5 at harvest compared to only 5.8 in the control. Increased soil pH resulting from BC application also induced decreases in phytoavailable metal pools (Fig. 4). Before lettuce cultivation, the immobilization rates for Cd, Cu, Pb, and Zn for BC10 were 86, 85, 96, and 93 %, respectively. After lettuce cultivation, while the immobilization rates for Cd, Pb, and Zn were similar to those determined prior to lettuce cultivation, the Cu immobilization rate had significantly declined from 85 to 49 %, implying that Cu phytoavailability had been significantly increased during lettuce cultivation. Indeed, phytoavailable Cu pools for BC1, BC2, BC5, and BC10 increased by 28, 41, 156, and 124 % from those determined before lettuce cultivation. This was partially attributed to increased soil DOC concentrations following lettuce cultivation due to root exudation (Kim et al. 2010c). Cu is known to form strong metal complexes with organic constitutes in soil solution which elevates Cu solubility. DOC concentrations after 4 weeks cultivation were 119, 139, 151, 151, and 143 mg kg−1 for control, BC1, BC2, BC5, and BC10, respectively (Table 2).
Metal uptake by lettuce and its growth
As expected from the observed decrease in soil phytoavailable metal pools following BC application, the lettuce tissue concentration of all metals, except Cu, decreased with the amount of BC applied (Fig. 5). At a treatment rate of only 1 % (w/w), BC application was effective in decreasing the concentration of Cd, Pb, and Zn in lettuce relative to the control, with the biggest decrease being observed for BC10. The accumulated Cd, Pb, and Zn concentrations in lettuce cultivated in BC10 were respectively, 88, 60, and 66 % lower than those of the control soil. For these three metals, the lowered metal accumulation by lettuce was attributed principally to increases in pH which induced a concomitant decrease in their phytoavailable pools following BC application. Similar decreases in metal uptake have previously been reported in the literature following application of a pH ameliorant. For example, application of red mud resulted in a pH increase which induced immobilization of heavy metals in soil, and subsequently decreased translocation of heavy metals to the above ground tissues of Festuca rubra (Gray et al. 2006). Application of rice-derived biochars also resulted in increased metal immobilization during rice cultivation as evidenced by declines in Cd, Pb, and Zn concentration in rice by 98, 72, and 83 % (Zheng et al. 2012).
In contrast to these three metals, the variation of Cu uptake by lettuce following BC application was very different. The concentration of Cu in lettuce cultivated in soils receiving <5 % BC was not significantly different from the control and only at a BC application rate of 10 % was a significant decrease (28 %) in accumulated Cu observed. This was due to an increase in the phytoavailable Cu pool in soils following lettuce cultivation (Fig. 4) since irrespective of the amount of BC applied; all soils experienced an increase in phytoavailable Cu following cultivation of lettuce for 4 weeks. The Cu solubility in soils is primarily governed by DOC concentrations, rather than soil pH, when the soil pH is above 7.5, so that Cu solubility increases as DOC concentrations increase, resulting in increased metal uptake by plants (Kim et al. 2009, 2012). In addition, many plants exude a wide range of organic substance from their roots into to the rhizosphere, which contributes to elevated DOC concentration in the soil (Kim et al. 2010b, c). While the incubation study had shown that BC was effective in Cu immobilization over an 8 weeks period, in direct contrast, the pot trial immobilization of Cu by BC was retarded within only 4 weeks of lettuce cultivation. This strongly suggested that lettuce was responsible for the increased DOC concentration in soil, most likely due to increased production of root exudates which increased the amount of phytoavailable Cu in the soil and consequently offset the beneficial immobilization effects of BC. As described in 3.3.1, lettuce cultivation for 4 weeks increased DOC concentrations, with the largest increases (18–70 mg kg−1) observed with BC-treated soils compared to only 14 mg kg−1 in the control for DOC concentrations (Table 2).
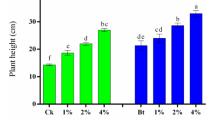
Despite decreases in metal accumulation in the above ground tissue of lettuce, the growth of lettuce, as determined by the total dry weight yield, was hindered as BC application rate increased above 0.5 % and the maximum dry weight yield of lettuce (1.07 g) was observed at 0.5 % (w/w) BC (Fig. 6). While the positive effects of BC as soil amendment to enhance plant growth have been evidenced in many previous studies (Atkinson et al. 2010; Major et al. 2010), in agreement with this study, some researchers have reported detrimental effects of BC application on plant growth. Rajkovich et al. (2012) found that for corn, irrespective of the type of BC, no growth promotion was observed when the BC application rate was >2 %. This lack of any beneficial effect at higher application rates has been attributed to a decline in available nutrients. For example, BC incorporated with soil for 25 days decreased nitrate concentrations in soil leachate proportional to the amount of BC applied indicating that BC application was likely to hinder plant growth by decreasing available inorganic nitrogen due to adsorption of nitrogen on to the BC surface (Novak et al. 2010).
