Abstract
The current study was conducted in order to examine the applicability of rice hull derived biochar (BC) to improve the properties of growing media (GM). Biochar was incorporated into a growing media composed of coir dust, perlite and vermiculite at 0, 1, 2 and 5 % (w/w). Subsequently, the physicochemical properties of the GM-BC mixtures were determined in the cultivation of kale (Brassica oleracea L. var. acephala) for 25 days through the observation of the plant growth response. During kale cultivation in the GM-BC mixtures, the leachates were collected and analyzed to determine the changes in nutrient levels due to BC amendment. Application of rice hull-derived BC increased the retention of nutrients in the growing media due to a biochar-induced increase in cation exchange capacity, in addition to the biochar nutrient supply such as potassium and phosphorus. Furthermore, a higher water content of the growing media was observed when BC was used as an amendment, mainly due to the increased proportion of pore space available for water storage. The growth rate of kale was also increased as the biochar incorporation rate was increased. For example, the dry weight of the kale shoots was 150 % higher when grown in media containing 5 % GM-BC mixture than with the control growing media (with no biochar). From these results, it can be concluded that the rice hull-derived biochar would be a practically applicable amendment to improve the properties of the growing media.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, growing media are used in horticulture for seedling production, plant propagation, and ornamental plant production. Hence, the components of the media are required to be stable with suitable physical, chemical and biological properties for supporting plant growth performance.
The growing media are composed mainly of different fractions of organic material such as coir dust and peat moss, and inorganic material such as perlite and vermiculite [1, 2]. However, due to the variation in quality of the raw materials produced in each country for the production of growing media, the properties of growing media are not consistent, and in some cases are not good enough for plant cultivation [3]. Therefore, investigation into prospective materials to improve the properties of growing media is required.
Addition of organic and inorganic materials such as agriculture waste, compost, clay and fly ash have been demonstrated to improve the physicochemical properties of growing media [4–7]. For example, Marianthi [5] reported that the application of rice hulls and ground kenaf core increased the total porosity and concentration of nutrients such as nitrogen (N), potassium (K) and magnesium (Mg) in the growing media. Ribeiro et al. [6] also showed that increasing the percentage of compost (forestry wastes to solid phase of pig slurry, 3:1 by volume) in growing media, increased the pH and availability of N, calcium (Ca), and Mg, consequently enhancing the growth of tomato seedlings. Currently, continuous investigation and performance evaluation of amendments to improve the quality of growing media are in progress and this study shares the same objective.
Biochar is a carbonaceous material produced through the pyrolysis of organic materials in the absence of oxygen, which has been used widely for carbon sequestration in soils [8–10]. In addition, many studies have demonstrated that biochar incorporation into soils induces improvement of the soil properties and structure, resulting in increased plant growth and crop yield [11–15]. Likewise, when used as a soil amendment, biochar is likely to improve the properties of growing media in the horticultural industry. Recently, Zhang et al. [16] reported an increase in the water holding capacity and macro- and micro nutrient contents of the growing media when mixed with coconut husk fiber derived biochar, and as a result the growth performance of the ornamental plant, Calathea insignis was improved. Furthermore, although biochar was not applied directly to the soil, biochar incorporation into growing media offers the opportunity to sequester carbon as part of the normal outplanting process [17]. To date, only a few studies have reported the potential use of biochar as a growing media amendment [17–19].
To be utilized in a growing media, the candidate component should (1) be available in large amounts, (2) be as homogeneous as possible, (3) be low in price and (4) have minimal transport costs [20]. This implies that feed materials for the production of biochar, practically used as a growing media amendment, should be stable and easy to collect. In addition, the bulk amount should be available within the local area in order to minimize the transport cost. From this perspective, rice hull derived from agriculture would be a good candidate for biochar production in Asia. The total generation of by-products derived from rice cultivation in Korea in 2009 is estimated to have been approximately 7.6 million tons, while the biomass by-products derived from agriculture is approximately 12 million tons [21]. In general, improvement in the physicochemical properties of growing media by the incorporation of biochar depends on the type of biochar used, because biochar derived from various feedstocks show different characteristics [13, 22]. It should be noted therefore, that each biochar from a different feedstock must be carefully evaluated before practical application.
