Abstract
In Korea, soils adjacent to abandoned mines are commonly contaminated by heavy metals present in mine tailings. Further, the disposal of oyster shell waste by oyster farm industries has been associated with serious environmental problems. In this study, we attempted to remediate cadmium (Cd)- and lead (Pb)-contaminated soils typical of those commonly found adjacent to abandoned mines using oyster shell waste as a soil stabilizer. Natural oyster shell powder (NOSP) and calcined oyster shell powder (COSP) were applied as soil amendments to immobilize Cd and Pb. The primary components of NOSP and COSP are calcium carbonate (CaCO3) and calcium oxide (CaO), respectively. X-ray diffraction, X-ray fluorescence and scanning electron microscope analyses conducted in this study revealed that the calcination of NOSP at 770°C converted the less reactive CaCO3 to the more reactive CaO. The calcination process also decreased the sodium content in COSP, indicating that it was advantageous to use COSP as a liming material in agricultural soil. After 30 days of incubation, we found that the 0.1 N HCl-extractable Cd and Pb contents in soil decreased significantly as a result of an increase in the soil pH and the formation of metal hydroxides. COSP was more effective in immobilizing Cd and Pb in the contaminated soil than NOSP. Overall, the results of this study suggest that oyster shell waste can be recycled into an effective soil ameliorant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contamination of soil with heavy metals is a worldwide problem. Mine and smelter discharges, sewage irrigation and sludge application to agricultural land, as well as gaseous emissions from industrial processes, are some of the major sources of heavy metal contamination in soil. The toxicity of these heavy metals may affect soil quality and agricultural productivity and ultimately becomes a hazard to human and animal health.
In Korea, soil contamination by heavy metals is directly or indirectly related to mining sites (Yang et al. 2000). Most mine tailings are located on abandoned slopes, and heavy metals that leach from these tailings are discharged directly into streams and agricultural lands that are adjacent to closed or abandoned mines (Yang et al. 2006). Metals leached from these abandoned mines are toxic to most plants and have adverse effects on human health. In particular, cadmium (Cd) and lead (Pb) in soil can be transferred to humans via the food chain and then cause chronic and acute diseases (Ok et al. 2004). According to the Soil Conservation Act of Korea (1996), soils with Cd and Pb concentrations >1.5 and >100 mg kg−1, respectively (based on 0.1 N HCl extraction), cannot be used for agriculture and must be continuously monitored (Ok and Kim 2007). However, a significant proportion of paddy soils near mining areas have Cd and Pb concentrations greater than the standard guidelines set by the Ministry of Environment (Yang et al. 2008).
In recent years, the use of various inorganic amendments to immobilize/stabilize heavy metals in contaminated soil has attracted attention (Hong et al. 2009). Lime-based stabilization has been considered as an effective remediation technique and has been widely applied (Hong et al. 2009; Moon et al. 2004; Dermatas et al. 2004; Moon et al. 2008; Dermatas and Moon 2006; Kostarelos et al. 2006). Lime increases the soil pH, leading to the formation of calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH), which can change the soil to a relatively impermeable layer reducing the mobility of heavy metals (Kostarelos et al. 2006). In addition, the use of readily available and cost-advantageous materials as immobilizing amendments becomes more desirable when the remediation is targeting vast amounts of contaminated soils, including those in mining areas (Ok et al. 2007b; Hashimoto et al. 2009).
Korea is the second largest producer of oysters in the world after China (Dore 1991). Financially, the oyster farming industry has considerably boosted the Korean economy; however, the disposal of oyster shell waste has led to serious environmental problems. Therefore, the recycling of oyster shell waste has become an issue of concern. To date, a portion of oyster shell waste has been recycled for use as spat and as a substitute for limestone in fertilizers and chicken feed (Kwon et al. 2004). However, this use is limited due to the high concentrations of sodium chloride in oyster shells (Lee et al. 2008). Hence, alternative approaches are required for recycling of oyster shell waste.