In agreement with this previous study, a decline in soil inorganic nitrogen concentration was also observed here following BC incorporation (Fig. 6). During the pot trial, while the same amount of nutrient solution, containing 91 mg L−1 N, was supplied to all treatment, residual soil concentrations of inorganic N following lettuce cultivation were higher in the soils which received more BC. This indicated that while lettuce was able to readily utilize the supplied nitrogen in the soil when BC contents were low, at higher BC application rates (>5 %) it became increasingly difficult for the lettuce to access the supplied nitrogen due to increased partitioning of nitrogen to the BC surface. The change in soil nitrogen content (ΔN) which was equivalent to (N concentration after lettuce cultivation—N concentration before lettuce cultivation) increased with elevated BC application rate resulting in retardation of lettuce growth (Fig. 6). Indeed, the growth of lettuce was hindered with >5 % BC application than with <1 % BC application being indicative with significantly higher dry weight of lettuce appeared with <1 % BC treatment. This result highlights the importance of accounting for the possibility of adsorption of inorganic nitrogen by BC as a limiting factor when BC is used as an amendment for agricultural soils, suggesting that appropriate nitrogen management should accompany with BC application.
Conclusion
When considering application of BC for heavy metal immobilization to agricultural soils, the immobilization efficiency of the BC and the availability of feed materials for producing bulk BC are important issues. Rice hull-derived BC is a promising material for agricultural soil amendment because it is an abundant agricultural by-product and as shown here an effective heavy metal (Cd, Pb, and Zn) immobilization agent. Incorporation of BC into soils between 1 and 10 % (w/w) induced significant declines in soil phytoavailable metal pools and consequential decreases in lettuce metal uptake. The decline in metal phytoavailability was attributed to rice hull-derived BC-induced elevation of soil pH and also increased surface area for metal sorption. Despite the beneficial effects of increased metal immobilization, no significant increases in yield were observed at high BC application rates due to concomitant decreases in phytoavailable soil nitrogen. Thus, soil nutrient status together with BC application rate need to be both managed to ensure optimum growth of the species of interest.
References
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Bech J, Poschenrieder C, Llugany M, Barceló J, Tume P, Tobias FJ, Barranzuela JL, Vásquez ER (1997) Arsenic and heavy metal contamination of soil and vegetation around a copper mine in Northern Peru. Sci Total Environ 203:83–91
Beesley L, Marmiroli M (2011) The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ Pollut 159:474–480
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158:2282–2287
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159:3269–3282
Bolan NS, Adriano DC, Mani PA, Duraisamy A (2003) Immobilization and phytoavailability of cadmium in variable charge soils. II. Effect of lime addition. Plant Soil 251:187–198
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J Harzard Mater 266:141–166
Bremner JM (1996) Nitrogen-total. In: Soil Science society of America and America Society of Agronomy (ed) Methods of soils analysis, part 3, chemical methods. SSSA Books Series 5, Madison, WI, pp 1085–1122
CEN (Committee for European Normalization) (2001) EN 13652: 2001 Soil improvers and growing media—Extraction of water soluble nutrients and elements. Belgium, Brussels
CEN (Committee for European Normalization) (2011a) EN 13037: 2011 Soil improvers and growing media—Determination of pH. Belgium, Brussels
CEN (Committee for European Normalization) (2011b) EN 13038: 2011 Soil improvers and growing media—Determination of electrical conductivity. Belgium, Brussels
Chan KY, Xu Z (2009) Biochar: nutrient properties and their enhancement. In: Lehmann J, Joseph S (eds) Biochar for environmental management. Earthscan, London, pp 67–84
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of green waste biochar as a soil amendment. Aust J Soil Res 45:629–634
DIN (Deutches Institute für Normung) (1995) DIN 19730 Soil quality extraction of trace elements with ammonium nitrate solution. Beuth, Berlin, Germany
Fellet G, Marchiol L, Delle Vedove G, Peressotti A (2011) Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 83:1262–1267
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35:219–230
Gray CW, Dunham SJ, Dennis PG, Zhao FJ, McGrath SP (2006) Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ Pollut 142:530–539
Jiang J, Xu RK, Jiang TY, Li Z (2012) Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J Hazard Mater 229–230:145–150
Jo IS, Koh MH (2004) Chemical changes in agricultural soils of Korea: data review and suggested countermeasures. Environ Geochem Health 26:105–117
Kim KR, Owens G, Naidu R (2009) Heavy metal distribution, bioaccessibility, and phytoavailability in long-term contaminated soils from Lake Macquarie, Australia. Aust J Soil Res 47:166–176
Kim KR, Owens G, Kwon SI (2010a) Influence of Indian mustard (Brassica juncea) on rhizosphere soil solution chemistry in long-term contaminated soils: a rhizobox study. J Environ Sci 22:98–105
Kim KR, Owens G, Naidu R (2010b) Effect of root-induced chemical changes on dynamics and plant uptake of heavy metals in rhizosphere soils. Pedosphere 20:494–504