The main objective of this study is to evaluate the suitability of rice hull-derived biochar as a growing media amendment, through the determination of physical and chemical properties together with a plant growth study.
Materials and Methods
Growing Media Preparation
The rice hull biochar (BC) pyrolyzed at 500 °C, was obtained from a commercial charcoal production facility (DAEWON GSI, Korea). The chemical properties of the biochar are shown in Table 1, and detailed information regarding the measurement methods has been presented in our previous study [23]. The base growing media (GM) composed of 70 % coir dust from India, 20 % perlite and 10 % vermiculite from China, on a volume basis was used in this study. Coir dust is the most widely used material for making growing media, either by itself or in combination with other materials such as perlite and vermiculite in Korea [24–26]. This growing media was incorporated with biochar at four different rates (w/w); 0 % as a control (GM-BC0), 1 % (GM-BC1), 2 % (GM-BC2), and 5 % (GM-BC5).
Determination of Physico-Chemical Properties
Before the pot experiment, physico-chemical properties of GM-BC mixtures were determined as follows. The pH and EC of the GM-BC mixtures were analyzed in a growing media distilled water suspension (growing media to water ratio, 1:5, v/v) using a pH meter (S220, Mettler Toledo, Switzerland) and an EC meter (S230, Mettler Toledo, Switzerland), as described by the European Committee for Standardization [27, 28]. Following the pH and EC measurements, the suspension was centrifuged and filtered through Whatman No. 42 paper in order to determine the water-soluble nutrient contents [29]. The ammonium (NH4 +) concentration was measured using a nitrogen distillation system (Kjeltec 2300, Foss Tecator, Sweden) and the NO3 − concentration was analyzed by ion chromatography (IC, ICS 1600, Dionex, USA). The phosphorus (P) concentration was determined via UV-Spectrophotometer (UV-160A, Shimadzu, Japan) at 880 nm after the extract was mixed with ammonium ascorbic acid, while water soluble cations (Ca2+, Mg2+ and K+) were determined using atomic absorption spectrometry (AAS, Analyst 400, Perkin Elmer, USA). Cation exchange capacity (CEC) was measured using the ammonium acetate extract method at pH 7.0 [30].
The physical properties including bulk density, particle density, total pore space, water volume, and air capacity were measured according to the method described by the European Committee for Standardization [31]. The GM-BC mixtures were added to a container (2 L), saturated in water and then equilibrated on a sand suction table (Eijkelkamp, The Netherlands) at 5 kPa water tension for 48 h. The equilibrated GM-BC mixtures were distributed into double ring cylinders, re-saturated and equilibrated at 1 kPa water tension because this value usually reflects container capacity. The double ring cylinders were removed from the sand suction table and then the upper ring with GM-BC mixtures were discarded using a sharp knife strike off the material level with the top of the lower ring. The GM-BC mixtures in the lower ring cylinders were dried in a fan-forced oven at 105 °C for 48 h and then weighed. The measurements obtained from the above procedure were used to calculate the bulk density, particle density, total pore space, water volume, and air capacity using the following equations [31]:
In order to calculate the particle density, organic matter content and ash content of the GM-BC mixtures were determined by loss on ignition at 450 °C [32]. The water retention curves of the GM-BC mixtures were determined using a sand suction table at 1, 5 and 10 kPa water tensions and a pressure plate (1600, soilmoisture, USA) at 100 and 500 kPa water tension. The quantity of water released, easily available water (difference between the water volume at 1 and 5 kPa water tension) and the water buffering capacity (difference between the water volume at 5 and 10 kPa water tension) were determined using the water retention curves [33].
