Abstract
In the past few decades, cadmium (Cd) as soil contaminant is a major problem for the mankind. Cd contamination of soil and food crops is a critical environmental concern as it deteriorates the soil quality and creates threat to the food safety and human health. High Cd concentration in soils pose negative effects on the plants at physiological, structural and molecular levels. Secretion of certain secondary metabolites in the rhizosphere is a survival mechanism adopted by plants to tolerate and encounter Cd toxicity. Under metal-stressed conditions, secretion of root exudates in soil increases the external detoxification strategies of the plants. The secreted phytochemicals are gaseous compounds, inorganic and especially organic in composition. In plants, the role of these metabolites to confront Cd toxicity and induce tolerance under Cd distress is underrated. The review paper focuses on Cd sources, factors that affect its bioavailability, uptake and toxicity in the plants. Furthermore, it also highlights the contemporary progression in our understanding on the mechanisms of root exudation in plants and the effect of Cd toxicity on the root exudation. Finally, the review provides important information on the role of different root exudates to subsist Cd stress in plants naturally, particularly by reducing the dependence on synthetic amendments to enhance Cd-tolerance and its aquisition in plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Overpopulation, urbanisation and intensive use of natural resources by humans have accelerated the rate of water, soil and land degradation throughout the world (Dharupaneedi et al. 2019; Reddy et al. 2019; Tavangar et al. 2020). Increasing pressure on land due to unfavourable technogenic activities is quickly degrading the vital land resources (Keesstra et al. 2018). Degradation of soils by heavy metals poses serious threat on the biotic and abiotic functioning of the soils, quality of crop plants and health of living organisms (Keesstra et al. 2018). The persistent nature and potential toxicity induced by heavy metals to the public health and soil ecosystems are major environmental concerns (Abdu et al. 2017; Dubey et al. 2018; Kumar et al. 2019). Cadmium (Cd) contamination in the environment has received substantial attention in the modern world research. Due to its high solubility in soil and prevalence of factors such as Cd speciation, its concentration in the medium and soil pH, it can be easily extracted by the plant roots (Sidhu et al. 2019a, b). Cd is not biologically important for plants, though along with the micronutrients it is acquired quite easily by plants from the rhizospheric soil, the root–soil interface (Riesen and Feller 2005; Shahid et al. 2016). Cd induces toxicity in plants by impeding the plant growth, nutrient uptake, photosynthesis and respiration (Sidhu et al. 2017). It promotes oxidative stress by inducing membrane disruption, lipid peroxidation and alterations in antioxidative response (Sidhu et al. 2017).
Various conventional/non-conventional techniques have been adopted to solve the problem of Cd-contaminated soil/water ecosystems (Table 1). The conventional practices involve high operational cost and are often labour intensive. On the contrary, non-conventional techniques are proving a boon to mitigate Cd-contaminated ecosystems. Phytoremediation is a one such clean and green approach that employs plants in conformity with the soil microbes for the alleviation of heavy metal contaminants from the soil systems (Ma et al. 2016; Muthusaravanan et al. 2018; Sidhu et al. 2018, 2020).
To enhance plant tolerance and acclimatisation towards increased heavy metal regimes various innovative biotechnological initiatives are on the rise. A variety of metal transporter proteins, sulphur metabolism enzymes and metal detoxification chelators such as phytochelatins and metallothioneins are critically involved in the tolerance and detoxification strategies of the plants to combat Cd stress. The number of studies has witnessed the potential utilisation of different plant species in the remediation of Cd-contaminated soils. In the assessment of phytoremedial potential of plant species towards increased Cd concentrations, Sidhu et al. (2017) demonstrated the potential ability of Coronopus didymus to accumulate and tolerate high-Cd regimes (100, 200 and 400 mg kg−1) in the soil. Besides, plant roots release certain phytochemicals/metabolites in the rhizosphere that altered the soil pH and enabled the formation of metal–metabolite complexes to confront Cd distress (Badri and Vivanco 2009; Chen et al. 2017). Lapie et al. (2019) documented that maize plants exposed to different Cd levels (10, 20 and 40 µM) in the nutritive medium tend to secrete different exudates. The tendency to exude excessive phytochemicals both qualitatively and quantitatively as rhizodeposits is the tolerance strategy adopted by maize plants to combat Cd stress (Lapie et al. 2019). According to Li ZR et al. (2019), the intercropping of hyperaccumulator Arabis alpina and winter crop Vicia faba enhanced the secretion of free amino acids and total content of organic acids (~ 578% and ~ 37%) from the roots of the plant species. The concentration of Pb and Cd acclimatised and bound to the cell wall and other organelles of intercropped plants was dramatically more compared to the monocropped plants. Additionally, it was found that alteration in composition and concentration of root exudates of Arabis alpina and Vicia faba under intercropping conditions enhanced the phytoremediation potency of the plant species towards Pb- and Cd-contaminated soils (Li ZR et al. 2019).
Under metal-stressed conditions, secretion of secondary metabolites as root exudates in soil increases the external detoxification strategies of the plants. The secretion of root exudates in the soil augments the tolerance potential of plants growing in Cd-contaminated soils (Fig. 1). The secreted root exudates include enzymes, various free inorganic ions and carbon-containing metabolites such as low molecular weight compounds (phenolics, amino acids, organic acids and sugars), while high molecular weight compounds include mucilage and proteins (Bais et al. 2006). Tolerance mechanisms adopted by plants to counteract excess Cd toxicity primarily include exclusion mechanisms that impede the entry of Cd ions to the root cell by secreting root exudates in the rhizosphere. Secondly, secondary metabolites such as low-molecular-weight organic acids (LMWOAs) can form chelate compounds with Cd ions at the soil–root interface and hence enable the plants to tolerate and withstand Cd-induced toxicity in the soil (Fig. 1).
The concept of root exudation particularly in plant physiology remains understudied (Preece and Peñuelas 2019). Very few researches have engaged the utilisation of root exudates against heavy metal stress in plants (Chen et al. 2017). Previous reviews have mainly focussed the role of root exudates in promoting mutualistic interactions (Rasmann and Turlings 2016) and their participation in plant defence against plant pathogens (Baetz and Martinoia 2014). In this review, Cd is employed as a model heavy metal contaminant because of its very high toxicity to plants and other living organisms. Nevertheless, no attention has been paid previously on the role of different root exudates in promoting plant tolerance against Cd stress. Thus, a knowledge gap exists to explore the functional aspects of root exudates to combat Cd-induced toxicity in plants. We have considered and discussed the principal findings from the recent research on different root exudates that show their efficacy to tolerate and reduce Cd toxicity in plants. By reviewing the available literature on root exudation, certain aspects on the mechanisms of action definitely insight the potency of root exudates to subsist Cd stress with a focus on reducing the utilisation of synthetic amendments. This review briefly concludes that future research in this field completely explicates their mechanism of action against metal stress and the studies related to secretion of root exudates in rhizosphere provide opportunities to understand the tolerance mechanisms adopted by plants in plant stress physiological studies.
Cadmium sources, contamination of soils and bioavailability
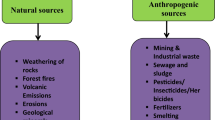
Cd with atomic number 48, atomic weight 112.41 and density 8.65 g/cm3 is a silvery bluish-grey metal. It is located at the end of the second row of the transition elements having melting point 320.9 °C and boiling point 765 °C. It belongs to group 12 of the periodic table with electronic configuration of [Kr] 4d10 5 s2. It is soft, malleable, ductile and insoluble in water (Asgher et al. 2015). Cd exists in + 1 and + 2 oxidation states; however, + 2 oxidation state is the most common. An amorphous substance cadmium oxide (CdO) is formed due to burning of Cd in air. High Cd levels are positively correlated with excessive industrialisation that has lead to increased production and consumption of Cd. Over use of Cd in electroplating, nickel–cadmium batteries, as pigments for colouring glass and plastic, stabilisers for processing PVC polymers and production of some alloys contribute equally towards its distribution in the environment (Fig. 2). Besides, mining activities, inappropriate wastewater irrigational practices, inadequate executions related to waste disposal, high inputs of agricultural fertilizers and industrial and vehicular emissions are the major causes for Cd contamination of agricultural soils (Kaplan et al. 2011; Asgher et al. 2015; Khan et al. 2016; Nawab et al. 2016; Khan et al. 2017) (Fig. 2).
Cadmium sources, uptake and toxicity in plants. DNA, deoxyribonucleic acid; MDA, malondialdehyde; ROS, reactive oxygen species: (A) cadmium sources in the soil; (B) cadmium uptake is modulated by various transporter proteins in plants; (C) cadmium toxicity induces a reduction in morphological, physiological and biochemical traits of plants; (D) cadmium toxicity induces oxidative distress, genotoxicity in plants
Both natural and anthropogenic factors contribute towards enhanced Cd contamination in the environment (Chen L et al. 2019). Cd occurrence in soils ranged from 0.07 to1.1 mg kg−1 soil (WHO 2007), although its threshold limit in agricultural soils is nearly 100 mg kg−1 (Asgher et al. 2015). According to Gallego et al. (2012), 30,000 tonnes of Cd is added to the environment annually, out of which technogenic activities contribute approximately 13,000 tonnes of Cd annually. During the past decade, undesirable anthropogenic activities have caused the release of Cd at an alarming rate that has reached up to 2.2 × 107 kg throughout the world (Costa et al. 2012). In China, Cd is considered as the most noxious heavy metal due to its high mobility and toxicity (Chen et al. 2015). Rafiq et al. (2014) statistically analysed more than 1.3 × 105 ha Cd-enriched soil area which accounts for 1/5 of the total farmland area. Due to wide distribution and Cd-enriched soils, Rafiq et al. (2014) demonstrated Cd induced grain contamination of approximately 1.2 × 105 kg and induced a reduction in the grain yield by approximately 1010 kg. Based on toxicity and potential for human exposure, Agency for Toxic Substances and Disease Registry (ATSDR) has ranked Cd to the seventh position in the priority list of hazardous substances (ATSDR 2017). The fraction of total Cd available for uptake by plants is a crucial scenario to assess the environmental risk associated with Cd contamination in soil. Cd is mainly present as Cd ions or found associated as inorganic and organic complexes in the soil solution. The major Cd uptake in plants occurs through contact with soil porewater, which is the net result of Cd partitioning between the solid and liquid phases of the soil. Nevertheless, foliar uptake and transfer of Cd have also been reported in the study of Mombo et al. (2016). The authors revealed high Cd and Pb concentration in vegetables grown in urban gardens near a Pb recycling company (Mombo et al. 2016).
Various chemical reactions such as precipitation/dissolution, adsorption/desorption and Cd ligand formation influence Cd partitioning in soil. These dynamic reactions are strongly affected by organic and inorganic ligands (Shahid et al. 2014), soil pH (Saeki and Kunito 2012), redox conditions (Zhang et al. 2012), metal concentration and temperature (Silber et al. 2012). In soil systems, Cd partitioning is an important factor in regulating Cd toxicity (Rizwan et al. 2017). Shahid et al. (2016) documented that total Cd concentration in the soil medium does not mandate Cd solubility, mobility and bioavailability, because the total chemical forms of Cd are not 100% bioavailable. Moreover, the biogeochemical behaviour of Cd is completely dependent upon the free Cd ion concentration in the soil medium (Shahid et al. 2016). Certain factors such as soil pH, organic matter content, cation exchange capacity and microbial activity of the soil effect Cd bioavailability (Shahid et al. 2016).
Effect of soil pH on Cd bioavailability
Soil pH is a crucial factor in regulating Cd partitioning and bioavailability (Yu et al. 2016). Under varied soil pH, Cd is known to exist in different chemical forms. Cd in soil is found mostly in combined state and ~ 99% of the total Cd is coupled with soil colloids. According to Kabata-Pendias and Sadurski (2004), Cd in soil occurs in both cationic and anionic forms. Cationic forms include CdCl+, CdOH+, CdHCO3+, while anionic forms include CdCl3−, Cd(OH)3−, Cd(HS) 2−4 . It has been estimated that ~ 99% of Cd is found in free ionic form in the soil solution (Kabata-Pendias 1993). It has been demonstrated that solubility of Cd in soil solution is greatly influenced by soil acidity. Mineralised soils have pH range from 4.0 to 4.5. A decrease in pH range of up to 0.2 units exhibits 3–5 times increase in labile Cd pool. An indirect correlation occurs between soil pH and Cd extraction in plant tissues. At low soil pH, the transformation of Cd from immobile combined form such as Fe and Mn oxides and carbonates to more phytoavailable and exchangeable form enables free Cd mobility and phytoavailability (Qi et al. 2018). In Elba muck soils of Western New York, Sullivan et al. (2013) reported a negative correlation (r = − 0.55) between CaCl2 extractable Cd fractions with pH of the soils. The findings probably corroborated with pH 6 that acts as a threshold point for Cd solubility in soil due to the formation of strong complex with organic matter and adsorption on mineral surfaces (Sullivan et al. 2013; Shahid et al. 2016). A positive correlation between the low soil pH with enhanced Cd phytoavailability revealed the possibility of deploying non-conventional techniques such as phytoremediation to ameliorate Cd uptake by plants from Cd-contaminated soils (Sidhu et al. 2019a).