Kim KR, Owens G, Naidu R, Kwon SI (2010c) Influence of plant roots on rhizosphere soil solution composition of long-term contaminated soils. Geoderma 155:86–92
Kim KR, Kim JG, Park JS, Kim MS, Owens G, Youn GH, Lee JS (2012) Immobilizer-assisted management of metal-contaminated agricultural soils for safer food production. J Environ Manage 102:88–95
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manage 28:215–225
Kuo O (1996) Phosphorus. In: Soil Science society of America and America Society of Agronomy (ed) Methods of soils analysis, part 3, chemical methods. SSSA Books Series 5, Madison, WI, pp 869–919
Lee CG, Chon HT, Jung MC (2001) Heavy metal contamination in the vicinity of the Daduk Au-Ag-Pb-Zn mine in Korea. Appl Geochem 16:1377–1386
Lee JS, Chon HT, Kim KW (2005) Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ Geochem Health 27:185–191
Lee SH, Lee JS, Choi YJ, Kim JG (2009) In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 77:1069–1075
Lee SS, Lim JE, El-Azeem SAMA, Choi B, Oh SE, Moon DH, Ok YS (2012) Heavy metal immobilization in soil near abandoned mines using eggshell waste and rapeseed residue. Environ Sci Pollut Res 20:1719–1726
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387
Lehmann J, da Silva JP Jr, Steiner C, Nehls T, Zech W, Glaser B (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—review. Mitig Adapt Strateg Global Change 11:395–419
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Lombi E, Hamon RE, McGrath SP, McLaughlin MJ (2003) Lability of Cd, Cu, and Zn in polluted soils treated with lime, beringite, and red mud and identification of a non-labile colloidal fraction of metals using isotopic techniques. Environ Sci Technol 37:979–984
Ma J, Takahashi E (1990) Effect of silicon on the growth and phosphorus uptake of rice. Plant Soil 126:115–119
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128
Matichekov V, Bocharnikova E (2004) Si in horticultural industry. In: Dris R, Jain SM (eds) Production practices and quality assessment of food crops, vol 2., Plant mineral nutrition and pesticide managementKluwer, Dordrecht, pp 217–228
Miller WP, Miller DM (1987) A micro-pipette method for soil mechanical analysis. Comm Soil Sci Plant Anal 18:1–15
MoE (Ministry of Environment) (2010) Soil environment conservation act. Gwacheon, Korea
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Mulvaney RL (1996) Nitrogen-inorganic forms. In: Soil Science society of America and America Society of Agronomy (ed) Methods of soils analysis, part 3, chemical methods. SSSA Books Series 5, Madison, WI, pp 1123–1184
Naidu R, Bolan NS, Kookana RS, Tiller KG (1994) Ionic-strength and pH effects on the adsorption of cadmium and the surface charge of soils. Eur J Soil Sci 45:419–429
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Soil Science society of America and America Society of Agronomy (ed) Methods of soils analysis, part 3, chemical methods. SSSA Books Series 5, Madison, WI, pp 961–1009
Novak JM, Busscher WJ, Watts DW, Laird DA, Ahmedna MA, Niandou MAS (2010) Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 154:281–288
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011a) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Park WK, Park NB, Shin JD, Hong SG, Kwon SI (2011b) Estimation of biomass resource conversion factor and potential production in agricultural sector. Korean J Environ Agric 30:252–260 (In Korean, with English abstract)
Rajkovich S, Enders A, Hanley K, Hyland C, Zimmerman AR, Lehmann J (2012) Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol Fertil Soils 48:271–284
Rim SK, Hur BK, Jung SJ, Hyeon GS (1997) Physico-chemical properties on the management groups of upland soils in Korea. Korean J Soil Sci Fert 30:67–71 (In Korean, with English abstract)
Savant NK, Korndorfer GH, Datnoff LE, Snyder GH (1999) Silicon nutrition and sugarcane production: a review 1. J Plant Nurt 22:1853–1903
Steiner C, Teixeira WG, Lehmann J, Nehls T, de Macêdo JLV, Blum WEH, Zech W (2007) Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 291:275–290
Sumner ME, Miller WP (1996) Cation exchange capacity and exchange coefficients. In: Soil Science society of America and America Society of Agronomy (ed) Methods of soils analysis, part 3, chemical methods. SSSA Books Series 5, Madison, WI, pp 1201–1230
Sun L, Gong K (2001) Silicon-based Materials from rice husks and their applications. Ind Eng Chem Res 40:5861–5877
Walker DJ, Clemente R, Bernal MP (2004) Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 57:215–224
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd form aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res 20:358–368
Yuan JH, Xu RK (2011) The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use and Manage 27:110–115
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497
Zheng RL, Cai C, Liang JH, Huang Q, Chen Z, Huang YZ, Arp HPH, Sun GX (2012) The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 89:856–862
Acknowledgments
This study was financially supported by the EI project (project No. 2012000210003), Ministry of Environment, Korea. Dr Gary Owens gratefully acknowledges the financial support of the Australian Research Council Future Fellowship Scheme (grant number FT120100799) for funding his salary.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, HS., Kim, KR., Kim, HJ. et al. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ Earth Sci 74, 1249–1259 (2015). https://doi.org/10.1007/s12665-015-4116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4116-1