Pot Trial
The pot experiment was conducted in a greenhouse (average temperature was 30 °C day/15 °C night) under natural light conditions. After measuring the laboratory compacted bulk density to convert weight to volume [34], a portion of each GM-BC mixture (500 ml) was distributed to plastic pots (diameter 11 cm × height 10 cm), followed by saturation with tap water and subsequently allowed to drain naturally for 24 h to attain their respective field capacities. Kale (Brassica oleracea L. var. acephala) seedlings (a week old), previously cultivated on a commercial horticultural growing substrate in a growth chamber (day time, 16 h, 25 °C; night time 8 h, 18 °C; light, 500 mol m−2 s−1), were then transplanted into each pot. Pot trials were carried out in triplicates for each GM-BC mixture. Kale was cultivated for 25 days and supplemented with a nutrient solution every 3 days after transplanting. The nutrient solution contained, major nutrients (9.5, 0.625, 3, 5.5, 4.5, 1.125 meq L−1 for NO3-N, NH4-N, P, K, Ca, and SO4-S, respectively), and minor nutrients (1, 0.005, 0.1, 0.1, 0.1, 0.25 mg L−1 for Fe, Cu, B, Mn, Zn, Mo, respectively). The nutrient solution was supplied on top of the pot media. In order to determine the released nutrients, the leachates were collected twice, on day 6 and 24 of the experiment, filtered through filter paper (Whatman No. 42) and subsequently analyzed for NH4 + using a nitrogen distillation system, and cations (Ca2+, Mg2+, K+) using a AAS and anions (nitrate (NO3 −), phosphate (PO4 2−), sulfate (SO4 2−), chloride (Cl−)) by IC.
Plant Analysis
The shoot and root tissues were harvested 25 days after transplantation. At harvest, shoots and roots were separated and washed once with tap water and twice with distilled water to remove any adhering particles. Subsequently, the plant tissues were dried in a fan-forced oven at 65 °C for 48 h and then the dried plant shoots and roots were weighed. The nutrient (N, P and K) contents of the plant shoot were analyzed following acid digestion based on Korean Standard Methods [35]. Powdered plant shoot (0.5 g) was digested in an Erlenmeyer flask (100 ml) with H2SO4 (1 ml) and 50 % HClO4 (10 ml) using a hot plate at 180 °C over 3 h [36], followed by filtration through filter paper (Whatman No. 6). The nitrogen content was measured using a nitrogen distillation system and the K content was analyzed using AAS. The phosphorus content was determined using a UV-Spectrophotometer (UV-160A, Shimadzu, Japan) at 470 nm after the filtrate was mixed with ammonium molybdate and ammonium meta vanadate.
Statistical Analysis
The results were calculated as an average of triplicate determinations together with the standard deviation of each treatment. Any significant differences among treatments were determined by ANOVA using the SAS 9.3 software (SAS for Windows v. 9.3, SAS Institute Inc., Cary, NC).
Results and Discussion
Chemical Properties
The selected chemical properties of the GM-BC mixtures are listed in Table 2. Biochar incorporation increased the pH of the growing media showing a proportional increment to the BC amount applied. The highest increase of 0.5 pH units, from pH 6.1 of the GM-BC0 (control), was observed in GM-BC5. As oxygen containing functional groups are generated on biochar surfaces, and the contents of carbonate increased during pyrolysis of feedstocks, in general biochar is alkaline [37]. Therefore, BC incorporation with GM likely resulted in a pH increment. Previously Vaughn et al. [19] showed that addition of straw biochars at 10 % (v/v) to growing media, consisting of 40 % (by volume) peat and 50 % vermiculite, had a higher pH value (0.5 units) than the control growing media (biochar untreated), which was attributed to the high pH of the biochar. In agreement with this study, the increased pH observed in the present study was most likely due to the high pH (10.2) of the rice hull-derived biochar. The pH of GM-BC0, GM-BC1, and GM-BC2 examined in this study were within the optimum pH range (5.3–6.5) for growing media proposed by Abad et al. [38], while the pH (6.6) of GM-BC5 was slightly higher than the proposed range. Biochar addition slightly changed growing media EC but the EC of all the GM-BC mixtures used in the present study were within the optimal EC range (0.6–2.0 dSm−1) for healthy, vigorous growth [39].