On the contrary, an increase in soil pH enhanced its alkalinity that effects and influences the adsorption of Cd ions on to the soil particles which in turn subsequently poses negative effect in the uptake of vital nutrients by the plants. Yu et al. (2016) studied 73 pairs of soil and rice samples contaminated with acid mine drainage containing Cd to assess the effect of soil pH and iron fractions in the bioavailability of Cd in the rice grains. Furthermore, assessment of soil properties for Cd acquisition in the rice grains revealed that pH of soil imparts a key role in acclimatising Cd in rice grains, while amorphous iron fractions were considered as the second important factor in contributing Cd bioavailability in the rice grains (Yu et al. 2016). Increased soil pH poses negative effect on the phytoavailability, as Cd precipitation and adsorption onto the soil particles reduce the free Cd availability in the soil solution (Meng et al. 2018). In soils having pH > 7.5, Cd precipitated and adsorbed onto the soil particles remains immobilised. The degree of net charge associated with the solid phase is directly related to the soil pH. In acidic soil solution, protons (H+) compete with Cd ions for binding sites, which induces Cd desorption from soil particles into the solution. But in the alkaline environment, the solid-phase exchange sites are freely available for binding with cationic metal ions. At high soil pH, Cd is hydrolysed to hydroxy species and is adsorbed readily with the solid phase and influence high-Cd adsorption, making Cd desorption a difficult task in alkaline medium (Shahid et al. 2016).
Effect of soil organic matter on Cd bioavailability
Soil organic matter derived from decomposition of plants and animals imparts a consequential role in Cd bioavailability, as it readily forms complex with Cd in the soil medium. Cd bioavailability is directly influenced by humus, and it varies with its concentration, source and various forms such as either suspended or dissolved and other physico-chemical properties. Humus in soil as organic matter is responsible for maintaining high soil pH, and component such as fulvic acid in it is very reactive which helps to maintain high metal bioavailability due to high cation exchange capacity, small size and high oxygen content (Shahid et al. 2012). It has been noticed that content of surface organic matter has a direct influence in binding and acclimatising Cd. Kirkham (2006) documented that soils rich in organic matter had high sorption potential which is about 30 times higher for Cd compared to mineral soils. In a recent finding, Rocco et al. (2018) assessed the potential utilisation of organic matter compounds (poultry manure, coal fly ash and biochar) to enhance bioavailability of Cd in Zea mays in two Australian soils with different pH and texture. However, the addition of amendments to Kapuda-acid loamy sand though enhanced the soil pH but subsequently reduced Cd bioavailability in Zea mays (Rocco et al. 2018).
Addition of biochar to Cd-contaminated soils effectively enhances the soil pH and reduces Cd bioavailability. The utilisation of biochar as organic material reduces Cd bioavailability in Lolium multiflorum by 6–14%, when the plants were grown under Cd-contaminated soils. Furthermore, the application of ˃ 10% biochar dramatically decreased Cd bioavailability in plants by immobilising Cd in soil (Xiao et al. 2019). In a similar study, Abbas et al. (2018) demonstrated the application of biochar efficaciously reduces Cd and salt stress in Triticum aestivum by decreasing Cd bioavailability and promoted the plant growth by regulating antioxidant enzyme activities in plants. Likewise, biochar application to soils contaminated with Cd significantly altered the rhizobacterial communities by stimulating the activities of growth-promoting bacteria in rice plants (Wang et al. 2019). The finding revealed that biochar application as organic matter reduced Cd mobilisation in soil and hence decreased Cd bioavailability in rice plants.
On the contrary, application of tea waste derived biochar to Cd-contaminated soil sediments enhanced the uptake and accumulation potency of ramie seedlings by altering Cd speciation in soil sediments and by modulating sub-cellular Cd distribution in plant cells (Gong et al. 2019). Yousaf et al. (2016) assessed the potential effect of traditional organic amendments such as press mud, biochar, farm manure, sewage sludge, poultry manure and compost as carbon sources on the bioavailability and uptake of Cd in Triticum aestivum. The results revealed that all the organic amendments significantly increased the organic content of the soil and Cd uptake in Triticum aestivum was enhanced predominantly with the application of sewage sludge, poultry manure and farm manure (Yousaf et al. 2016). Amendment of un-entrapped inorganic fertilizers such as urea and bio-fertilizers such as Bacillus subtilis and Azotobacter chrocoocum increased Cd concentration in roots and shoots of Brassica juncea and Ricinus communis plant species (Bauddh and Singh 2015). The vermicompost as organic fertilizer could bind Cd and promote high biomass production and microbial consortium that ultimately effect the enhanced Cd extraction and growth of both the plant species (Bauddh and Singh 2015). Additionally, it was noticed that the application of vermicompost obtained from pig manure to soils co-contaminated with Cd and polycyclic aromatic hydrocarbons dramatically enhanced the Cd phytoextraction efficiency of Sedum alfredii due to enhanced root–shoot biomass and growth of the plants that ultimately induce rapid dissemination of polycyclic aromatic hydrocarbons in soil probably due to increased generation of root exudates which subsequently favours the bacterial community structure in the rhizosphere (Wang et al. 2012).
Effect of cation exchange capacity of soil on Cd bioavailability
Cd mobility in soil and its bioavailability are influenced by cation exchange capacity of the soil. According to the study conducted by Gusiatin and Klimiuk (2012), the loamy soil and loamy sand soils in Warmia and Mazury Province, north-eastern Poland, bound Cd to the exchangeable and acid-soluble fraction having small Cd concentration was found to be associated with organic matter fraction. Though, in silt–clay soil, Cd was found to be bound with reducible fractions followed by exchangeable acid-soluble fractions (Gusiatin and Klimiuk 2012). In clayey soils, Hong et al. (2002) demonstrated the low mobility of Cd due to the strong affinity of Cd with the surface of clay minerals, humus and Fe–Al oxides. The presence of other mineral ions in the soil medium has direct influence on Cd bioavailability. This is directly correlated with the ionic strength, complexation and competition for soil exchange sites or root surface exchange sites. Moreover, the ionic strength of the growth medium inversely affects Cd bioavailability, which shows that low ionic strength in the growth medium enhanced the Cd extraction by the plants (Gothberg et al. 2004). In another study, Kim et al. (2017) elucidated an increase in soil pH and negative surface charge of the soils treated with combination of both 30 mg/ha bottom ash and animal manure compost ultimately enhanced Cd adsorption on soil surface and decreased Cd bioavailability in Lactuca sativa plants.
Effect of soil microbial activity on Cd bioavailability
Certain microorganisms develop potential resistance towards Cd, dominate the rhizospheric conditions and promote the growth of the plants under Cd stress. To overcome the Cd stress, the microbes have developed inherent mechanisms to bind free Cd ions and actively colonise rhizosphere and resistance towards Cd excess in the soil (Table 2). Cd availability in the soil is enhanced by soil microbes as the later are highly involved in the subsequent solubilisation of Cd-bearing minerals and excretion of chelate-forming organic acids (Liu et al. 2020) (Table 2). Cd-solubilising microorganisms such as plant growth-promoting bacteria (PGPR) as an amendment in the soil, thus impart a crucial role for increasing Cd bioavailability (Wu et al. 2020). For instance, Li X et al. (2019) documented dsrA and soxB gene and Thiobacillus sp. was the dominant bacterial species involved in the effective oxidation of sulphur, assisting the uptake and accumulation of Cd up to ~ 66%, 46% and 42% in leaves, stems and roots of tobacco plants. On the parallel, a study conducted by Sangthong et al. (2016) postulated the potential of bioaugmenting agent Micrococcus sp. TISTR2221 to regulate high Cd uptake in the stem and root part of the Zea mays plants grown under high Cd stress. The above findings support the work conducted by Li X et al. (2019) who demonstrated the inoculation of Lycopersicon esculentum with arbuscular mycorrhizal fungi (Funneliformis mosseae) and Cd-resistant bacteria strain (Enterobacter sp. EG 16) enhanced root–shoot biomass along with the increased Cd uptake in the roots of the plants under 50 and 100 mg kg−1 Cd treatment. Arbuscular mycorrhizal fungi impart a vital role in Cd mobility in plants. The co-inoculation of Solanum nigrum with arbuscular mycorrhizal fungi and earthworms enhanced the plant biomass and acquisition of phosphorus and Cd in the shoots (Wang et al. 2019). The results of their study showed that co-inoculation (arbuscular mycorrhizal fungi and earthworms) increased Cd phytoavailability up to 149.3% under 120 mg kg−1 Cd-spiked soils by changing the Cd chemical fractions (Wang et al. 2019).
On the contrary, to curb Cd toxicity in plants, microbes in the form of plant growth-promoting bacteria and arbuscular mycorrhizal fungi impart an active participation in limiting Cd uptake in roots and its translocation to foliar plant parts. Sharma and Archana (2016) postulated that plant growth-promoting bacteria are not directly involved in Cd sequestration but reduce the plant stress based on the plant growth-promoting traits. A significant decline in Cd phytoavailability was observed by bioaugmentation of soils, employing free and immobilised Cd-resistant bacteria and fungi in the rhizosphere (Sharma and Archana 2016). Soils inoculated with certain microbes such as Pseudomonas aeruginosa, Burkholderia gladioli (Khanna et al. 2019); Cupriavidus sp. strain ZSK (Zeng et al. 2020) and arbuscular mycorrhizal fungi (Zhang X et al. 2019) have proclaimed decrease Cd phytoavailability. The strategies adopted for reducing Cd bioavailability in plants depend upon the potency of microbes to transform mobile states of Cd into inert forms by the mechanisms such as (1) reducing Cd solubility in soil by altering soil pH and metal valency; (2) binding or sequestering Cd with the components present on the cell surface; (3) releasing a variety of organic compounds in the rhizosphere that form complex with the Cd ions; and (4) formation of insoluble CdS by releasing H2S. The microbes achieve Cd resistance and decrease Cd bioavailability to plants by biosorption, bioaccumulation, precipitation, complexation and efflux of Cd ions. In bacterial defence mechanisms, intracellular binding proteins such as metallothioneins (cystein-rich protein) and metallochaperones function as cytoplasmic Cd-binding proteins that assist in lowering the concentration of free Cd ions within the cytoplasm and hence decrease Cd bioavailability to the plants and promote the number of friendly microbial community in the rhizosphere. In a recent study, Xu et al. (2019) documented a substantial reduction in Cd bioavailability to Brassica chinensis when the Cd-contaminated soil was inoculated with Raoultella sp. strain X13. The functional trait of X13 strain in releasing indole acetic acid and solubilising phosphate dramatically improved the growth and production of Brassica chinensis plants (Xu et al. 2019). The inoculation of Cd-contaminated soil with sulphate-reducing bacteria (SRB-1) strain isolated from metal(loid) contaminated paddy fields significantly reduced the bioavailability of Cd and Pb by 29.5% and 26.2%, respectively, in the rice grains (Shan et al. 2019). In a similar work, combined effect of indigenous soil arbuscular mycorrhizal fungi and zeolite addition on Cd uptake in the grains of bread wheat was assessed by Baghaie et al. (2019) who found a noticeable decrease in Cd uptake to the bread wheat grains from 8.9 to 3.3 mg Cd kg−1, respectively. Additionally, a remarkable increase in growth and phosphorus and nitrogen concentration in plants grown under Cd stress was observed (Baghaie et al. 2019). The reduction in Cd bioavailability to plants by microbes might also be attributed to the Cd immobilisation in the soil by forming less soluble organic fractions. This hypothesis is corroborated with the study conducted by Xu et al. (2012), who reported ~ 8–25% Cd transformation into less bioavailable organic-bound fraction, with the assistance of bacteria. The role of microorganisms in transforming Cd to less soluble organic fractions immobilises Cd in the soil, decreases its phytoavailability and enables the plants to tolerate and overcome Cd-induced toxicity.
Cd uptake and toxicity in plants
Cd is a non-essential trace element and has no physiological importance in plants. Cd occurs in the form of Cd ions in soil (Tudoreanu and Phillips 2004) and enters the root cells with the help of zinc-regulated transporters/iron-regulated transporters (Asgher et al. 2015) (Fig. 2). The epidermal layer of the root is the first site for the contact of free Cd ions in soil (Sidhu et al. 2019a). The root hairs provide large surface area and aid in absorption of Cd ions from the soil through diffusion (Seregin and Ivanov 1997). Plant roots also secrete certain organic compounds such as chelates that complex with Cd ions to form ligands, allowing its entry into root epidermis (Sidhu et al. 2019a). Additionally, certain protein transporters such as natural resistance-associated macrophage proteins (NRAMPs) impart a critical role in Cd transport across the cell membrane (Song et al. 2014). In general, after Cd uptake by plant roots, maximum portion of Cd gets fractionised into the root cells and only a small portion gets fractionised to the upper aerial plant parts and grains (Song et al. 2014). In the process of Cd transport in plants, commonly intracellular transport occurs in plant vacuole. Cd-chelating components such as metallothioneins, phytochelatins and organic acids are crucial for the transport. Sulphydryl and carboxylic groups in these chelating compounds form complex with Cd and bind it before its transport to the vacuole. Vacuolar transporter proteins such as natural resistance-associated macrophage proteins, ATP-binding cassette transporters (ABC transporters) and P-type ATPase are involved in Cd–chelant complex into the vacuole. The intracellular Cd transport facilitates Cd detoxification in the roots and hence promotes Cd tolerance in plants against Cd toxicity.
Cd transport process in plants may be a short or long-distance transport. From the soil, Cd is taken up by the plant roots and then the short-distance transportation of water occurs through both apoplastic and symplastic pathways. Parrotta et al. (2015) documented that the apoplastic pathway facilitates Cd binding in the cell wall, while the carboxyl group present in the cell wall binds and immobilises Cd. In apoplastic movement, the plasticity of cell wall imparts a decisive role in Cd movement. During Cd transport through apoplastic route, suberin layer acts as a barrier for Cd transport (Tao et al. 2017). The symplastic route enables Cd transport from epidermis to water-conducting elements. Ueno et al. (2010) revealed that certain transporter proteins such as Oryza sativa heavy metal P-type ATPase 3 (OsHMA3) play a consequential role in decreasing Cd transport to the shoots. Oryza sativa heavy metal P-type ATPase 3 transporter protein enhanced Cd sequestration in the vacuole of root cells and decreased Cd transport to upper aerial parts. Another metal transporter protein from low-affinity cation transporter 1 (LCT1) family was found to remobilise Cd via phloem by forming association with metal complexes (Uraguchi et al. 2011). Low-affinity cation transporter 1 protein exists in the nodes and leaf blades and functions as a Cd transporter (Uraguchi et al. 2011). Nevertheless, previous studies have revealed the active participation of P1B-ATPase and zinc/iron permease (ZIP) family transporter proteins in Cd transport across the plasmalemma into the shoots by utilising energy obtained from ATP hydrolysis to transport Cd against the concentration gradient (Hanikenne et al. 2008; Wong and Cobbett 2009). In phloem, certain Cd-binding agents such as glutathione and phytochelatins are predominantly present. After prolonged Cd exposure, the concentration of amino acid cysteine increased in the phloem and influenced long-distance Cd transport in the plants (Jozefczak et al. 2012).