Recently, several studies have been conducted in Korea to evaluate possible means of reusing oyster shell waste material. For example, Lee et al. (2009) and Kwon et al. (2004) used oyster shell waste to remove phosphorus from wastewater, while Yang et al. (2005) and Yoon et al. (2004) studied its reuse as a construction material. The effects of oyster shell application on soil quality and crop production have also been studied by Lee et al. (2008). However, no studies have been conducted to evaluate the reuse of oyster shell waste outside of Korea.
The eastern part of the Korean peninsula is rich in lime resources; however, mining and processing of limestone is expensive and causes damage to the environment (Lee et al. 2008). In previous studies (Lee et al. 2008; Yang et al. 2005; Yoon et al. 2004), it was reported that oyster shells are primarily composed of calcium carbonate (CaCO3). Therefore, in this study, we evaluated the use of oyster shell waste as a liming material for the stabilization of metal-contaminated soil. Further, changes in the natural oyster shell composition due to calcination were investigated.
Materials and methods
Preparation of natural and calcined oyster shell powders (NOSP and COSP)
Oyster shell waste was collected from a representative shellfish farm in Hadong-gun in the westernmost part of Gyeongsangnam-do, Republic of Korea during the winter of 2008. The oyster shells were rinsed several times with deionized water and then heated at 95°C using hot water for several hours to remove any possible interfering materials and impurities from the surface of the shells. The cleaned oyster shells were then dried at 105°C in a forced air oven for 72 h, after which they were mechanically crushed and passed through a 1.0-mm sieve to analyze their chemical properties and conduct incubation experiments. Calcination of the sieved oyster shell powder was performed in a Carbolite® furnace (Sheffield, UK) at 900°C for 4 h using the method described by Park et al. (2007).
Characterization of NOSP and COSP
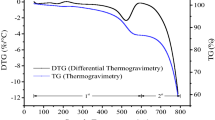
A thermogravimetric analysis (TGA) of NOSP was conducted in an inert (N2 gas) atmosphere using a thermogravimetric analyzer (SDT Q600, TA Instruments, USA) and the weight loss that occurred as a result of combustion of the oyster shell powder was measured to determine its thermal stability (Singh and Kolay 2002). To accomplish this, approximately 10 mg of oyster shell powder was taken for analysis and heated to 1,000°C at 10°C/min.
X-ray fluorescence (XRF-1700, Shimadzu, Japan) was used for the qualitative and quantitative analyses of the inorganic compositions of NOSP and calcined oyster shell powders (COSP), and the major oxides were considered. The NOSP and COSP were further evaluated for their mineralogical composition by conducting XRD spectrometry (X’pert PRO MPD, PANalytical, Netherlands) studies using a graphite monochromator and Cu Kα radiation (Singh and Kolay 2002). Briefly, the samples were scanned for 2θ ranging from 10 to 80°, after which the data files prepared by the Inorganic Crystal Structure Database (ICSD) and Joint Committee on Powder Diffraction Standards (JCPDS) were used to identify the minerals present in each sample. Scanning electron microscopy (SEM, S-4300, Hitachi, Japan) analyses were performed to observe the morphology of NOSP and COSP.
Preparation and characterization of Cd- and Pb-contaminated soil
Cd- and Pb-contaminated soils were collected from a paddy field adjacent to the Seosung mine, Seosan, Chungcheongnam-do, Republic of Korea at a depth of 0–30 cm during the spring of 2008. In the early 1960s, elements such as gold, silver, copper and zinc were being extracted from the Seosung mine; however, the mine was closed in 1970. Most of the paddy fields are contaminated by heavy metals in mine tailings and other wastes generated from the closed mine (Yang et al. 2006).
The soil samples were air dried, ground to pass through a 2-mm sieve and then analyzed for selected chemical properties. The properties of the paddy soil used in this experiment were similar to those of typical Korean rice paddy soils, with a pH of 6.21, cation exchange capacity (CEC) of 15.11 cmol(+) kg−1 and organic matter content of 38.69 g kg−1 (Jo and Koh 2004). The total Cd and Pb concentrations in the soil analyses conducted based on aqua regia extraction were 17 and 1,246 mg kg−1, respectively.