Application of biochar at 5 % increased the water soluble P and K concentration from 1.9 and 118.5 mg L−1 in the control to 7.2 and 132.7 mg L−1, respectively, while the Ca concentration decreased. This was attributed to a high concentration of P and K embedded in the biochar (Table 1). In general, the P and K concentration in biochar is significantly increased by increasing the pyrolysis temperature [40, 41], and consequently, application of biochar can improve soil or growing media fertility. For example, Peng et al. [41] demonstrated that incorporation of rice straw biochar into soil at 1 % (w/w) was equivalent to P and K fertilizer treatment of ~0.02 and 1.89–2.78 g kg−1 respectively. In addition, Dumroese et al. [17] reported that increasing the level of biochar pellets corresponded to increasing P and K amounts in the growing media.
Application of biochar increased the CEC of the growing media by a significant amount from 82 cmolc kg−1 in the GM-BC0 to 96 cmolc kg−1 in the GM-BC5. This increase in CEC was mainly attributed to the large surface area and high charge density of the applied biochar [13, 42]. Many previous studies have shown that application of biochar to soil increases soil CEC [43, 44]. Moreover, Headlee et al. [45] reported that biochar from red oak increased the CEC of peat moss growing media when mixed at 25 % (v/v).
Physical Properties
Physical properties of the growing media directly affect plant growth and are not significantly changed during the plant growth period when compared with the chemical properties. Thus, the improved physical properties of the growing media could enhance the growing conditions, resulting in an increased water and nutrient uptake by the plant [1].
Table 3 shows the physical properties of the GM-BC mixtures examined in the present study. The bulk density was in the range of 158–174 kg m−3 and increased as the biochar application rate increased. The particle density ranged from 2031 to 2072 kg m−3 and decreased with an increase in the application of biochar. The bulk density of the growing media influences the growth of the plant. If the bulk density becomes too high, it can limit plant root growth due to a reduction in porosity, and a high bulk density also increases the transport cost of the growing media [46, 47]. Conversely, a very low bulk density can cause excessive aeration of the growing media and concomitantly decrease the available water content. Since biochar itself has a higher bulk density than other growing media materials such as peat moss and vermiculite [19], it increases the bulk density of the GM-BC mixtures, while additions of biochar soil induces a decrease in the bulk density [48]. Nevertheless, the bulk density of all the GM-BC mixtures used in the present study were within the recommended range (<400 kg m−3) for plant growth [38].
The increased bulk density of the growing media via BC incorporation results in a proportional decrease in the total pore space [48, 49]. However, the total pore space of all the GM-BC mixtures used in the present study were also within the recommended range (>85 %) for plant growth [38].
The water volume at 1 kPa water tension in the GM-BC mixtures increased from 63 % in the GM-BC0 to 73 % in the GM-BC5 (Table 3). This result is in agreement with Dumroese et al. [17], who showed that a mixture of 75 % peat and 25 % biochar pellets increased the water volume at 10 cm water tension by 13 % compared to 100 % peat. The water volume of the growing media with biochar incorporation, at each water tension, also showed increased values as the amount of BC was increased (Fig. 1). As a result, the easily available water (the difference between the water volume at 1 and 5 kPa water tension) was significantly higher with biochar incorporation than with the control, while the water buffering capacity (the difference between the water volume at 5 and 10 kPa water tension) was not significantly different between the control and the biochar-treated growing media (Fig. 2). The specific increase in the easily available water observed with BC treatment was possibly caused by the large amounts of micro- and/or meso-pores within the biochar particles, because easily available water is known to be stored in micro (<2 nm) and meso (2–50 nm) pores [50]. For optimal plant growth, a growing media must contain enough easily available water [51], as indicated by Papafotiou et al. [52] who reported that a decrease in the amount of easily available water in growing media, following the addition of olive-mill wastes compost, resulted in a decreased growth of poinsettia (Euphorbia pulcherrima cv.). Therefore, it was expected that the observed increases in the easily available water with biochar treatment would be a key factor in enhancing plant growth.