Cd adversely disrupts the plant metabolic functions by affecting photosynthesis, respiration, opening and closing of stomata, hinders nutrient and water uptake and cell division and impedes nitrogen metabolism and protein expression (Gallego et al. 2012; He et al. 2015). Cd excess inhibits the plant growth and production by posing toxic effects on the enzymes involved in Calvin cycle and carbohydrate metabolism (Shi et al. 2010; Javed et al. 2017). Elevated Cd level impedes plant growth, promotes chlorosis by restricting chlorophyll biosynthesis and alters uptake of vital micronutrients (Sidhu et al. 2017). In leaves, Cd alters the photosynthetic machinery by interfering with enzymes of Calvin cycle, retarding photosystem I (PS I) and photosystem II (PS II) activity or by causing dissociation of ribulose-1, 5-bisphosphate carboxylase oxygenase enzyme (RuBisCO) (Zoghlami et al. 2011) (Fig. 3). Inside the plant cell, Cd accumulation hinders the activities of various mitochondrial enzymes such as isocitrate dehydrogenase, succinate dehydrogenase and malate dehydrogenase (Bansal et al. 2002) (Fig. 3). Cd distress in plants stimulates the formation of reactive oxygen species (ROS) that causes protein oxidation by rupturing peptide bonds, induces damage to lipid and nucleic acids and alters carbohydrate metabolism (Sobrino-Plata et al. 2014) (Fig. 3). Additionally, excess Cd imparts oxidative stress, alters the gene expression and cell cycle and instigates apoptosis in plants (Kapoor et al. 2014; Sidhu et al. 2017).
An overview of the toxic effect of cadmium on different cell organelles of plant cell. Cd2+, bivalent cadmium ion; ROS, reactive oxygen species; OH., hydroxyl radical; DNA, deoxyribonucleic acid; PS I, photosystem I; PS II, photosystem II; ETRs, electron transport rates; RuBisCO, ribulose-1,5-bisphosphate carboxylase oxygenase: (A) cadmium entry inside the cytosol of the plant cell; (B) cadmium toxicity affects photosynthetic machinery of the plant cell; (C) toxic Cd ions damages cytosolic proteins; (D) toxic Cd ions affect the genetic material by generating reactive oxygen species, induce changes in the gene expression and cause genotoxicity
Mechanism of root exudation and effect of Cd stress on exudation
Plants exude secondary metabolites in the rhizosphere through both active and passive transport pathways. However, the chemical properties of the exuded compounds influence their secretion at plant–soil interface (Vives-Peris et al. 2019). Baetz and Martinoia (2014) opined that passive transport system imparts a critical role in root exudation through varied pathways: (1) transport through vesicles, (2) diffusion from the root membrane and (3) formation of ionic gradient. Root exudates like carbohydrates and carboxylates (malate, citrate, oxalate) are exuded by ionic channels and are transported across the membrane through various transporter proteins. Two anionic channels like slow anionic channels (SLACs), S-type (take time to be activated), and quick anionic channels (QUAC), R-type (activated in few milliseconds), are involved in exudate transport across the membrane (Dreyer et al. 2012). In association with these anionic channels, aluminium-activated malate transporters (ALMT) are involved in various plant physiological activities such as malate secretion in soil under Al stress. Sharma et al. (2016) studied the activation of anionic channels under Al stress and concluded the research by revealing the role of these channels in inducing plant tolerance towards Al stress. Likewise, maximised autotransporter-mediated expression (MATE) family of transporter proteins induces citrate secretion in soil under metal stress (Liu et al. 2009). Recently, Maruyama et al. (2019) opined that phosphorus deficiency in soil caused the activation of aluminium-activated malate transporter protein responsible to induce malate secretion in soil. The secretion of low molecular weight molecules such as carboxylic acids, sugars, amino acids and phenolics by the roots in the rhizosphere is mediated by diffusion. A concentration gradient is formed between the cytoplasm of the root cells and the rhizosphere that enable the release of exudates in the rhizosphere down the concentration gradient. However, polarity of the compounds, root membrane permeability and membrane integrity of the root cells effect the root exudation in the rhizosphere (Badri and Vivanco 2009).
Proteins present on root plasma membrane are involved in the transport of exuded secondary metabolites from roots through passive transport mechanism (Baetz and Martinoia 2014). ATP-binding cassette transporters and maximised autotransporter-mediated expression are the two family transporter proteins involved in root secretions and transport (Kang et al. 2011). The primary transporter protein family includes ATP-binding cassette transporters as they use the energy from ATP hydrolysis to translocate variety of solutes (Orelle et al. 2018). The researchers have appraised the importance and the potential role of ATP-binding cassette transporters in root exudation. Five knockout mutants Atmrp2, Atpgp4-1, Atpdr2, Atath6 and Atpdr6 obtained from ATP-binding cassette transporters display their role as transporter protein that promotes root exudation in Arabidopsis thaliana plants, though a composition difference prevails between the mutants and those obtained from control plants (Badri et al. 2008). Rhizospheric microbiota of Atabcg30 mutant showed variations in the secretion of phenolics and sugars in the rhizosphere. Atabcg30 mutant exudes less concentration of sugars and more concentration of phenolics in the rhizosphere compared to the wild mutants (Badri et al. 2008).
Maximised autotransporter-mediated expression family transporter proteins were first identified in Arabidopsis thaliana plants which act as secondary active transporters and allow the movement of various compounds across the plasma membrane by forming an electrochemical gradient (Weston et al. 2012). The participation of maximised autotransporter-mediated expression transporter proteins in secretion of secondary metabolites has been observed in various genotypes having genes in barley (HvAACT1), Arabidopsis (AtMATE1) (Yokosho et al. 2011; Zhou et al. 2013) that modulate citrate secretion in rhizosphere under Al stress. Additionally, genotypes of rice such as OsPEZ1 and OsPEZ2 influence the secretion and transport of phenolics as secondary metabolites in rhizosphere (Takanashi et al. 2014).
Presence of Cd ions in the growth medium increased the exudation of secondary metabolites in the rhizosphere of hyperaccumulator plant species. In a study conducted by Luo et al. (2014), Cd stress influences the secretion of root exudates in a hyperaccumulator plant Sedum alfredii. Furthermore, sample analysis by GC–MS revealed the release of 20 compounds by Sedum alfredii in the rhizosphere on exposure to Cd stress. Certain secondary metabolites such as trehalose, erythritol, naphthalene and n-octacosane were found to be involved in Cd stabilisation in the soil, while phosphoric acid, threonic acid, oxalic acid and glycine were probably found to be related to Cd mobilisation (Luo et al. 2014). Likewise, a study conducted by Pinto et al. (2008) postulated the high secretion of malate from roots of sorghum and citrate from roots of maize plants when the plants were supplemented with 5.0 mg Cd l−1 grown in hydroponic solution. The role of organic acids exuded from plant roots in decreasing Cd bioavailability by inducing Cd–organic acid complexation in the medium was observed which enhanced the stabilisation of free Cd ions in the rhizosphere (Pinto et al. 2008). In a recent study, Lapie et al. (2019) noticed the exudation of proteins, sugars, amino acids and about 40 molecules by maize plants in the rhizosphere when the plants were exposed for 6 weeks under 10, 20 and 40 µM Cd in nutrient solution. Furthermore, it was revealed that increased Cd levels reduced the content of total carbon, amino acids and sugars in the rhizodeposits of maize plants, while protein secretion was constant and the exudation of organic acids was increased. Lapie et al. (2019) concluded the active participation of exuded secondary metabolites to combat Cd toxicity and induce Cd tolerance in maize plants.
Role of different root exudates to combat Cd toxicity in plants
The modifications and promotion of root exudation can assist the plants to cope with the adverse abiotic stress factors by altering soil pH, chelation of toxic metal ions and assimilation of micronutrients by solubilising them. The exudation of different phytochemicals by plants as amendment in rhizosphere effects Cd mobilisation and reduces the leaching risk of its toxic concentrations. Furthermore, exuded phytochemicals allow the colonisation of beneficial microbiota and favours enhanced plant biomass by increasing the soil organic matter. Enhanced biomass yield is the critical condition for the plant species to be employed in phytoremediation-based studies. The profitable role of root exudates in Cd tolerance and its acquisition has been exploited for phytoremediation and soil reclamation purposes. In recent studies, the secretion of organic acids and amino acids in maize cultivar (cv. 3062) and rice cultivar (Lu 527-8) improved the nutrient uptake, plant growth and antioxidant activities and enabled the Cd-tolerant cultivars to acclimatise in Cd-contaminated environment (Javed et al. 2017; Fu et al. 2018). Furthermore, in an intercropping agricultural system, cultivation of agricultural crops along with Cd hyperaccumulators increases the nutrient uptake in crop plants and better farmland yield and can result in better agricultural products.
Phytosiderophores
Phytosiderophores are low molecular weight iron-selective chelants secreted as root exudates by the plants into the rhizosphere (Fig. 4). They are known as carriers of Fe3+ under conditions where mobilisation of Fe is poor. Moreover, phytosiderophores also exhibit high affinity for metals such as Cu, Zn, Cd and Ni (Sharma et al. 2018), solubilise them readily in soil, enable the plants to extract metals and ameliorate remediation potential of the plants (Sharma et al. 2018). Although their potential to solubilise metals depends upon the biogeochemical soil properties, microbial enrichment in rhizosphere and the quantity of phytosiderophores secreted in the soil. Phytosiderophores act as chelants having strong binding constant to form complex formation with certain metals such as Cd, enabling its mobilisation within the root tissues (Shenker et al. 2001) (Fig. 4). The potential to acclimatise Cd from soil and its mobilisation mark phytosiderophores as effective mediators to accomplish Cd phytoextraction from the contaminated soils. However, only few study reports have demonstrated the effect of phytosiderophores as metabolites in conferring Cd tolerance and its phytoextraction from Cd-contaminated soils. One of the few experiments conducted by Awad and Römheld (2000) and Römheld and Awad (2000) revealed that phytosiderophore exudation elevated Cd uptake in Fe-deficient wheat plants grown in calcareous soils containing Cd. Their studies concluded the possible implication of phytosiderophores that can be employed as chelants in chelant-mediated phytoextraction. On the contrary, there are certain findings that rejected phytosiderophores role in Cd influx into the root cells. In a series of experiments, Kudo et al. (2007) investigated the role of phytosiderophores in chelant assisted phytoextraction of Cd in hydroponically grown barley plants. Negative results were obtained for Cd uptake by barley plants when the plants were grown under Fe-sufficient (10, 100 µmol l−1) conditions for a period of 7 days. However, in Fe-deficient conditions (0 µmol l−1), a drastic increment in Cd uptake was observed. The authors further suggested the involvement of phytosiderophores in mobilising insoluble Cd in the rhizosphere but unable to convey phytosiderophores–Cd complex entry into the root cells. On the parallel, Puschenreiter et al. (2017) made a relative study by conducting a RHIZO test and batch extraction for analysing phytosiderophores exudation, heavy metal mobilisation and their accumulation in wheat plants. It was noticed that Fe-deficient wheat plants exhibited more phytosiderophore exudation in soils inducing peaked Zn, Cu and Ni concentrations in wheat shoots, while the Cd and Pb concentrations remained uneffected. Moreover, under limited Fe supply Meda et al. (2007) examined the interaction between Cd and phytosiderophore-imparted Fe uptake in hydroponically grown Zea mays plants. Cd exposure under Fe-deficient conditions increased the expression of Fe–phytosiderophore transporter gene ZmYS1 in roots. Additionally, Fe deficiency under Cd stress induced the secretion of 2′-deoxymugineic acid (DMA), a phytosiderophore from the roots of maize plants. 2′-Deoxymugineic acid constitutes a weak complex formation with Cd and enables relatively low Cd mobilisation in the medium compared to other metals such as Zn, Cu and Fe (Meda et al. 2007). The results conveyed that hindrance of ZmYS1-imparted Fe–2´-deoxymugineic acid translocation by Cd is not correlated with Cd–2´-deoxymugineic acid complex formation and Cd-induced phytosiderophore secretion did not exhibit any protective role in maize plants against Cd stress.
Root exudates secreted in the rhizosphere influence Cd tolerance in plants. (A) Secretion of root exudates such as low-molecular-weight organic acids, phytosiderophores, phenols, amino acids, proteins, mucilage in the rhizosphere; (B) plant growth-promoting bacteria regulate siderophore secretion in the rhizosphere; (C) formation of Cd–chelant stable complex can immobilise Cd ions in the rhizosphere or can enhance the transport and accumulation of relatively less toxic Cd ions in the plant tissues; (D) secreted exudates induce Cd tolerance in plants. LMWOAs low-molecular-weight organic acids
Conversely, in the literature it seemed that Cd excess in the growth medium has induced pessimistic effects on the secretion of phytosiderophores from roots of plants. For instance, Kudo et al. (2013) compared the effect of Cu on phytosiderophores release in barley plants with or without the supplementation of Cd. Under Cu toxicity, the release of phytosiderophores was enhanced in roots of barley plants. They opined that Cu toxicity in barley is a signal that triggers the phytosiderophore secretion by plant roots, but their release imparted by Cu stress is dramatically reduced under Cd toxicity. They proposed that inhibition of phytosiderophores secretion is one of the detrimental effects of Cd toxicity in the plants.