Incubation experiment
Experiments were conducted using 13 treatments in three replicates, as shown in Table 1. The treatments consisted of 100 g samples placed in unsealed polyethylene bags that were incubated for 30 days in the dark in a growth chamber (25°C ± 2°C). The soil moisture level was controlled at 70% of the water holding capacity and maintained by periodic weighing of the beakers, and the weight was adjusted by adding distilled water (Chander and Joergensen 2002; Hong et al. 2007).
After 30 days, subsamples from different treatments were collected and evaluated for the soil pH and bioavailability of Cd and Pb by performing single extractions with HCl for each soil (Isoyama and Wada 2007). The single extraction scheme was based on shaking 10 g of air-dried soil (2-mm sieve) with 50 mL of 0.1 N HCl (Korean Standard Method). The extracts were then filtered through a Whatman no. 42 filter paper and <0.45-μm Millipore filter paper, after which they were analyzed by ICP-OES (XL-3100, Perkin Elmer, USA) (Cheng and Hseu 2002).
Statistical analysis
The SAS package (ver. 9.1) was used to perform all statistical analysis (SAS 2004). The means and standard deviations were presented based on original data. ANOVA was conducted to compare the means of untreated soil with those of soils containing CaCO3, CaO, NOSP and COSP.
Results
Characterization of NOSP and COSP
Yoon et al. (2003) reported that calcite (CaCO3) was the major component in NOSP. To convert the relatively non-reactive form, CaCO3, into the more reactive form, CaO, a calcination process was adopted in this study (Kwon et al. 2004). TGA was used to monitor the calcination of the natural oyster shells and the TGA profile is shown in Fig. 1. We observed a phase change at 600–770°C, which indicates a complete thermal decomposition of the natural oyster shells. A weight loss of 46.96% occurred after complete combustion of the material at 770°C (Fig. 1).
The chemical compositions of both NOSP and COSP were determined by XRF, and the results are shown in Fig. 2. The increase in CaO contents (93.6%) was greater in COSP than in NOSP. Additionally, the loss on ignition (LOI) as well as the sodium (Na2O), magnesium (MgO) and phosphorous (P2O5) contents decreased in the oyster shell powder following calcination. However, a slight increase in potassium (K2O), silica (SiO2), iron (Fe2O3), manganese (MnO) and titanium (TiO2) was observed in the COSP.
The mineralogical compositions of both NOSP and COSP are shown as an XRD pattern in Fig. 3. In the case of NOSP, the main peak appeared at 2θ = 29.3, which corresponded to a limestone peak (CaCO3) in the JCPDS database, confirming that the major component of NOSP was CaCO3. In contrast, in the XRD peak of COSP, the main peak was observed at 2θ = 37.4, which corresponded to lime (CaO).
The surface structures of both NOSP and COSP were visualized by SEM, as shown in Fig. 4. NOSP had an irregular crystal structure, while numerous pores were observed in the crystal structure of COSP. A similar porous structure was also observed in heated oyster shells by Kwon et al. (2004).
Soil pH
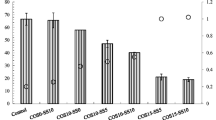
Changes in the soil pH in response to various treatments with CaCO3, CaO, NOSP and COSP are shown in Fig. 5. Treatment with 1 wt% CaCO3 and NOSP resulted in an increase in the soil pH from 6.5 to 8.3, and there was no further increase observed in response to 3 and 5 wt% treatments. However, both CaO and COSP increased the soil pH in a dose-dependent fashion, with a significant increase from 6.5 to 12.5 being observed in response to the 5 wt% treatment.