Effect on GM Fertility
As shown in Fig. 3, the NH4 + concentration in leachates from GM-BC1, GM-BC2 and GM-BC5 on day 6 post-transplantation were 14, 28 and 55 % lower than that of the control, respectively. In addition, the concentration of NO3 − in the leachates collected 6 and 24 days after planting decreased with the amount of biochar applied (Fig. 3). These results were in agreement with the report by Altland and Locke [53] who evaluated the effect of biochar on nutrient retention and release in growing media, where they showed that the application of biochar increased the NO3 − adsorption capacity and decreases NO3 − leaching more slowly over time. Beck et al. [54] also showed that amendment of a green roof growing media with 7 % biochar decreased the NO3 − concentration of the rainfall runoff by 79 % compared with the control growing media (without biochar). The decreased NO3 − in the leachate following biochar application was primarily attributed to biochar-induced NO3 − immobilization. Application of biochar encouraged microbial populations and functions, thus NO3 − immobilization occurred due to the fact that NO3 − can be immobilized by microorganisms [55]. Also, due to adsorption of NH4 + on to the BC surface, Laird et al. [56] hypothesized that the application of biochar reduced the rate of N mineralization and hence the rate of NO3 − leaching. In contrast to N, the concentration of PO4 2− and K in leachates increased with increasing BC, likely due to the increased P and K that originated from the BC. For example, PO4 2− in the leachate collected 6 days after planting increased with the amount of biochar applied, and ranged from 108–122 mg L−1 on day 24 after planting. The concentrations of Ca2+ and Mg2+ in the leachate were significantly lower in the growing media mixed with biochar than those in the control. Decreasing cations such as NH4 +, Ca2+ and Mg2+ in the leachate with BC treatment were attributed to increased CEC in the GM-BC mixtures in comparison with the control. Since biochar itself has a high CEC value, the application of biochar increased the CEC of the GM-BC mixtures as described in “Chemical Properties”, and subsequently favored the adsorption of cations [56, 57]. This phenomenon was supported by the linear regression analysis in the current study, where a negative correlation was shown between the CEC and the concentration of NH4 +, Ca2+ and Mg2+ in the leachate on day 6 (NH4 +: r = −0.97, p < 0.05; Ca2+: r = −0.96, p < 0.05; Mg2+: r = −0.97, p < 0.05) and 24 (Ca2+: r = −0.95, p < 0.05; Mg2+: r = −0.97, p < 0.05) after transplantation. The concentration of SO4 2− and Cl− in the leachate collected 6 and 24 days after planting were not significantly different (p < 0.05) between the control and the biochar treatments (data not shown). Overall, the application of biochar to the growing media increased essential plant nutrients such as P and K, and also maintained fertility by the retention of supplied nutrients due to an increased CEC.
Plant Response
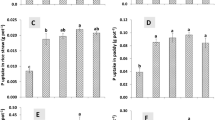
Biochar incorporation into GM enhanced kale growth performance proportional to the BC application rate. The maximum shoot and root dry weight of kale was observed in the growing media that received BC at 5 % (w/w), where the shoot and root dry weight were 150 and 192 % higher than those of the control growing media, respectively (Fig. 4). In agreement with this study, Tian et al. [58] found that urban green waste biochar and peat mixture (50 % peat and 50 % biochar, v/v) improved the growth of Calathea rotundifola cv. Fasciata compared to the growth in peat only (100 %). In addition, Graber et al. [18] reported that citrus wood biochar improved the growth and productivity of pepper when incorporated into the growing media (1–5 %, w/w) when compared with the control. The increase in the dry weight of kale with biochar application was most probably caused by an improvement in the physical properties of the GM, as indicated by a positive relationship between the easily available water and the dry weight of both the shoot (p < 0.001) and the root (p < 0.001).
The increased nutrient and water retention of the growing media with biochar application contributed to the increased N content in the shoots (Table 4). The N content in the shoots was negatively correlated with the concentration of NO3 − in the leachate collected 24 days after planting (p < 0.01), while it showed a positive correlation with water volume at 1 kPa water tension (p < 0.0005). This implies that the N retention increased by biochar incorporation provided more opportunity for N to be taken up by the plant through the increased easily available water that was also induced by biochar incorporation. Like N, both P and K content in the shoots also increased with increasing biochar application, which was attributed to the high concentrations of P and K in the applied biochar, as described earlier. Consequently, the maximum N, P and K content in the kale shoots were observed in GM-BC5, implying that the use of rice hull-derived BC as a growing media amendment is a practically applicable approach to improve and/or maintain the fertility of the growing media.