The nutritional status in some crop plants is directly related to phytosiderophore which induced enhanced endosperm loading of some vital microelements and by lemmatising Cd accumulation in the endosperm. The potential of phytosiderophores such as nicotianamine (NA) and 2´-deoxymugenic acid to restrict Cd accumulation in rice endosperm was probed by Banakar et al. (2017), delineating an increment in twofold Zn concentration and fourfold Fe concentration in the rice endosperm compared to the wild ecotypes. Concomitantly, in the presence of phytosiderophores, the transgenic rice plants employed in this study accumulated 1.5- to 2.0-fold lower Cd levels in the seeds and endosperm, respectively. However, the mechanism by which plants in the presence of phytosiderophores and other micronutrients impede Cd transport and accumulation is not clearly understood. Though, studies conducted by Uraguchi and Fujiwara (2012) and Hazama et al. (2015) revealed that endosperm is the potential area for Cd acclimatisation in crop plants. In the presence of phytosiderophores, Fe and Zn exert competitive environment with Cd in the growth medium that showed a tolerance strategy adopted by transgenic rice plants to hinder Cd transport and its acclimatisation in seed endosperm (Banakar et al. 2017).
Some studies have evidenced the positive correlation between the activation of genes involved in biosynthesis of phytosiderophores with increased Cd levels in the growth medium. Astolfi et al. (2014) studied the relationship between transporter genes involved in phytosiderophores secretion and Cd excess in barley plants. They noticed that the transporter genes (HvDMAS1, HvNAAT-A, HvNAS4, HvNAS6, HvNAS7, HvTOM1 and HvNAS3) induced the biosynthesis and secretion of phytosiderophores and their activity was triggered under Cd excess in Fe-sufficient condition (Astolfi et al. 2014). The up-regulated expression of these genes in roots under Cd excess was followed by an enhanced phytosiderophores secretion. Moreover, the authors proposed that Cd exposure induced the over-expression of HvIRO2 and down-regulation of HvIDEF1 and HvIRT1 genes that mediate HvIRO2-induced biosynthesis of phytosiderophores under Cd-stressed conditions which may not be due to Fe deficit (Astolfi et al. 2014). Adding to it, the down-regulation of transporter genes such as HvIRT1 and HvNramp5 depicted the defensive strategy opted at transcription level against Cd translocation in barley seedlings (Astolfi et al. 2014). Furthermore, the up-regulated transporter genes mediate phytosiderophore secretion and influence enhanced Fe uptake under Cd stress.
Various authors in their research have appraised the bacterial-strain induced siderophore production in rhizosphere of Cd-contaminated soils that enabled the plant to cope Cd toxicity. In Cd-contaminated soils, the bacterial strains isolated from the plant roots and rhizospheric area are effective in releasing siderophores and relieving Cd stress in Cd-tolerant plants. The plant-related siderophore-generating bacteria mounted the micronutrient availability to the plants, which increases Cd mobility, its transport, tolerance and phytoextraction potential (Sinha and Mukherjee 2008). The authors studied the effect of siderophore-producing bacterial strain of Pseudomonas aeruginosa in relieving Cd stress of mustard and pumpkin plants. Siderophore produced by bacterial strain in the rhizosphere reduced Cd uptake in roots of mustard and pumpkin plants by ~ 52% and 59%, respectively, but in shoots by ~ 37% and 47%, respectively. The reduced Cd uptake in both the plants is ascribed to the bacterial Cd accumulation and its immobilisation inducing low Cd availability in the soil (Sinha and Mukherjee 2008). On the contrary, rhizospheric secretions of bacterial siderophores increase the phytoextraction potential of Cd-tolerant plants grown under Cd-contaminated soils. Dimkpa et al. (2009) demonstrated that secretion of siderophores such as hydroxamate by Streptomyces tendae F4 in the rhizosphere tremendously enhanced the growth of Helianthus annuus and significantly mounted Cd and Fe uptake in plants grown under high-Cd regimes. Furthermore, the authors recommended the employment of siderophores in bio-chelator-assisted phytoremediation of Cd-contaminated soils. The secretion of siderophores in the rhizosphere by bacterial strains increased the plant biomass as well as Cd accumulation in tissue parts of Helianthus annuus grown under high-Cd regimes (Dimkpa et al. 2009).
Organic acids
The generation, accumulation and balance of organic acids in rhizosphere are governed by secretions from plant roots, microbial mineralisation of organic compounds and sorption–desorption processes (Jones 1998) (Fig. 4). Organic acids, especially low-molecular-weight organic acids, display a critical part in various biochemical pathways, such as energy production, trigger progenitor for amino acids biosynthesis and regulate adaptations against various abiotic stresses (Kaur et al. 2018). According to Hinsinger et al. (2005), photosynthates exuded by roots in the rhizosphere contain certain link of ligands capable of forming chelation with heavy metals present in soil and water ecosystems. In rhizosphere, organic acids secreted by plant roots actively participate in Cd uptake, prompt its dissolution from insoluble mineral phases in the soil, increase its solubility and mobility in the vicinity of roots and thereby enhance its phytoavailability to the plants (Kaur et al. 2017, 2018) (Fig. 4). The enhanced secretion of organic acids under Cd stress corroborates Cd-modulated induction of metabolic pathways and determines their role in immobilisation and detoxification of Cd in plants (Chen et al. 2003). Furthermore, for Cd-related detoxification mechanisms, organic acids released at root–soil interface exhibit two-way defensive tolerance strategies for plants. Cardinal strategy involves organic acids inducing efficacious Cd precipitation by chelation in the rhizosphere and secondly by facilitating Cd quenching in the vacuole (Ryan et al. 2001; Dresler et al. 2014). Specific low-molecular-weight organic acids can quench and form a complex with Cd and hinder Cd-induced toxicity in the plants (Adeleke et al. 2017; Kaur et al. 2017).
Organic acids play a key role in reducing Cd availability to the plants by forming chelation and prevent its entry in free form into the roots (Meach and Martin 1991). To combat Cd-induced stress in plants, some Cd-resistant plant cultivars adopt exclusion mechanisms in the rhizosphere to restrict Cd entry inside the roots. Exuded organic acids keep the toxic metals such as Cd excluded from being entered the plant tissues through roots. Pinto et al. (2008) evaluated the Cd speciation with HYPERQUAD speciation programme and reported that under Cd stress, malate or citrate exuded from roots of maize and sorghum effectively decline the free Cd ion concentration in the solution, hence contributing towards Cd-resistant mechanisms in maize and sorghum plants. For instance, Zhu et al. (2011) found that oxalate secreted from root apex by a cultivar (Micro-Tom) of Lycopersicon esulentum helps to exclude Cd from being entered inside the roots and contribute towards Cd resistance in Cd-resistant tomato cultivar. However, the exclusion of Cd by plant roots with the assistance of organic acids has not been well documented in the literature.
When the exclusion mechanism is broken down, organic acids are capable of chelating with free metal ions in cytoplasm, restricting their noxious effects on metabolic activities of the cell and promoting their diffusion in chelated form to the vacuoles (Chen et al. 2003). Organic acids induced Cd complexation, and compartmentalisation in the vacuole is the intracellular mechanism adopted by plants to cope Cd detoxification (Chaffai et al. 2006). In some cases, it has been documented that increased Cd concentration in plant tissues induces a parallel mount in the secretion of organic acids in their tissues. This can be attributed to the strong defence strategy acquired by the plants to subsist Cd toxicity. Pot experiments with Helianthus annuus under Cd stress conducted by Saber et al. (1999) revealed an increase in the generation of malic and citric acids in the roots and shoots, hence enabling the plants to tolerate and detoxicate Cd distress. Likewise, Irtelli and Navari-Izzo (2006) demonstrated that citric acid and NTA addition (20 mmol kg−1) to Cd amended soils (150 mg kg−1) instigated the production of oxalic, malic and malonic acids in shoots of Brassica juncea plants. Furthermore, the researchers positively correlated the increased production of OAs with peaked Cd levels in shoots. Likewise, in another member of Brassicacae family, the root hair of Cd hyperaccumulator Thlaspi caerulescens, contains high levels of citric and malonic acid as demonstrated by Boominathan and Doran (2003), who reported high Cd localisation in cell walls and ensured the plant tolerance to withstand stress under 178 µM Cd treatment. In addition, Sun et al. (2006) reported that leaves of Solanum nigrum can accumulate 167.8 µg Cd g−1 dry weight, when the soil was amended with 100 µg Cd g−1 dry weight soil. Malic acid forms chelation with Cd and transports it as carrier into the leaf vacuoles of Solanum nigrum where it is stored as a stable compound. Furthermore, a positive correlation between total Cd accumulated with water-soluble organic acids such as acetic and citric acid concentrations in leaves indicates their role related to Cd tolerance and hyperaccumulation in Solanum nigrum plants. Similarly, studies conducted by Sun et al. (2013) demonstrated the Cd concentration at 25 µg g−1 dry weight induced a significant increase in Cd accumulation of up to 158.2 µg g−1 dry weight in leaves of Rorippa globosa plants. The authors opined that elevated Cd regimes in growth medium significantly enhance the concentrations of water-soluble tartaric acid and malic acids along with non-protein thiols in leaves and showed a correlation between the tolerance and hyperaccumulation potential of Rorippa globosa towards Cd excess. In hydroponic experiments, Fernándezf et al. (2014) studied the interaction between organic acids exuded from roots and Cd using Dittrichia viscosa and elucidated the retention of Cd in the cell wall of root cells along with secretion of citric acid and non-protein thiols as the tolerance strategy adopted by plants in response to Cd toxicity.
Based upon the tolerance strategies exhibited by plants to combat metal toxicity, some studies have investigated the concentration of organic acids accumulated in plant tissues also depends upon the type of metal. For instance, the level of Cu and Cd in the mature and old leaf section of maize increased when the maize seedlings grown in nutrient medium treated with Cd and Cu (50, 100 µM) (Dresler et al. 2014). Interestingly, the authors found that Cu (100 µM) increased the concentration of succinate and tartrate in mature leaf sections, while Cd (100 µM) enhanced the citrate content. Additionally, excess Cd induced high concentration of tartrate and malate in roots, while Cu (100 µM) peaked the citrate level.
Organic acid–Cd complexes in rhizosphere are relatively less noxious and are more soluble in the soil than free Cd ions. Cd detoxification by the exuded organic acids from plants has been meticulously studied by Adeniji et al. (2010) who pointed exudation of high concentration of fumaric, malic, oxalic and succinic acid in roots of low Cd-accumulating isoline of Triticum turgidum (W9260-BC). A direct linkage between differential Cd acclimatisation in tissues of Triticum turgidum with Cd partitioning and confinement in roots of low Cd-accumulating isolines was observed. The enhanced Cd confinement in roots of low Cd-accumulating isolines is related to the formation of strong low-molecular-weight organic acid–Cd complexes (Adeniji et al. 2010). Furthermore, Cd chelation with OAs prevents its translocation to aerial parts of low Cd-accumulating isolines of Triticum turgidum (Adeniji et al. 2010). In some previous experiments, peaked Cd regimes were positively correlated with high efflux of organic acids by the roots of Setaria italica (Chiang et al. 2011) and Cd-tolerant cultivars of hot pepper (Xin et al. 2015), which strongly pointed the detoxification of Cd through organic acid complexation of Cd ions. Under different treatments of Cd, Ni and Cu, marsh plant Phragmites australis was capable of exuding oxalic, citric and malic acid, while Halimione portulacoides exuded oxalic and malic acids from the roots (Rocha et al. 2016). Cd and Ni accumulation and translocation in a halophyte Sesuvium portulacastrum were directly correlated with the significant liberation of citric acid in xylem sap which by forming metal chelation contributes to metal tolerance in the plant species (Mnasri et al. 2015). The formation of organic acid–Cd complexes in the rhizosphere effectively enables the plants to tolerate and combat Cd toxicity.
Studies have also demonstrated the role of organic acids in promoting hyperaccumulation potential of plants towards Cd. In the literature, studies have assessed the interactions between the organic acids secreted as secondary metabolites in the rhizosphere and hyperaccumulator plants (Table 3). Wang et al. (2015) revealed the enhanced secretion of citrate and oxalate with the increased Cd regimes from the roots of Beijingxin3 cultivar of Chinese cabbage. The high acclimatisation of organic acids, especially oxalate, induces an effective internal tolerance mechanism in Beijingxin3 cultivar. In a similar study, the roots of Cd-tolerant ecotype of Sedum alfredii exuded about twofold increase in oxalate compared to Cd non-tolerant ecotype (Tao et al. 2016). Javed et al. (2017) illustrated that Cd-tolerant maize cultivar (cv. 3062) exhibited a dramatic increase in the exudation of citric acid, glutamic acid, acetic acid, succinic acid and oxalic acid along with the increased Cd concentration in the plant roots. Furthermore, exuded organic acids as phytochemicals assist in improving the nutrient uptake and up-regulating antioxidant enzyme response (SOD and POD), hence enabling Cd-tolerant maize cultivar (cv. 3062) to withstand and acclimatise in Cd-contaminated soil.
Phenolics
Phenolics are ubiquitous and an important class of secondary metabolites that are required for lignin and pigment synthesis in plants (Elguera et al. 2013). Phenolics are a group of aromatic compounds categorised on the basis of benzene rings and hydroxyl groups (Bhattacharya et al. 2010). Phenolics are involved in plant defence mechanism, impart structural integrity and support plants (Bhattacharya et al. 2010). Phenolics stimulate antioxidant and antitumor properties in plants that elevate their nutritional quality (Elguera et al. 2013). They are prospective ligands for metal ions with more affinity for hydroxyl groups (Llugany et al. 2013). Phenolics have high tendency to chelate metals under heavy metal stress and due to their strong antioxidant activity, and phenolics are reported to be involved in conferring metal tolerance in plants (Singh et al. 2016). The hydroxyl and carbonyl groups present in phenolics bind the metal ions and protect the plants from metal toxicity. Increased production of phenolics in response to metal stress might be correlated with increased synthesis of enzymes involved in phenolic biosynthesis (Singh et al. 2016).