Concentration of heavy metals
The effects of different treatments on the chemical extractability of Cd and Pb were evaluated with 0.1 N HCl (Isoyama and Wada 2007; Yang et al. 2008). Figure 6 shows the variations in the extractability of heavy metals in soil as a function of soil amendments with different treatment levels after incubation for 30 days. The relative percentage when compared to the initial concentration is shown on the y-axis. Treatment with the two common liming materials, CaCO3 and CaO, resulted in the extractability of both heavy metals being reduced when the amendment levels were increased from 0 to 5 wt%. A similar reduction in chemical extractability occurred during both oyster shell powder treatments, which indicates the potential for the use of oyster shell powder as a metal immobilizing amendment.
Evaluation of the extractability of Cd (Fig. 6a) revealed that the COSP treatment led to the highest reduction in chemical extractability for any given level of amendment addition (significance level = 0.05), and these effects were statistically equivalent to those of CaO. In contrast, NOSP treatment resulted in the lowest immobilization effect, with less than 35% of the initial Cd being stabilized in response to the 5 wt% treatment. The overall stabilization efficiency of Cd occurred in the following order: COSP ≥ CaO > CaCO3 > NOSP.
The general trend in the extractability reduction of Pb occurred in the following order: CaO > COSP > CaCO3 ≥ NOSP. It should be noted that the initial concentration of 0.1 N HCl-extractable Pb concentrations (i.e., 0 wt% level) was 1,012 mg kg−1. In the case of all the treatments with 5 wt% application, the extractability was reduced to less than 100 mg kg−1 after 30-days of incubation (Fig. 6b). In other words, a >95% reduction in the initial Pb concentration was observed following treatment with 5 wt% of each amendment.
Discussion
Calcination of oyster shell powders
CaCO3 can be converted to CaO by employing a simple chemical decomposition reaction during the calcination process:
The calcination reaction occurs at or above the thermal decomposition temperature, i.e., the temperature at which the standard Gibb’s free energy of the reaction is equal to zero (Gilchrist 1989). Therefore, calcination of the oyster shell powder was performed at 900°C for 4 h to completely decompose the CaCO3. A sharp decrease in the weight of the oyster shell powder at 600–770°C indicated a phase change from CaCO3 to CaO (Fig. 1), and complete thermal decomposition was achieved at 770°C as the TGA curve became parallel to the X-axis, indicating that most of the CaCO3 was converted to CaO. Kwon et al. (2004) adopted the calcination process at 650–800°C to convert the CaCO3 in the raw oyster shell, which is in a relatively non-reactive form, into a form that readily reacts with soil particles. They found that calcination under an air atmosphere leads to a mass loss of 45%, which was comparable to the calcination results observed in the present study (47% weight loss). Similarly, Park et al. (2007) reported that calcination began at 650°C and was completed at 770°C.
Instrumental analysis was conducted to characterize the oyster shell powder before (as NOSP) and after (as COSP) calcination using XRF, XRD and SEM (Figs. 2, 3, 4). The XRD pattern clearly indicated that the major component in NOSP was CaCO3; however, CaO became the major component in COSP following calcination (Fig. 3). These XRD results were confirmed by XRF, which revealed that there was a significantly higher content of CaO in COSP than in NOSP (Fig. 2). These findings indicate that the oyster shell powder composition can be changed by calcination and that a relatively pure form of the sample can be obtained by the removal of organic compounds. Yoon et al. (2003), Yoon et al. (2004), and Yang et al. (2005) also reported that raw oyster shells consisted of CaCO3, whereas the pyrolyzed oyster shells primarily consisted of CaO (Kwon et al. 2004).
The inorganic composition of oyster shells has differed among studies (Lee et al. 2008; Yang et al. 2005; Yoon et al. 2004; Kwon et al. 2004; Moon et al. 2009); however, calcium was the major component of oyster shells in all studies that have been conducted to date (including this one), and minor quantities of other cations such as sodium and magnesium were also presented. The relatively higher content of sodium in oyster shells has limited its use as a liming material in agricultural soils (Lee et al. 2008). However, in this study, the calcination process led to a significant decrease in the sodium content from 1.52 to 0.056%. Lee et al. (2008), Yoon et al. (2004), Yang et al. (2005), and Kwon et al. (2004) used raw oyster shells with sodium contents ranging from 0.58 to 0.98%; hence, calcination contributed to a decrease in the sodium contents in oyster shells, thereby making it more advantageous for use as a liming material in agricultural soils. The significant drop in LOI (Fig. 2), observed in the case of COSP, could be attributed to CO2 emission during the calcination process at higher temperatures (900°C).