Conclusions
The current study demonstrates that rice hull-derived biochar, which can be easily obtained via agricultural byproducts, is a suitable candidate for a growing media amendment as indicated by the improved fertility and physical properties of the base growing media. In summary, biochar application increases the bulk density and water content, while decreasing particle density and total pore space. As a result, the easily available water content, which is a key factor for appropriate plant growth, was significantly increased as observed by a 122 % increment with 5 % biochar incorporation compared with the control growing media that received no biochar. In addition, biochar amendment maintained or improved the fertility of the growing media through the increased retention of nutrients supplied in the growing media, and through the additional supply of P and K from the biochar. These ameliorated the physical and chemical properties of the growing medium, resulting in increased plant growth.
References
Verdonck, O., Demeyer, P.: The influence of the particle sizes on the physical properties of growing media. Acta Hort. 644, 99–101 (2004)
Papadopoulos, A.P., Bar-Tal, A., Silber, A., Saha, U.K., Raviv, M.: Inorganic and synthetic organic components of soilless culture and potting mixes. In: Raviv, M., Lieth, J.H. (eds.) Soilless culture theory and practice, pp. 505–543. Elsevier, Burlington (2008)
Shin, B.K., Son, J.E., Choi, J.M.: Physico-chemical properties of peatmoss and coir dust currently used as root medium components for crop production in Korean plant factories. J. Bio-Environ. Control 21, 362–371 (2012)
Chen, J., Li, Y.: Coal fly ash as an amendment to container substrate for Spathiphyllum production. Bioresour. Technol. 97, 1920–1926 (2006)
Marianthi, T.: Kenaf (Hibiscus cannabinus L.) core and rice hulls as components of container media for growing Pinus halepensis M. seedlings. Bioresour. Technol. 97, 1631–1639 (2006)
Ribeiro, H.M., Romero, A.M., Pereira, H., Borges, P., Cabral, F., Vasconcelos, E.: Evaluation of a compost obtained from forestry wastes and solid phase of pig slurry as a substrate for seedlings production. Bioresour. Technol. 98, 3294–3297 (2007)
Michel, J.C.: Influence of clay addition on physical properties and wettability of peat-growing media. HortScience 44, 1694–1697 (2009)
Lehmann, J., Gaunt, J., Rondon, M.: Bio-char sequestration in terrestrial ecosystems—a review. Mitig. Adapt. Strat. Glob. Change 11, 403–427 (2006)
Lehmann, J.: A handful of carbon. Nature 447, 143–144 (2007)
Mohan, D., Sarswat, A., Ok, Y.S., Pittman, C.U.: Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresour. Technol. 160, 191–202 (2014)
Glaser, B., Lehmann, J., Zech, W.: Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol. Fertil. Soils 35, 219–230 (2002)
Steiner, C., Teixeira, W.G., Lehmann, J., Nehls, T., de Macêdo, J.L.V., Blum, W.E.H., Zech, W.: Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 291, 275–290 (2007)
Atkinson, C.J., Fitzgerald, J.D., Hipps, N.A.: Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337, 1–18 (2010)
Major, J., Rondon, M., Molina, D., Riha, S.J., Lehmann, J.: Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333, 117–128 (2010)
Ahmad, M., Lee, S.S., Yang, J.E., Ro, H.M., Lee, Y.H., Ok, Y.S.: Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol. Environ. Saf. 79, 225–231 (2012)
Zhang, L., Sun, X.Y., Tian, Y., Gong, X.Q.: Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 176, 70–78 (2014)
Dumroese, R.K., Heiskanen, J., Englund, K., Tervahauta, A.: Pelleted biochar: chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenerg. 35, 2018–2027 (2011)