Phenolics in soil are broken down by microorganisms into elements that cause mineralisation of soil. Exudation of phenolics by plants serves as a protective mechanism against metal distress by hindering the toxic effects of the metal at cellular levels such as membrane disruption and oxidative damage which ultimately affect the electron transport or even cause cell death (Okem et al. 2015). The exudation and accumulation of phenolics in plant tissues are efficacious responses to the stress induced by heavy metals, either by combating oxidative distress or by forming chelation with heavy metals. The antioxidative response of phenolics depends upon chemical structure, position and number of functional groups that consequently portray their electron donating activity and radical stability (Cheng et al. 2002). Exudation of phenolics is an important mechanism in Cd acquisition (Kováčik and Bačkor 2007). The experiments conducted by Guo et al. (2016) revealed the strong evidence associated with phenolic production that is directly related to enhanced Cd mobilisation, uptake, tolerance and translocation in plants.
In the previous years, studies have reported the role of phenolics to combat oxidative damage with the specific aim to promote plant tolerance and detoxification against Cd toxicity (Table 4). Manquián-Cerda et al. (2016) investigated the effect of Cd (50 and 100 µM) on phenolics exudation, oxidative status and antioxidative response in Vaccinium corymbosum plantlets. With the increased Cd levels in nutrient medium, positive results were obtained for the exudation of phenolics, especially chlorogenic acid, in the roots and leaves of Vaccinium corymbosum, enabling the plantlets to cope oxidative distress caused by Cd excess by triggering strong antioxidative response (Manquián-Cerda et al. 2016). In this context, the authors correlate the increased phenolic exudation and accumulation with the activation of phenylalanine ammonia lyase (PAL) enzyme, the major biosynthetic pathway of phenolics. Similarly, Manquián-Cerda et al. (2018) conducted the experiments to elucidate the interactive effects of Al and Cd (100 µM Al; 100 µM Al + 50 µM Cd and 100 µM Al + 100 µM Cd) upon the exudation of phenolics and their tolerance response to combat metal-induced oxidative distress in Vaccinium corymbosum plantlets during 7, 14, 21 and 30 days. The exudation of three phenolic compounds (chlorogenic, ellagic and gallic acid) in Vaccinium corymbosum plantlets exposed to combined supplementation of Al and Cd enhanced over the time and were 2–3 times higher in comparison with Al-alone treatment (Manquián-Cerda et al. 2018). Additionally, the study explicated the exudation of phenolics in plantlets was assessed by the presence of Cd not by Al, and the antioxidant response as a defence strategy in Vaccinium corymbosum plantlets was consequently determined by the concentration of phenolics, type of metal and the exposure time (Manquián-Cerda et al. 2018). Earlier, Okem et al. (2015) assessed the effects of Al and Cd alone/combined on the accumulation of secondary metabolites (phenolics, flavonoids) and their effect on antioxidant machinery of Hypoxis hemerocallidea. The findings of the work illustrated an increase in the concentration of phenolics at moderate Cd treatment (5 mg l−1), elucidating the tolerance strategy of Hypoxis hemerocallidea against Cd stress. Conversely, high-Cd exposure (10 mg l−1) exhibited toxic effects on the plants by reducing the synthesis of phenolics and also hampered the tolerance mechanisms. Therefore, the exudation of phenolics in plants could induce effective tolerance mechanisms in plants to counteract enhanced Cd concentrations (Okem et al. 2015). Likewise, a comparative study related to the effect of Cd and Cu on the accumulation of phenolics in Marticaria chamomilla plants exposed to 60 and 120 µM for 7 days was studied by Kováčik et al. (2009). The findings revealed a positive correlation of increased Cd concentration in plant tissues with enhanced accumulation of cinnamate derivatives as well as a strong antioxidative response against Cd stress (Kováčik et al. 2009).
Along with tolerance, concentration of phenolic compounds inside the plant tissues also increases the phytoremediation potential of plants for Cd-contaminated soils. The formation of relatively less toxic and highly mobile phenolics–Cd complex is extracted by the plant roots and can be translocated to the aerial parts with quite ease in the hyperaccumulators. Ali and Hadi (2015) conducted pot experiments to assess the effects of gibberellic acid and EDTA on phytoextraction potential of Parthenium hysterophorus grown under 100 mg kg−1 Cd dose in soil. Gibberellic acid-treated plants showed high concentration of endogenous phenolic compounds which has been reported to improve the phytoextraction potential of Parthenium hysterophorus for Cd-contaminated soils which was attributed to the strong antioxidant activity of phenolics under Cd-stressed conditions (Ali and Hadi 2015). Similarly, Ahmad et al. (2015) conducted the experiments to assess the comparative effect of NPK fertilizers, plant growth regulators and sodium chloride on Cd phytoaccumulation in Cannabis sativa plants grown under 100 mg kg−1 Cd-contaminated soil. The study demonstrated a positive correlation with the increased concentration of total phenolics and phytoaccumulation of Cd in tissue parts of Cannabis sativa plants grown under the combined treatment of NPK fertilizers and Cd. The accumulation of phenolics in plant tissues induces effective antioxidative response that enables the plants to cope and withstand stress caused by Cd excess. Earlier, Uraguchi et al. (2006) reported high concentration of phenolics in leaves of Crotalaria juncea that imparted high-Cd accumulation and tolerance potential to the plant.
Silicon (Si) has also been reported to regulate phenolic exudation from roots of the plants grown under Cd stress. In this context, Guo et al. (2016) appraised the potential of Si to elevate phenolic exudation in two cypress varieties and to avoid Cd toxicity in them. Using rhizobag technique, pot experiments for 220 days using Juniperus chinensis and Platycladus orientalis under 100 mg kg−1 Cd and 40 mg kg−1 Si treatments evidenced a considerable increase in phenolics secretions from the roots of two cypress varieties in the rhizosphere. The most important phenolic compound exuded was cinnamic acid, a crucial indicator of lignin degradation (He et al. 2011) that might be derived during root rejuvenation from the highly lignified epidermal tissues in Cd-treated woody roots. In another similar work, Huang et al. (2016) conducted a pot experiments on Thujopsis dolabrata for 210 days to study the interaction between Si and root exuded phenolics on Cd bioavailability, tolerance and detoxification. The authors observed that combination of Cd (100 mg kg−1) and Si (40 mg kg−1) treatments showed a positive correlation with increased phenolics exudation from the roots in the rhizosphere. The results showed that Si-induced efflux of phenolics from roots is species specific. Moreover, in this study, the stability constant for the formation of Cd complex with phenolic compounds was very high compared to the low-molecular-weight organic acids and enhanced Cd mobility and consequently its uptake and tolerance in Thujopsis dolabrata plants (Huang et al. 2016). The limited literature in this field has revealed the effective participation of exuded phenolic compounds in alleviating Cd distress in plants grown under Cd excess.
Amino acids
Amino acids are organic compounds that provide structural stability to large macromolecules and maintain vital functions in plants. They are reported as potential ligands for binding of metal ions in plants (Pohlmeier 2004). Upon metal exposure, plants synthesise a number of metabolites, especially amino acids such as proline and histidine, peptides such as glutathione and phytochelatins (Sharma and Dietz 2006). Many researchers in their studies have reported exudation of amino acids in response to Cd stress as a tolerance strategy adapted by plants to detoxicate Cd stress (Table 5). Xie et al. (2013) compiled a wide list of amino acids (15) exuded by Kandelia obovata roots under varied Cd treatments (2.5, 5, 10, 20 and 40 mg l−1) which includes methionine, tyrosine, cysteine, isoleucine and arginine as dominants, constituting ~ 85% of the total amount after both 24 h and 7 days time, respectively, while threonine, serine and glutamic acid were exuded only after treatment with 10, 20 and 40 mg l−1 Cd, respectively, for 24 h. The other alkaline amino acids (lysine, arginine and histidine) and neutral amino acids such as alanine, valine, leucine, isoleucine and methionine accounted ~ 24% and ~ 91% of the total amino acids exuded by roots of Kandelia obovata plants. In another study, two culturing mode experiments (water-culture and sand-culture medium) on Triticum aestivum conducted by Zhang and Wang (2002) assessed the physiological responses of root exudates to varied Cd regimes (0.5, 5, 15, 50 mg l−1). The findings explicated that the root exudation of amino acids was increased at lower Cd regime, while the inhibitory effects were noticed at higher Cd regimes. Amino acid exudation under Cd stress is an adaptive mechanism in plants to confront Cd toxicity.
Proline, a proteinogenic amino acid, acts as an osmolyte, stabilises large molecules and is a component of plant cell wall (Sharma and Dietz 2006). Proline combats heavy metal stress by detoxifying free radicals through the formation of stable complex with them and hence maintains the normal ratio of NAD(P)+/NAD(P)H (Kováčik et al. 2010). Increased concentration of amino acids along with proline is often encountered in plants under Cd stress and hence contributes to ameliorate the catastrophic effects of Cd toxicity (Konotop et al. 2012). Costa and Morel (1994) reported thirteen fold increase in root proline content in Lactuca sativa upon exposure to 100 μΜ Cd dose. The higher proline production in response to Cd stress might be correlated with increased expression of gene P5CS (∆1-pyrroline-5-carboxylate synthetase) responsible for stimulating the first step in proline synthesis (Siripornadulsil et al. 2002). Amino acids exuded from roots of Cd hyperaccumulators actively participate towards its mobility, uptake and tolerance and thereby efficaciously curb phytotoxicity against Cd excess. Luo et al. (2014) performed the hydroponic experiments to assess the variety of root exudates secreted from Cd hyperaccumulator Sedum alfredii under 5, 10, 40 and 400 µM Cd levels. The compounds exuded from the roots include six organic acids (oxalic acid, threonic acid, phosphoric acid, oleic acid, tetradecanoic acid and 3-hydroxybutanoic acid), one amino acid (glycine), two sugars (trehalose and fructose), six alcohol compounds (cholesterol, ribitol, erythritol, mannitol, d-pinitol and octacosanol) and five other compounds. The exudation of amino acid glycine enhanced at low Cd levels but later on declined with the increased Cd levels. Moreover, Cd mobilisation, uptake, accumulation as well as tolerance of Sedum alfredii towards low Cd levels were increased (Luo et al. 2014). Glycine secretion at high Cd levels was decreased which in turn reduced Cd uptake and assisted Sedum alfredii plants to mitigate Cd toxicity.
According to Fu et al. (2019), Cd phytotoxicity promoted the exudation of histidine, methionine, phenylalanine and lysine from roots in high Cd-accumulating rice line (Lu527-8) and normal rice line (Lu527-4), respectively. Histidine was reported to be the major constituent accounting for ~ 50–70% of the total concentration of exuded amino acids. The authors observed a significant positive correlation between high-histidine content with increased Cd levels in both the rice lines, respectively. Likewise, the authors also observed increased methionine and phenylalanine content in Lu527-8 which actively participated in plant defence mechanisms under Cd toxicity. Nevertheless, peroxides generated under Cd distress have been reported to be scavenged by a metabolite of methionine metabolic pathway (She et al. 2015), or by the generation of lignin and isoflavone in the phenylalanine metabolism pathway (Maeda et al. 2011), elucidating the role of methionine and phenylalanine to combat Cd distress. Though, lysine content in xylem sap of both the rice lines reduced dramatically with the elevated Cd levels, but interestingly Lu527-8 always maintained high Cd concentration and exhibited high tolerance in comparison with Lu527-4. Additionally, the elevated histidine content with increased Cd accumulation in both the rice lines indicates histidine secretion as a contributing factor in Cd uptake and its long-distance transport in plant tissues (Fu et al. 2019). Moreover, exogenous application of histidine elevated Cd content in both Solanum nigrum and Solanum torvum plants, thus supporting positive role of histidine in Cd uptake and tolerance (Xu et al. 2012).
Conclusion
Cd-contaminated soil ecosystems pose serious threats on the morphological and physiological attributes of the plants. The exudation of secondary metabolites from roots is an effective mechanism adopted by plants to react and modify their environment. Cd extraction and acclimatisation in plants depends upon the potential of a plant species and its rhizospheric environment that enables to restrict Cd entry inside the roots through immobilisation or promotes Cd chelation with ionic species in rhizosphere that influence its solubilisation and mobilisation. A better understanding of root exudation pattern of plants under Cd stress can provide an effective approach to optimise various techniques in reducing Cd acquisition and promote tolerance in plants and/or phytoremediation of Cd-contaminated soils that in turn prove beneficial for agriculture and environment development. However, the knowledge about the root exudates and their functional aspects to ameliorate Cd tolerance in plants has not been completely developed yet. Some questions remain unanswered due to lack of research on the use of exuded phytochemicals in Cd tolerance and its phytoextraction studies along with various regulatory mechanisms that lead to their secretion in the rhizosphere. Therefore, future research should be focused on recognising different genes or pathways that get modulated in the plants to secrete more secondary metabolites which aid them to confront Cd toxicity when grown under Cd-contaminated soils. Besides, screening and identification of more crucial secondary metabolites having the potency to confer plant tolerance towards Cd excess should be emphasised. This would definitely assist in devicing novel strategies for bettering plant performance and increasing their tolerance potential against Cd excess. The information communicated in this review could illuminate the scientific community and flicker interest in future research on the employment of diverse root exudates in combating plant tolerance towards Cd and other heavy metal stress.