Following calcination, a porous surface structure was developed in COSP, whereas the NOSP developed a smooth surface (Fig. 4). The development of pores in COSP was due to the emission of CO2 as a result of the thermal decomposition of CaCO3 to CaO, as shown in Eq. 1. Similarly, Kwon et al. (2004) observed highly convoluted and macaroni-shaped structures in pyrolyzed oyster shells. Studies by Park et al. (2007) also revealed that the irregular crystal structure in natural egg shells was changed by the development of pores in calcined egg shells.
Effects of NOSP and COSP treatments on soil pH
One of the most important factors affecting the mobility and bioavailability of heavy metals in soil is pH (Zhao and Masaihiko 2007; Ok et al. 2008; Yang et al. 2009). The soil pH governs the solid–solution equilibria of metals in soil (Zhao and Masaihiko 2007; Ok et al. 2007a, b). In the present study, all treatments increased the soil pH; however, a significant increase in the soil pH (up to 12.5) was observed in soil treated with COSP and CaO when compared to soil treated with CaCO3 and NOSP. The dissolution of CaCO3 and CaO in water produces hydroxyl ion (OH−) in accordance with the following chemical reactions:
The hydroxyl ion is responsible for increasing the soil pH. In the case of CaO and COSP, two hydroxyl ions are generated, which results in a rapid and more significant increase in soil pH. The application of NOSP and COSP to acidic soil improved the soil quality since the soil pH was increased. Lee et al. (2008) applied oyster shell fertilizer to two different soils to determine their physical and biological properties and found that the soil pH increased with the amount of oyster shell fertilizer added. Similarly, Park et al. (2007) reported an increase in the pH of wastewater following the addition of calcined egg shells. However, calcined materials led to a greater increase in the pH of the matrix than raw materials, as was the case in the present study. Similarly, Cao et al. (2008) reported that quicklime (CaO) treatment increased the soil pH to 11.5 in metal-contaminated soil, resulting in a decrease in TCLP-Pb and soil Pb leachability. The authors revealed that calcite was formed, and further, that a high pH enhanced the Pb adsorption on the calcite surface in lime-treated soil.
Immobilization of Cd and Pb
The characterization of oyster shell powder indicated that CaCO3 and CaO were the major components of the NOSP and COSP, respectively (Fig. 2). As shown in Fig. 6, when an incubation experiment was conducted for 30 days using different application levels (1, 3, and 5 wt%), the most dramatic decrease in the Cd and Pb extractability was observed in response to treatment with 3 and 5 wt% COSP. This phenomenon can be attributed to the formation of insoluble metal hydroxides in alkaline pH (Zhao and Masaihiko 2007). An increase in soil pH also causes an increase in the net negative soil surface charge, which leads to an increased capacity of cationic metal adsorption (Zhao and Masaihiko 2007). In general, the precipitations of soluble Cd and Pb as Cd(OH)2 and Pb(OH)2, respectively, are expected at pH values above 8 and 6, respectively (Lee et al. 2008; Park et al. 2007). In the present study, COSP was found to be more effective in immobilizing Cd and Pb in soil than NOSP, which likely occurred because the hydroxyl group (−OH) in soils increased after COSP treatment. Indeed, treatment with 3 and 5 wt% COSP stabilized both the Cd and Pb to levels sufficiently below the soil pollution standards in Korea (1.5 and 100 mg kg−1 for HCl-extractable Cd and Pb, respectively).