Graber, E.R., Harel, Y.M., Kolton, M., Cytryn, E., Silber, A., David, D.R., Tsechansky, L., Borenshtein, M., Elad, Y.: Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 337, 481–496 (2010)
Vaughn, S.F., Kenar, J.A., Thompson, A.R., Peterson, S.C.: Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crops Prod. 51, 437–443 (2013)
Verdonck, O.: Composts from organic waste materials as substitutes for the usual horticultural substrates. Biol. Wastes 26, 325–330 (1988)
Park, W.K., Park, N.B., Shin, J.D., Hong, S.G., Kwon, S.I.: Estimation of biomass resource conversion factor and potential production in agricultural sector. Korean J. Environ. Agric. 30, 252–260 (2011). (In Korean, with English abstract)
Rajapaksha, A.U., Vithanage, M., Zhang, M., Ahmad, M., Mohan, D., Chang, S.X., Ok, Y.S.: Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour. Technol. 166, 303–308 (2014)
Kim, H.S., Kim, K.R., Kim, H.J., Yoon, J.H., Yang, J.E., Ok, Y.S., Owens, G., Kim, K.H.: Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 74, 1249–1259 (2015)
Kang, J.Y., Lee, H.H., Kim, K.H.: Physical and chemical properties of organic horticultural substrates used in Korea. Acta. Hort. 644, 231–235 (2004)
Kang, J.Y., Lee, H.H., Kim, K.H.: Physical and chemical properties of inorganic horticultural substrates used in Korea. Acta. Hort. 644, 237–241 (2004)
Park, C.L., Kim, B.G., Jeon, H.S., Lee, G.S., Lee, J.R.: Improved calcined clay with a spherical layered structure and its characteristics as a medium for plant growth. Appl. Clay Sci. 49, 298–305 (2010)
CEN (Committee for European Normalization): EN 13037:2011 Soil improvers and growing media—determination of pH. Brussels (2011)
CEN (Committee for European Normalization): EN 13038:2011 Soil improvers and growing media—determination of electrical conductivity. Brussels (2011)
CEN (Committee for European Normalization): EN 13652:2001 Soil improvers and growing media—extraction of water soluble nutrients and elements. Brussels (2001)
Sumner, M.E., Miller, W.P.: Cation exchange capacity and exchange coefficients. In: Sparks, D.L. (ed.) Methods of soils analysis, part 3, chemical methods, pp. 1201–1230. Soil Science society of America and America Society of Agronomy, Madison (1996)
CEN (Committee for European Normalization): EN 13041:2011 Soil improvers and growing media—dry bulk density, air volume, water volume, shrinkage value and total pore space. Brussels (2011)
CEN (Committee for European Normalization): EN 13039:2011 Soil improvers and growing media—determination of organic matter content and ash. Brussels (2011)
Benito, M., Masaguer, A., De Antonio, R., Molimer, A.: Use of pruning waste compost as a component in soilless growing media. Bioresour. Technol. 96, 597–603 (2005)
CEN (Committee for European Normalization): EN 13040:2007 Soil improvers and growing media—sample preparation for chemical and physical tests, determination of dry matter content, moisture content and laboratory compacted bulk density. Brussels (2001)
NIAST (National Institute of Agricultural Science and Technology): method of soil and plant analysis. Suwon, Korea (In Korean) (2000)
Cresser, M.S., Parsons, J.W.: Sulfuric-perchloric acid digestion of plant material for the determination of nitrogen, phosphorus, potassium, calcium and magnesium. Anal. Chim. Acta 109, 431–436 (1979)
Yuan, J.H., Xu, R.K., Zhang, H.: The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 102, 3488–3497 (2011)
Abad, M., Noguera, P., Burés, S.: National inventory of organic wastes for use as growing media for ornamental potted plant production: case study in Spain. Bioresour. Technol. 77, 197–200 (2001)
Lemaire, F., Dartigues, A., Riviere, L.M.: Properties of substrate made with spent mushroom compost. Acta Horticult. 172, 13–29 (1985)