Abbreviations
- ABC transporters:
-
ATP-binding cassette transporters
- ALMT:
-
Aluminium-activated malate transporters
- ATSDR:
-
Agency for Toxic Substances and Disease Registry
- AMF:
-
Arbuscular mycorrhizal fungi
- CdO:
-
Cadmium oxide
- DMA:
-
2′-Deoxymugineic acid
- DNA:
-
Deoxyribonucleic acid
- LCT1:
-
Low-affinity cation transporter 1
- LMWOAs:
-
Low-molecular-weight organic acids
- MATE:
-
Maximised autotransporter-mediated expression
- NA:
-
Nicotianamine
- NRAMPs:
-
Natural resistance-associated macrophage proteins
- OsHMA3:
-
Oryza sativa heavy metal P-type ATPase 3
- PGPB:
-
Plant growth-promoting bacteria
- P5CS:
-
∆1-Pyrroline-5-carboxylate synthetase
- QUAC:
-
Quick anionic channels
- ROS:
-
Reactive oxygen species
- RuBisCO:
-
Ribulose-1, 5-bisphosphate carboxylase oxygenase
- SLAC:
-
Slow anionic channels
- SRB-1:
-
Sulphate-reducing bacteria
- ZIP:
-
Zinc/iron permease
References
Abbas T, Rizwan M, Ali S, Adrees M, Zia-ur-Rehman M, Qayyum MF, Ok YS, Murtaza G (2018) Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ Sci Pollut Res 25:25668–25680. https://doi.org/10.1007/s11356-017-8987-4
Abdu N, Abdullahi AA, Abdulkadir A (2017) Heavy metals and soil microbes. Environ Chem Lett 15:65–84. https://doi.org/10.1007/s10311-016-0587-x
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles and fate of organic acids in soils: a review. S Afr J Bot 108:393–406. https://doi.org/10.1016/j.sajb.2016.09.002
Adeniji BA, Budimir-Hussey MT, Macfie SM (2010) Production of organic acids and adsorption of Cd on roots of durum wheat (Triticum turgidum L. var. durum). Acta Physiol Plant 32:1063–1072. https://doi.org/10.1007/s11738-010-0498-6
Agency for Toxic Substances and Disease Registry (2017) CERCLA priority list of hazardous substances. http://www.atsdr.cdc.gov/spl/index.html
Ahmad A, Hadi F, Ali N (2015) Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L.) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int J Phytoremediation 17:56–65. https://doi.org/10.1080/15226514.2013.828018
Ahsan MT, Tahseen R, Ashraf A, Mahmood A, Arslan M, Afzal M (2019) Effective plant-endophyte interplay can improve the cadmium hyperaccumulation in Brachiaria mutica. World J Microbiol Biotechnol 35:188. https://doi.org/10.1007/s11274-019-2757-z
Ali N, Hadi F (2015) Phytoremediation of cadmium improved with the high production of endogenous phenolics and free proline contents in Parthenium hysterophorus plant treated exogenously with plant growth regulator and chelating agent. Environ Sci Pollut Res 22:13305–13318. https://doi.org/10.1007/s11356-015-4595-3
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252:399–413. https://doi.org/10.1007/s00709-014-0710-4
Astolfi S, Ortolani MR, Catarcione G, Paolacci AR, Cesco S, Pinton R, Ciaffi M (2014) Cadmium exposure affects iron acquisition in barley (Hordeum vulgare) seedlings. Physiol Plant 152:646–659. https://doi.org/10.1111/ppl.12207
Awad F, Römheld V (2000) Mobilization of heavy metals from contaminated calcareous soils by plant born, microbial and synthetic chelates and their uptake by wheat plants. J Plant Nutr 23:1847–1855. https://doi.org/10.1080/01904160009382147
Awasthi MK, Wang Q, Chen H, Liu T, Awasthi SK, Duan Y, Varjani S, Pandey A, Zhang Z (2019) Role of compost biochar amendment on the (im) mobilization of cadmium and zinc for Chinese cabbage (Brassica rapa L.) from contaminated soil. J Soil Sediment 19:3883–3897. https://doi.org/10.1007/s11368-019-02277-8
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant, Cell Environ 32:666–681. https://doi.org/10.1111/j.1365-3040.2009.01926.x
Badri DV, Loyola-Vargas VM, Broeckling CD, De-la-Peña C, Jasinski M, Santelia D, Martinoia W, Summer LW, Banta LM, Stermitz F, Vivanco JM (2008) Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol 146:762–771. https://doi.org/10.1104/pp.107.109587
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98. https://doi.org/10.1016/j.tplants.2013.11.006
Baghaie AH, Aghili F, Jafarinia R (2019) Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ Sci Pollut Res 26:30794–30807. https://doi.org/10.1007/s11356-019-06237-0
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Banakar R, Alvarez Fernandez A, Díaz-Benito P, Abadia J, Capell T, Christou P (2017) Phytosiderophores determine thresholds for iron and zinc accumulation in biofortified rice endosperm while inhibiting the accumulation of cadmium. J Exp Bot 68:4983–4995. https://doi.org/10.1093/jxb/erx304
Bansal P, Sharma P, Goyal V (2002) Impact of lead and cadmium on enzyme of citric acid cycle in germinating pea seeds. Biol Plant 45:125–127. https://doi.org/10.1023/A:1015173112842
Bao T, Sun T, Sun L (2011) Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L. and nonhyperaccumulator Solanum lycopersicum L. Afr J Biotechnol 10:17180–17185. https://doi.org/10.5897/AJB11.1617
Bauddh K, Singh RP (2015) Effects of organic and inorganic amendments on bio-accumulation and partitioning of Cd in Brassica juncea and Ricinus communis. Ecol Eng 74:93–100. https://doi.org/10.1016/j.ecoleng.2014.10.022
Belimov AA, Zinovkina NY, Safronova VI, Litvinsky VA, Nosikov VV, Zavalin AA, Tikhonovich IA (2019) Rhizobial ACC deaminase contributes to efficient symbiosis with pea (Pisum sativum L.) under single and combined cadmium and water deficit stress. Environ Exp Bot 167:103859. https://doi.org/10.1016/j.envexpbot.2019.103859
Berbar Y, Hammache ZE, Bensaadi S, Soukeur R, Amara M, Van der Bruggen B (2019) Effect of functionalized silica nanoparticles on sulfonated polyethersulfone ion exchange membrane for removal of lead and cadmium ions from aqueous solutions. J Water Process Eng 32:100953. https://doi.org/10.1016/j.jwpe.2019.100953
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol Plant Pathol 11:705–719. https://doi.org/10.1111/j.1364-3703.2010.00625.x
Boominathan R, Doran PM (2003) Cadmium tolerance and antioxidative defenses in hairy roots of the cadmium hyperaccumulator, Thlaspi caerulescens. Biotechnol Bioeng 83:158–167. https://doi.org/10.1002/bit.10656
Chaffai R, Tekitek A, El Ferjani E (2006) A comparative study on the organic acid content and exudation in maize (Zea mays L.) seedlings under conditions of copper and cadmium stress. Asian J Plant Sci 5:598–606. https://doi.org/10.3923/ajps.2006.598.606
Chen YX, Lin Q, Luo YM, He YF, Zhen SJ, Yu YL, Tian GM, Wong MH (2003) The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 50:807–811. https://doi.org/10.1016/S0045-6535(02)00223-0
Chen B, Zhang Y, Rafiq MT, Khan KY, Pan F, Yang X, Feng Y (2014) Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: effects on plant growth and root exudates. Chemosphere 117:367–373. https://doi.org/10.1016/j.chemosphere.2014.07.078
Chen H, Teng Y, Lu S, Wang Y, Wang J (2015) Contamination features and health risk of soil heavy metals in China. Sci Total Environ 512:143–153. https://doi.org/10.1016/j.scitotenv.2015.01.025
Chen J, Shafi M, Wang Y, Wu J, Ye Z, Liu C, Zhong B, Guo H, He L, Liu D (2016) Organic acid compounds in root exudation of Moso Bamboo (Phyllostachys pubescens) and its bioactivity as affected by heavy metals. Environ Sci Pollut Res 23:20977–20984. https://doi.org/10.1007/s11356-016-7323-8
Chen YT, Wang Y, Yeh KC (2017) Role of root exudates in metal acquisition and tolerance. Curr Opin Plant Biol 39:66–72. https://doi.org/10.1016/j.pbi.2017.06.004
Chen H, Lei J, Tong H, Gu M, Fang Y, Wang X, Tang C, Li Z, Liu C (2019) Effects of Mn(II) on the oxidation of fe in soils and the uptake of cadmium by rice (Oryza sativa). Water Air Soil Pollut 230:190. https://doi.org/10.1007/s11270-019-4237-3
Chen L, Li F, Wei Y, Li G, Shen K, He HJ (2019) High cadmium adsorption on nanoscale zero-valent iron coated Eichhornia crassipes biochar. Environ Chem Lett 17:589–594. https://doi.org/10.1007/s10311-018-0811-y
Cheng Z, Ren J, Li Y, Chang W, Chen Z (2002) Study on the multiple mechanisms underlying the reaction between hydroxyl radical and phenolic compounds by qualitative structure and activity relationship. Bioorg Med Chem 10:4067–4073. https://doi.org/10.1016/S0968-0896(02)00267-5
Chiang PN, Wang MK, Chiu CY, Chou SY (2006) Effects of cadmium amendments on low-molecular-weight organic acid exudates in rhizosphere soils of tobacco and sunflower. Environ Toxicol 21:479–488. https://doi.org/10.1002/tox.20210
Chiang PN, Chiu CY, Wang MK, Chen BT (2011) Low-molecular-weight organic acids exuded by Millet (Setaria italica (L.) Beauv.) roots and their effect on the remediation of cadmium-contaminated soil. Soil Sci 176:33–38. https://doi.org/10.1097/SS.0b013e318202fdc9
Chuaphasuk C, Prapagdee B (2019) Effects of biochar-immobilized bacteria on phytoremediation of cadmium-polluted soil. Environ Sci Pollut Res 26:23679–23688. https://doi.org/10.1007/s11356-019-05661-6
Costa G, Morel JL (1994) Water relations, gas exchange and amino acid content in Cd-treated lettuce. Plant Physiol Biochem 32:561–570
Costa G, Michaut JC, Guckert A (1997) Amino acids exuded from axenic roots of lettuce and white lupin seedlings exposed to different cadmium concentrations. J Plant Nutr 20:883–900. https://doi.org/10.1080/01904169709365303
Costa ET, Guilherme LRG, de Melo ÉEC, Ribeiro BT, Euzelina dos Santos BI, da Costa Severiano EC, Faquin V, Hale BA (2012) Assessing the tolerance of castor bean to Cd and Pb for phytoremediation purposes. Biol Trace Elem Res 145:93–100. https://doi.org/10.1007/s12011-011-9164-0
Deng Y, Liu XD, Liu HW, Jiang HD, Xu LF, Xiao YH, Yin HQ, Liang YL (2017) Bioleaching of cadmium from contaminated paddy fields by consortium of autotrophic and indigenous cadmium-tolerant bacteria. Solid State Phenom 262:617–621. https://doi.org/10.4028/www.scientific.net/SSP.262.617
Dharupaneedi SP, Nataraj SK, Nadagouda M, Reddy KR, Shukla SS, Aminabhavi TM (2019) Membrane-based separation of potential emerging pollutants. Sep Purif Technol 210:850–866. https://doi.org/10.1016/j.seppur.2018.09.003
Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E (2009) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696. https://doi.org/10.1111/j.1365-2672.2009.04355.x
Dresler S, Hanaka A, Bednarek W, Maksymiec W (2014) Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol Plant 36:1565–1575. https://doi.org/10.1007/s11738-014-1532-x
Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3:263. https://doi.org/10.3389/fpls.2012.00263
Dubey S, Shri M, Gupta A, Rani V, Chakrabarty D (2018) Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ Chem Lett 16:1169–1192. https://doi.org/10.1007/s10311-018-0741-8
Elguera JCT, Barrientos EY, Wrobel K, Wrobel K (2013) Effect of cadmium (Cd(II)), selenium (Se(IV)) and their mixtures on phenolic compounds and antioxidant capacity in Lepidium sativum. Acta Physiol Plant 35:431–441. https://doi.org/10.1007/s11738-012-1086-8
Fernándezf R, Fernández-Fuego D, Bertrand A, González A (2014) Strategies for Cd accumulation in Dittrichia viscosa (L.) Greuter: role of the cell wall, non-protein thiols and organic acids. Plant Physiol Biochem 78:63–70. https://doi.org/10.1016/j.plaphy.2014.02.021
Fu H, Yu H, Li T, Zhang X (2018) Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol Environ Saf 150:168–175. https://doi.org/10.1016/j.ecoenv.2017.12.014
Fu H, Yu H, Li T, Wu Y (2019) Effect of cadmium stress on inorganic and organic components in xylem sap of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol Environ Saf 168:330–337. https://doi.org/10.1016/j.ecoenv.2018.10.023
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. https://doi.org/10.1016/j.envexpbot.2012.04.006
Gong X, Huang D, Liu Y, Zeng G, Chen S, Wang R, Xue W (2019) Biochar facilitated the phytoremediation of cadmium contaminated sediments: metal behavior, plant toxicity, and microbial activity. Sci Total Environ 666:1126–1133. https://doi.org/10.1016/j.scitotenv.2019.02.215
Gothberg A, Greger M, Holm K, Bengtsson BE (2004) Influence of nutrient levels on uptake and effects of mercury, cadmium, and lead in water spinach. J Environ Qual 33:1247–1255. https://doi.org/10.2134/jeq2004.1247
Guo J, Chi J (2014) Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 375:205–214. https://doi.org/10.1007/s11104-013-1952-1