Conclusion
To remediate large amounts of contaminated soils such as those in mining areas, it is important to develop cost-effective and readily available soil amendments in place of conventional methods. In this study, oyster shell waste, which is rich in CaCO3, was applied as an alternative soil amendment for the treatment of Cd- and Pb-contaminated soils near a closed mine. The calcination process changed the composition of powdered oyster shell waste from the less active CaCO3 to the more active CaO and also decreased the Na content in oyster shell powder. Both NOSP and COSP decreased the 0.1 N HCl-extractable Cd and Pb from soils; however, COSP was more effective at stabilizing Cd and Pb in the contaminated soil. Furthermore, the soil quality in terms of soil nutrients can also be improved by applying oyster shell powder containing Ca, Na, Mg and K as nutrients. Finally, the results of this study indicate that oyster shell waste can be used as an effective soil amendment for the remediation of metal-contaminated soil. Our future research will focus on determination of the phytoavailability and speciation of Cd and Pb in contaminated soils through the use of chemical equilibrium models and advanced analytical tools such as EXAFS.
References
Cao X, Dermatas D, Xu X, Shen G (2008) Immobilization of lead in shooting range soils by means of cement, quick lime, and phosphate amendments. Environ Sci Pollut R 15:120–127
Chander K, Joergensen RG (2002) Decomposition of 14C labelled glucose in a Pb-contaminated soil remediated with synthetic zeolite and other amendments. Soil Biol Biochem 34:643–649
Cheng SF, Hseu ZY (2002) In situ immobilization of cadmium and lead by different amendments in two contaminated soils. Water Air Soil Poll 140:73–84
Dermatas D, Moon DH (2006) Chromium leaching and immobilization in treated soil. Environ Eng Sci 23(1):77–87
Dermatas D, Moon DH, Menouno N, Meng X, Hires R (2004) An evaluation of arsenic release from monolithic solids using a modified semi-dynamic leaching test. J Hazard Mater B116:25–38
Dore I (1991) Shellfish A guide to oysters, mussels, scallops, clams and similar products for the commercial user. Van Nostrand Reinhold, New York
Gilchrist JD (1989) Extraction metallurgy. Pergamon Press, Oxford
Hashimoto Y, Matsufuru H, Takaoka M, Tanida H, Sato T (2009) Impacts of chemical amendments and plant growth on lead speciation and enzyme activities in a shooting range soil: an X-ray absorption fine structure investigation. J Environ Qual 38:1420–1428
Hong CO, Lee DK, Chung DY, Kim PJ (2007) Liming effects on cadmium stabilization in upland soil affected by gold mining activity. Arch Environ Contam Toxical 52:496–502
Hong CO, Gutierrez J, Yun SW, Lee YB, Yu C, Kim PJ (2009) Heavy metal contamination of arable soil and corn plant in the vicinity of a zinc smelting factory and stabilization by liming. Arch Environ Contam Toxical 56:190–200
Isoyama M, Wada SI (2007) Remediation of Pb-contaminated soils by washing with hydrochloric acid and subsequent immobilization with calcite and allophonic soil. J Hazard Mater 143:636–642
Jo IS, Koh MH (2004) Chemical changes in agricultural soils of Korea: data review and suggested countermeasures. Environ Geochem Health 26:105–117
Kostarelos K, Reale D, Dermatas D, Rao E, Moon DH (2006) Optimum dose of lime and fly ash for treatment of hexavalent chromium-contaminated soil. Water Air Soil Poll 6:171–189
Kwon HB, Lee CW, Jun BS, Yun JD, Weon SY, Koopman B (2004) Recycling waste oyster shell for eutrophication control. Resour Conserv Recy 41:75–82
Lee CH, Lee DK, Ali MA, Kim PJ (2008) Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming material. Waste Manage 28:2702–2708
Lee CW, Kwon HB, Jeon HP, Koopman B (2009) A new recycling material for removing phosphorous from water. J Clean Prod 17:683–687
Moon DH, Dermatas D, Menounou N (2004) Arsenic immobilization by calcium–arsenic precipitates in lime treated soils. Sci Total Environ 330(1–3):171–185