Hossain, M.K., Strezov, V., Chan, K.Y., Ziolkowski, A., Nelson, P.F.: Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manage. 92, 223–228 (2011)
Peng, X., Ye, L.L., Wang, C.H., Zhou, H., Sun, B.: Temperature- and duration-dependent rice straw-derived biochar: characteristics and its effects on soil properties of an Ultisol in southern China. Soil Till. Res. 112, 159–166 (2011)
Liang, B., Lehmann, J., Solomon, D., Kinyangi, J., Grossman, J., O’Neill, B., Skjemstad, J.O., Thies, J., Luizão, F.J., Petersen, J., Neves, E.G.: Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 70, 1719–1730 (2006)
Van Zwieten, L., Kimber, S., Morris, S., Chan, K.Y., Downie, A., Rust, J., Joseph, S., Cowie, A.: Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327, 235–246 (2010)
Yuan, J.H., Xu, R.K., Qian, W., Wang, R.H.: Comparison of the ameliorating effects on an acidic ultisol between four crop straws and their biochars. J. Soils Sediments 11, 741–750 (2011)
Headlee, W.L., Brewer, C.E., Hall, R.B.: Biochar as a substitute for vermiculite in potting mix for hybrid poplar. Bioenerg Res 7, 120–131 (2014)
Corti, C., Crippa, L., Genevini, P.L., Centemero, M.: Compost use in plant nurseries: hydrological and physicochemical characteristics. Compost Sci. Util. 6, 35–45 (1998)
Jeyaseeli, D.M., Raj, S.P.: Physical characteristics of coir pith as a function of its particle size to be used as soilless medium. American-Eurasian J. Agric. Environ. Sci. 8, 431–437 (2010)
Alburquerque, J.A., Calero, J.M., Barrón, V., Torrent, J., del Campillo, M.C., Gallardo, A., Villar, R.: Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 177, 16–25 (2014)
Hernández-Apaolaza, L., Gascó, A.M., Gascó, J.M., Guerrero, F.: Reuse of waste materials as growing media for ornamental plants. Bioresour. Technol. 96, 125–131 (2005)
Herath, H.M.S.K., Camps-Arbestain, M., Hedley, M.: Effect of biochar on soil physical properties in two contrasting soils: an Alfisol and an Andisol. Geoderma 209–210, 188–197 (2013)
Verdonck, O., Penninck, R., De Boodt, M.: Physical properties of different horticultural substrates. Acta Hortic. 150, 155–160 (1983)
Papafotiou, M., Phsyhalou, M., Kargas, G., Chatzipavlidis, I., Chronopoulos, J.: Olive-mill wastes compost as growing medium component for the production of poinsettia. Sci. Hortic. 102, 167–175 (2004)
Altland, J.A., Locke, J.C.: Biochar affects macronutrient leaching from a soilless substrate. HortSci. 47, 1136–1140 (2012)
Beck, D.A., Johnson, G.R., Spolek, G.A.: Amending greenroof soil with biochar to affect runoff water quantity and quality. Environ. Pollut. 159, 2111–2118 (2011)
Ippolito, J.A., Novak, J.M., Busscher, W.J., Ahmedna, M., Rehrah, D., Watts, D.W.: Switchgrass biochar affects two Aridisols. J. Environ. Qual. 41, 1123–1130 (2012)
Laird, D., Fleming, P., Wang, B., Horton, R., Karlen, D.: Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158, 436–442 (2010)
Major, J., Steiner, C., Downie, A., Lehmann, J.: Biochar effects on nutrient leaching. In: Lehmann, J., Joseph, S. (eds.) Biochar for environmental management science and technology, pp. 271–287. Earthscan, London (2009)
Tian, Y., Sun, X.Y., Li, S.Y., Wang, H.Y., Wang, L.Z., Cao, J.X., Zhang, L.: Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 143, 15–18 (2012)
Acknowledgments
This study was supported by Postdoctoral Fellowship Program (PJ011161) of National Academy of Agricultural Science, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.S., Kim, K.R., Yang, JE. et al. Amelioration of Horticultural Growing Media Properties Through Rice Hull Biochar Incorporation. Waste Biomass Valor 8, 483–492 (2017). https://doi.org/10.1007/s12649-016-9588-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9588-z