Guo B, Liu C, Ding N, Fu Q, Lin Y, Li H, Li N (2016) Silicon alleviates cadmium toxicity in two cypress varieties by strengthening the exodermis tissues and stimulating phenolic exudation of roots. J Plant Growth Regul 35:420–429. https://doi.org/10.1007/s00344-015-9549-y
Guohua NI, Peng ZH, Yiman JI, Yuedong M (2012) Vitrification of MSWI fly ash by thermal plasma melting and fate of heavy metals. Plasma Sci Technol 14:813. https://doi.org/10.1088/1009-0630/14/9/08
Gusiatin ZM, Klimiuk E (2012) Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere 86:383–391. https://doi.org/10.1016/j.chemosphere.2011.10.027
Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U (2008) Evolution of metal hyperaccumulation required cis regulatory changes and triplication of HMA4. Nature 453:391–395. https://doi.org/10.1038/nature06877
Hashem A, Abd-Allah EF, Alqarawi AA, Malik JA, Wirth S, Egamberdieva D (2019) Role of calcium in AMF-mediated alleviation of the adverse impacts of cadmium stress in Bassia indica [Wight] AJ Scott. Saudi J Biol Sci 26:828–838. https://doi.org/10.1016/j.sjbs.2016.11.003
Hazama K, Nagata S, Fujimori T, Yanagisawa S, Yoneyama T (2015) Concentrations of metals and potential metal-binding compounds and speciation of Cd, Zn and Cu in phloem and xylem saps from castor bean plants (Ricinus communis) treated with four levels of cadmium. Physiol Plant 154:243–255. https://doi.org/10.1111/ppl.12309
He JL, Qin JJ, Long LY, Ma YL, Li H, Li K, Jiang XN, Liu TX, Polle A, Liang ZS (2011) Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus canescens. Physiol Plant 143:50–63. https://doi.org/10.1111/j.1399-3054.2011.01487.x
He S, He Z, Yang X, Stoffella PJ, Baligar VC (2015) Soil biogeochemistry, plant physiology, and phytoremediation of cadmium-contaminated soils. Adv Agron 134:135–225
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW (2005) Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol 168:293–303. https://doi.org/10.1111/j.1469-8137.2005.01512.x
Hong K, Tokunaga S, Kajiuchi T (2002) Evaluation of remediation process with plant derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 49:379–387. https://doi.org/10.1016/S0045-6535(02)00321-1
Huang YC, Chen H, Zhao WJ, Li W, Yang NH, Sun Y, Wang L, Cao SH (2016) Silicon-mediated alleviation of Cadmium toxicity on Thujopsis dolabrata. Phyton Int J Exp Bot 85:283–290
Huang X, Wang L, Zhu S, Ho SH, Wu J, Kalita PK, Ma F (2018) Unraveling the effects of arbuscular mycorrhizal fungus on uptake, translocation, and distribution of cadmium in Phragmites australis (Cav.) Trin. ex Steud. Ecotoxicol Environ Saf 149:43–50. https://doi.org/10.1016/j.ecoenv.2017.11.011
Irtelli B, Navari-Izzo F (2006) Influence of sodium nitrilotriacetate (NTA) and citric acid on phenolic and organic acids in Brassica juncea grown in excess of cadmium. Chemosphere 65:1348–1354. https://doi.org/10.1016/j.chemosphere.2006.04.014
Itusha A, Osborne WJ, Vaithilingam M (2019) Enhanced uptake of Cd by biofilm forming Cd resistant plant growth promoting bacteria bioaugmented to the rhizosphere of Vetiveria zizanioides. Int J Phytoremediation 21:487–495. https://doi.org/10.1080/15226514.2018.1537245
Jan R, Khan MA, Asaf S, Lee IJ, Kim KM (2019) Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress related genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 8:363. https://doi.org/10.3390/plants8100363
Javed MT, Akram MS, Tanwir K, Chaudhary HJ, Ali Q, Stoltz E, Lindberg S (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf 141:216–225. https://doi.org/10.1016/j.ecoenv.2017.03.027
Johansson EM, Fransson PM, Finlay RD, van Hees PA (2008) Quantitative analysis of root and ectomycorrhizal exudates as a response to Pb, Cd and As stress. Plant Soil 313:39–54. https://doi.org/10.1007/s11104-008-9678-1
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44. https://doi.org/10.1023/A:1004356007312
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175. https://doi.org/10.3390/ijms13033145
Kabata-Pendias A (1993) Behavioural properties of trace metals in soils. Appl Geochem 8:3–9. https://doi.org/10.1016/S0883-2927(09)80002-4
Kabata-Pendias A, Sadurski W (2004) Trace elements and compounds in soil. In: Merian E, Anke M, Ihnat M, Stoeppler M (eds) Elements and their compounds in the environment: Occurrence, analysis and biological relevance. Wiley, Hoboken, pp 79–99
Kang J, Park J, Choi H, Burla B, Kretzschmar T, Lee Y, Martinoia E (2011) Plant ABC transporters. Am Soc Plant Biol 9:e0153. https://doi.org/10.1199/tab.0153
Kaplan O, Ince M, Yaman M (2011) Sequential extraction of cadmium in different soil phases and plant parts from a former industrialized area. Environ Chem Lett 9:397–404. https://doi.org/10.1007/s10311-010-0292-0
Kapoor D, Kaur S, Bhardwaj R (2014) Physiological and biochemical changes in Brassica juncea plants under Cd-induced stress. Biomed Res Int 2014:1–13. https://doi.org/10.1155/2014/726070
Katoh M, Moriguchi S, Takagi N, Akashi Y, Sato T (2018) Simultaneous control of cadmium release and acidic pH neutralization in excavated sedimentary rock with concurrent oxidation of pyrite using steel slag. J Soil Sediment 18:1194–1204. https://doi.org/10.1007/s11368-017-1892-0
Kaur R, Yadav P, Sharma A, Thukral AK, Kumar V, Kohli SK, Bhardwaj R (2017) Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol Environ Saf 145:466–475. https://doi.org/10.1016/j.ecoenv.2017.07.067
Kaur R, Yadav P, Thukral AK, Sharma A, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018) Castasterone and citric acid supplementation alleviates cadmium toxicity by modifying antioxidants and organic acids in Brassica juncea. J Plant Growth Regul 37:286–299. https://doi.org/10.1007/s00344-017-9727-1
Keesstra S, Mol G, de Leeuw J, Okx J, de Cleen M, Visser S (2018) Soil-related sustainable development goals: four concepts to make land degradation neutrality and restoration work. Land 7:133. https://doi.org/10.3390/land7040133
Khan S, Munir S, Sajjad M, Li G (2016) Urban park soil contamination by potentially harmful elements and human health risk in Peshawar City, Khyber Pakhtunkhwa, Pakistan. J Geochem Explor 165:102–110. https://doi.org/10.1016/j.gexplo.2016.03.007
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601:1591–1605. https://doi.org/10.1016/j.scitotenv.2017.06.030
Khanna K, Jamwal VL, Gandhi SG, Ohri P, Bhardwaj R (2019) Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-41899-3
Kim SU, Owens VN, Kim SY, Hong CO (2017) Effect of different way of bottom ash and compost application on phytoextractability of cadmium in contaminated arable soil. Appl Biol Chem 60:353–362. https://doi.org/10.1007/s13765-017-0287-7
Kirkham MB (2006) Cadmium in plants on polluted soils: effects of soil factors, hyperaccumulation, and amendments. Geoderma 137:19–32. https://doi.org/10.1016/j.geoderma.2006.08.024
Konotop Y, Mészáros P, Spieß N, Mistríková V, Piršelová B, Libantová J, Moravčíková J, Taran N, Hauptvogel P, Matušíková I (2012) Defense responses of soybean roots during exposure to cadmium, excess of nitrogen supply and combinations of these stressors. Mol Biol Rep 39:10077–10087. https://doi.org/10.1007/s11033-012-1881-8
Kotoky R, Nath S, Maheshwari DK, Pandey P (2019) Cadmium resistant plant growth promoting rhizobacteria Serratia marcescens S2I7 associated with the growth promotion of rice plant. Environ Sustain 2:135–144. https://doi.org/10.1007/s42398-019-00055-3
Kováčik J, Bačkor M (2007) Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Pollut 185:185–193. https://doi.org/10.1007/s11270-007-9441-x
Kováčik J, Klejdus B, Hedbavny J, Štork F, Bačkor M (2009) Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 320:231. https://doi.org/10.1007/s11104-009-9889-0
Kováčik J, Klejdus B, Hedbavny J (2010) Effect of aluminium uptake on physiology, phenols and amino acids in Matricaria chamomilla plants. J Hazard Mater 178:949–955. https://doi.org/10.1016/j.jhazmat.2010.02.029
Kudo K, Kudo H, Kawai S (2007) Cadmium uptake in barley affected by iron concentration of the medium: role of phytosiderophores. Soil Sci Plant Nutr 53:259–266. https://doi.org/10.1111/j.1747-0765.2007.00131.x
Kudo K, Kudo H, Fujikawa YK, Kawai S (2013) The release of copper-induced phytosiderophores in barley plants is decreased by cadmium stress. Botany 91:568–572. https://doi.org/10.1139/cjb-2013-0035
Kumar V, Sharma A, Kaur P, Sidhu GPS, Bali AS, Bhardwaj R, Thukral AK, Cerda A (2019) Pollution assessment of heavy metals in soils of India and ecological risk assessment: a state-of-the-art. Chemosphere 216:449–462. https://doi.org/10.1016/j.chemosphere.2018.10.066
Lapie C, Leglize P, Paris C, Buisson T, Sterckeman T (2019) Profiling of main metabolites in root exudates and mucilage collected from maize submitted to cadmium stress. Environ Sci Pollut Res 26:17520–17534. https://doi.org/10.1007/s11356-019-05168-0
Li WC, Ye ZH, Wong MH (2007) Effects of bacteria on enhanced metal uptake of the Cd/Zn-hyperaccumulating plant, Sedum alfredii. J Exp Bot 58:4173–4182. https://doi.org/10.1093/jxb/erm274
Li X, Yu H, Sun X, Yang J, Wang D, Shen L, Pan Y, Wu Y, Wang Q, Zhao Y (2019) Effects of sulfur application on cadmium bioaccumulation in tobacco and its possible mechanisms of rhizospheric microorganisms. J Hazard Mater 368:308–315. https://doi.org/10.1016/j.jhazmat.2018.12.099
Li ZR, Wang JX, An LZ, Tan JB, Zhan FD, Wu J, Zu YQ (2019) Effect of root exudates of intercropping Vicia faba and Arabis alpina on accumulation and sub-cellular distribution of lead and cadmium. Int J Phytoremediation 21:4–13. https://doi.org/10.1080/15226514.2018.1523867
Liu J, Qian M, Cai G, Zhu Q, Wong MH (2007) Variations between rice cultivars in root secretion of organic acids and the relationship with plant cadmium uptake. Environ Geochem Health 29:189–195. https://doi.org/10.1007/s10653-006-9063-z
Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57:389–399. https://doi.org/10.1111/j.1365-313X.2008.03696.x
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194:495–503. https://doi.org/10.1016/j.chemosphere.2017.12.025
Liu H, Xie Y, Li J, Zeng G, Li H, Xu F, Feng S, Xu H (2020) Effect of Serratia sp. K3 combined with organic materials on cadmium migration in soil-Vetiveria zizanioides L. system and bacterial community in contaminated soil. Chemosphere 242:125164. https://doi.org/10.1016/j.chemosphere.2019.125164
Llugany M, Tolrà R, Martín SR, Poschenrieder C, Barceló J (2013) Cadmium-induced changes in glutathione and phenolics of Thlaspi and Noccaea species differing in Cd accumulation. J Plant Nutr Soil Sci 176:851–858. https://doi.org/10.1002/jpln.201300096
Luo Q, Sun L, Hu X, Zhou R (2014) The variation of root exudates from the hyperaccumulator Sedum alfredii under cadmium stress: metabonomics analysis. PLoS ONE 9:e115581. https://doi.org/10.1371/journal.pone.0115581
Luo Q, Sun LN, Wang H, Hu XM (2015) Metabolic profiling analysis of root exudates from the Cd hyperaccumulator Sedum alfredii under different Cd exposure concentrations and times. Anal Methods 7:3793–3800. https://doi.org/10.1039/C5AY00159E
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavymetal phytoremediation. J Environ Manag 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
Ma Q, Li J, Lee CC, Long X, Liu Y, Wu QT (2019) Combining potassium chloride leaching with vertical electrokinetics to remediate cadmium-contaminated soils. Environ Geochem Health 41:2081–2091. https://doi.org/10.1007/s10653-019-00259-w
Maeda H, Yoo H, Dudareva N (2011) Prephenate aminotransferase directs plant phenylalanine biosynthesis via arogenate. Nat Chem Biol 7:19–21. https://doi.org/10.1038/nchembio.485
Manquián-Cerda K, Escudey M, Zúñiga G, Arancibia-Miranda N, Molina M, Cruces E (2016) Effect of cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets grown in vitro. Ecotoxicol Environ Saf 133:316–326. https://doi.org/10.1016/j.ecoenv.2016.07.029
Manquián-Cerda K, Cruces E, Escudey M, Zúñiga G, Calderon R (2018) Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol Environ Saf 150:320–326. https://doi.org/10.1016/j.ecoenv.2017.12.050
Márquez-García B, Fernández-Recamales M, Córdoba F (2012) Effects of cadmium on phenolic composition and antioxidant activities of Erica andevalensis. J Bot 2012:1–6. https://doi.org/10.1155/2012/936950