Moon DH, Wazne M, Yoon IH, Grubb DG (2008) Assessment of cement kiln dust (CKD) for stabilization/solidification (S/S) of arsenic contaminated soils. J Hazard Mater 159(2–3):512–518
Moon DH, Cheong KH, Choi SB, Khim J, Kim KW, Ko I, Grubb DG (2009) Assessment of waste oyster shells for the stabilization of Pb-contaminated mine tailings in the Republic of Korea. In: 10th International symposium on environmental geotechnology and sustainable development, Bochum
Ok YS, Kim JG (2007) Enhancement of cadmium phytoextraction from contaminated soils with Artemisia princeps var. orientalis. J Appl Sci 7(2):263–268
Ok YS, Lee H, Jung J, Song H, Chung N, Lim S, Kim JG (2004) Chemical characterization and bioavailability of cadmium in artificially and naturally contaminated soils. Agric Chem Biotechnol 47(3):143–146
Ok YS, Chang SX, Feng Y (2007a) Sensitivity to acidification of forest soils in two contrasting watersheds in the oil sands region of Alberta. Pedosphere 17(6):747–757
Ok YS, Yang JE, Zhang YS, Kim SJ, Chung DY (2007b) Heavy metal adsorption by a formulated zeolite–Portland cement mixture. J Hazard Mater 147:91–96
Ok YS, Chang SX, Feng Y (2008) The role of atmospheric N deposition in soil acidification in forest ecosystems. In: Sanchez ML (ed) Ecological research progress. Nova Science Publishers, New York, pp 341–369
Park HJ, Jeong SW, Yang JK, Kim BG, Lee SM (2007) Removal of heavy metals using waste eggshell. J Environ Sci 19:1436–1441
SAS (2004) SAS user’s guide, version 9.1. SAS Institute, Cary
Singh DN, Kolay PK (2002) Simulation of ash–water interaction and its influence on ash characteristics. Prog Energ Combust 28(3):267–299
Yang JE, Kim YK, Kim JH, Park YH (2000) Environmental impacts and management strategies of trace metals in soil and groundwater in the Republic of Korea. In: Huang PM, Iskandar IK (eds) Soils and groundwater pollution and remediation: Asia, Africa, and Oceania. Lewis Publishers, Boca Raton, pp 270–289
Yang EI, Yi ST, Leem YM (2005) Effect of oyster shell substituted for fine aggregate on concrete characteristics: part I. Fundamental properties. Cement Concrete Res 35:2175–2182
Yang JE, Skousen JG, Ok YS, Yoo KR, Kim HJ (2006) Reclamation of abandoned coal mine wastes using lime cake by-products in Korea. Mine Water Environ 25(4):227–232
Yang JE, Ok YS, Kim WI, Lee JS (2008) Heavy metal pollution, risk assessment and remediation in paddy soil environment: research and experiences in Korea. In: Sanchez ML (ed) Ecological research progress. Nova Science Publishers, New York, pp 341–369
Yang JE, Lee WY, Ok YS, Skousen J (2009) Soil nutrient bioavailability and nutrient content of pine trees (Pinus thunbergii) in areas impacted by acid deposition in Korea. Environ Monit Assess 157:43–50
Yoon GL, Kim BT, Kim BO, Han SH (2003) Chemical–mechanical characteristics of crushed oyster shell. Waste Manage 23:825–834
Yoon H, Park S, Lee K, Park J (2004) Oyster shell as substitute for aggregate in mortar. Waste Manage Res 22:158–170
Zhao XL, Masaihiko S (2007) Amelioration of cadmium polluted paddy soils by porous hydrated calcium silicate. Water Air Soil Poll 183:309–315
Acknowledgments
This study was supported by the National Research Foundation of Korea Grant funded by the Korean Government (Project number: 2009-0071439). Instrumental analysis was supported by a grant from the Institute of Environmental Research, the Research Institute of Agricultural Science, and the Central Laboratory of Kangwon National University, Korea. The authors also thank Jung Eun Lim for conducting incubation experiments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ok, Y.S., Oh, SE., Ahmad, M. et al. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ Earth Sci 61, 1301–1308 (2010). https://doi.org/10.1007/s12665-010-0674-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0674-4