Maruyama H, Sasaki T, Yamammoto Y, Wasaki J (2019) AtALMT3 is involved in malate efflux induced by phosphorus deficiency in Arabidopsis thaliana root hairs. Plant Cell Physiol 60:107–115. https://doi.org/10.1093/pcp/pcy190
Meach M, Martin E (1991) Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L., Nicotiana tabacum L. and Nicotiana rustica L. Plant Soil 132:187–196. https://doi.org/10.1007/BF00010399
Meda AR, Scheuermann EB, Prechsl UE, Erenoglu B, Schaaf G, Hayen H, Weber G, von Wirén N (2007) Iron acquisition by phytosiderophores contributes to cadmium tolerance. Plant Physiol 143:1761–1773. https://doi.org/10.1104/pp.106.094474
Meng J, Zhong L, Wang L, Liu X, Tang C, Chen H, Xu J (2018) Contrasting effects of alkaline amendments on the bioavailability and uptake of Cd in rice plants in a Cd-contaminated acid paddy soil. Environ Sci Pollut Res 25:8827–8835. https://doi.org/10.1007/s11356-017-1148-y
Mnasri M, Ghabriche R, Fourati E, Zaier H, Sabally K, Barrington S, Lutts S, Abdelly C, Ghnaya T (2015) Cd and Ni transport and accumulation in the halophyte Sesuvium portulacastrum: implication of organic acids in these processes. Front Plant Sci 6:156. https://doi.org/10.3389/fpls.2015.00156
Mombo S, Foucault Y, Deola F, Gaillard I, Goix S, Shahid M, Schreck E, Pierart A, Dumat C (2016) Management of human health risk in the context of kitchen gardens polluted by lead and cadmium near a lead recycling company. J Soil Sediment 16:1214–1224. https://doi.org/10.1007/s11368-015-1069-7
Moreira H, Marques AP, Franco AR, Rangel AO, Castro PM (2014) Phytomanagement of Cd-contaminated soils using maize (Zea mays L.) assisted by plant growth-promoting rhizobacteria. Environ Sci Pollut Res 21:9742–9753. https://doi.org/10.1007/s11356-014-2848-1
Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Gayathri V, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environl Chem Lett 16:1339–1359. https://doi.org/10.1007/s10311-018-0762-3
Nawab J, Khan S, Aamir M, Shamshad I, Qamar Z, Din I, Huang Q (2016) Organic amendments impact the availability of heavy metal (loid) s in mine-impacted soil and their phytoremediation by Penisitum americanum and Sorghum bicolor. Environ Sci Pollut Res 23:2381–2390. https://doi.org/10.1007/s11356-015-5458-7
Nesler A, DalCorso G, Fasani E, Manara A, Di Sansebastiano GP, Argese E, Furini A (2017) Functional components of the bacterial CzcCBA efflux system reduce cadmium uptake and accumulation in transgenic tobacco plants. New Biotechnol 35:54–61. https://doi.org/10.1016/j.nbt.2016.11.006
Ning CH, Li WB, Xu QK, Li M, Guo SX (2019) Arbuscular mycorrhizal fungi enhance cadmium uptake of wetland plants in contaminated water. J Appl Ecol 30:2063–2071. https://doi.org/10.13287/j.1001-9332.201906.019
Okem A, Stirk WA, Street RA, Southway C, Finnie JF, Van Staden J (2015) Effects of Cd and Al stress on secondary metabolites, antioxidant and antibacterial activity of Hypoxis hemerocallidea Fisch. & CA Mey. Plant Physiol Biochem 97:147–155. https://doi.org/10.1016/j.plaphy.2015.09.015
Okolie CU, Chen H, Zhao Y, Tian D, Zhang L, Su M, Jiang Z, Li Z, Li H (2020) Cadmium immobilization in aqueous solution by Aspergillus niger and geological fluorapatite. Environ Sci Pollut Res 27:7647–7656. https://doi.org/10.1007/s11356-019-07500-0
Orelle C, Durmort C, Mathieu K, Duchêne B, Aros S, Fenaille F, André F, Junot C, Vernet T, Jault JM (2018) A multidrug ABC transporter with a taste for GTP. Sci Rep 8:2309. https://doi.org/10.1038/s41598-018-20558-z
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF (2015) Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci 6:133. https://doi.org/10.3389/fpls.2015.00133
Pérez M, Ramírez G, Serralde O, Peñaranda R, Wilches O, Ramírez L, Rengifo E (2019) Arbuscular mycorrhizal fungi (AMF) as a strategy to reduce the absorption of cadmium in cocoa (Theobroma cacao) plants. Terra Latinoam 37:121–130. https://doi.org/10.28940/terra.v37i2.479
Pinto AP, Simes I, Mota AM (2008) Cadmium impact on root exudates of sorghum and maize plants: a speciation study. J Plant Nutr 31:1746–1755. https://doi.org/10.1080/01904160802324829
Pohlmeier A (2004) Metal speciation, chelation and complexing ligands in plants. In: Prasad MNV (ed) Heavy metal stress in plants: from biomolecules to ecosystems. Springer, New Delhi, pp 28–46
Preece C, Peñuelas J (2019) A return to the wild: root exudates and food security. Trends Plant Sci 25:14–21. https://doi.org/10.1016/j.tplants.2019.09.010
Puschenreiter M, Gruber B, Wenzel WW, Schindlegger Y, Hann S, Spangl B, Schenkeveld WD, Kraemer SM, Oburger E (2017) Phytosiderophore-induced mobilization and uptake of Cd, Cu, Fe, Ni, Pb and Zn by wheat plants grown on metal-enriched soils. Environ Exp Bot 138:67–76. https://doi.org/10.1016/j.envexpbot.2017.03.011
Qi F, Lamb D, Naidu R, Bolan NS, Yan Y, Ok YS, Rahman MM, Choppala G (2018) Cadmium solubility and bioavailability in soils amended with acidic and neutral biochar. Sci Total Environ 610:1457–1466. https://doi.org/10.1016/j.scitotenv.2017.08.228
Qing LUO, Li-Na SUN, Xiao-Min HU (2015) Metabonomics study on root exudates of cadmium hyperaccumulator Sedum alfredii. Chin J Anal Chem 43:7–12. https://doi.org/10.1016/S1872-2040(15)60795-2
Rafiq MT, Aziz R, Yang X, Xiao W, Rafiq MK, Ali B, Li T (2014) Cadmium phytoavailability to rice (Oryza sativa L.) grown in representative Chinese soils. A model to improve soil environmental quality guidelines for food safety. Ecotoxicol Environ Saf 103:101–107. https://doi.org/10.1016/j.ecoenv.2013.10.016
Raghavan PS, Potnis AA, Bhattacharyya K, Salaskar DA, Rajaram H (2020) Axenic cyanobacterial (Nostoc muscorum) biofilm as a platform for Cd(II) sequestration from aqueous solutions. Algal Res 46:101778. https://doi.org/10.1016/j.algal.2019.101778
Rasmann S, Turlings TC (2016) Root signals that mediate mutualistic interactions in the rhizosphere. Curr Opin Plant Biol 32:62–68. https://doi.org/10.1016/j.pbi.2016.06.017
Reddy KR, Reddy CV, Nadagouda MN, Shetti NP, Jaesool S, Aminabhavi TM (2019) Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: synthesis methods, properties and photocatalytic applications. J Environ Manag 238:25–40. https://doi.org/10.1016/j.jenvman.2019.02.075
Riesen O, Feller U (2005) Redistribution of nickel, cobalt, manganese, zinc, and cadmium via the phloem in young and maturing wheat. J Plant Nutr 28:421–430. https://doi.org/10.1081/PLN-200049153
Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-ur-Rehman M, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health 39:259–277. https://doi.org/10.1007/s10653-016-9826-0
Rocco C, Seshadri B, Adamo P, Bolan NS, Mbene K, Naidu R (2018) Impact of waste-derived organic and inorganic amendments on the mobility and bioavailability of arsenic and cadmium in alkaline and acid soils. Environ Sci Pollut Res 25:25896–25905. https://doi.org/10.1007/s11356-018-2655-1
Rocha J, Tacão M, Fidalgo C, Alves A, Henriques I (2016) Diversity of endophytic Pseudomonas in Halimione portulacoides from metal (loid)-polluted salt marshes. Environ Sci Pollut Res 23:13255–13267. https://doi.org/10.1007/s11356-016-6483-x
Rohani N, Daneshmand F, Vaziri A, Mahmoudi M, Saber-Mahani F (2019) Growth and some physiological characteristics of Pistacia vera L. cv Ahmad Aghaei in response to cadmium stress and Glomus mosseae symbiosis. S Afr J Bot 124:499–507. https://doi.org/10.1016/j.sajb.2019.06.001
Römheld V, Awad F (2000) Significance of root exudates in acquisition of heavy-metals from a contaminated calcareous soil by graminaceous species. J Plant Nutr 23:1857–1866. https://doi.org/10.1080/01904160009382148
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Biol 52:527–560. https://doi.org/10.1146/annurev.arplant.52.1.527
Saber NE, Abdel-Moneim AM, Barakat SY (1999) Role of organic acids in sunflower tolerance to heavy metals. Biol Plantarum 42:65–73. https://doi.org/10.1023/A:1002115425544
Saeki K, Kunito T (2012) Influence of chloride ions on cadmium adsorptions by oxides, hydroxides, oxyhydroxides, and phyllosilicates. Appl Clay Sci 62–63:58–62. https://doi.org/10.1016/j.clay.2012.04.018
Saengwilai P, Meeinkuirt W, Phusantisampan T, Pichtel J (2019) Immobilization of cadmium in contaminated soil using organic amendments and its effects on rice growth performance. Expos Health. https://doi.org/10.1007/s12403-019-00312-0
Sangthong C, Setkit K, Prapagdee B (2016) Improvement of cadmium phytoremediation after soil inoculation with a cadmium-resistant Micrococcus sp. Environ Sci Pollut Res 23:756–764. https://doi.org/10.1007/s11356-015-5318-5
Seregin IV, Ivanov VB (1997) Histochemical investigation of cadmium and lead distribution in plants. Russ J Plant Physiol 44:791–796
Shahid M, Pinelli E, Dumat C (2012) Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater 219:1–12. https://doi.org/10.1016/j.jhazmat.2012.01.060
Shahid M, Austruy A, Echevarria G, Arshad M, Sanaullah M, Aslam M, Nadeem M, Nasim W, Dumat C (2014) EDTA-enhanced phytoremediation of heavy metals: a review. Soil Sediment Contam 23:389–416. https://doi.org/10.1080/15320383.2014.831029
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PM (2016) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Shan S, Guo Z, Lei P, Wang Y, Li Y, Cheng W, Zhang M, Wu S, Yi H (2019) Simultaneous mitigation of tissue cadmium and lead accumulation in rice via sulfate-reducing bacterium. Ecotoxicol Environ Saf 169:292–300. https://doi.org/10.1016/j.ecoenv.2018.11.030
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl Soil Ecol 107:66–78. https://doi.org/10.1016/j.apsoil.2016.05.009
Sharma SS, Dietz KJ (2006) The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot 57:711–726. https://doi.org/10.1093/jxb/erj073
Sharma T, Dreyer I, Kochian L, Pineros MA (2016) The ALMT family of organic acid transporters in plants and their involvement in detoxification and nutrient security. Front Plant Sci 7:1488. https://doi.org/10.3389/fpls.2016.01488
Sharma R, Bhardwaj R, Gautam V, Kohli SK, Kaur P, Bali RS, Saini P, Thukral AK, Arora S, Vig AP (2018) Microbial siderophores in metal detoxification and therapeutics: recent prospective and applications. In: Egamberdieva D, Ahmad P (eds) Plant microbiome: stress response. Springer, Singapore, pp 337–350
She W, Zhu S, Jie Y, Xing H, Cui G (2015) Expression profiling of cadmium response genes in ramie (Boehmeria nivea L.) root. Bull Environ Contam Toxicol 94:453–459. https://doi.org/10.1007/s00128-015-1502-z
Shen Y, Zhu W, Li H, Ho SH, Chen J, Xie Y, Shi X (2018) Enhancing cadmium bioremediation by a complex of water-hyacinth derived pellets immobilized with Chlorella sp. Bioresour Technol 257:157–163. https://doi.org/10.1016/j.biortech.2018.02.060
Shenker M, Fan TWM, Crowley DE (2001) Phytosiderophores influence on cadmium mobilization and uptake by wheat and barley plants. J Environ Qual 30:2091–2098. https://doi.org/10.2134/jeq2001.2091
Shi G, Liu C, Cai Q, Liu Q, Hou C (2010) Cadmium accumulation and tolerance of two safflower cultivars in relation to photosynthesis and antioxidative enzymes. Bull Environ Contam Toxicol 85:256–263. https://doi.org/10.1007/s00128-010-0067-0
Shi M, Zhao Z, Song Y, Xu M, Li J, Yao L (2020) A novel heat-treated humic acid/MgAl-layered double hydroxide composite for efficient removal of cadmium: fabrication, performance and mechanisms. Appl Clay Sci 187:105482. https://doi.org/10.1016/j.clay.2020.105482
Sidhu GPS, Singh HP, Batish DR, Kohli RK (2017) Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol Environ Saf 135:209–215. https://doi.org/10.1016/j.ecoenv.2016.10.001
Sidhu GPS, Bali AS, Singh HP, Batish DR, Kohli RK (2018) Ethylenediamine disuccinic acid enhanced phytoextraction of nickel from contaminated soils using Coronopus didymus (L.) Sm. Chemosphere 205:234–243. https://doi.org/10.1016/j.chemosphere.2018.04.106
Sidhu GPS, Bali AS, Bhardwaj R (2019a) Use of fungi in mitigating cadmium toxicity in plants. In: Hasanuzzaman M, Prasad MNV, Fujita M (eds) Cadmium toxicity and tolerance in plants. Academic Press, Cambridge, pp 397–426
Sidhu GPS, Bali AS, Bhardwaj R (2019b) Role of organic acids in mitigating cadmium toxicity in plants. In: Hasanuzzaman M, Prasad MNV, Fujita M (eds) Cadmium tolerance in plants. Academic Press, Cambridge, pp 255–279
Sidhu GPS, Bali AS, Singh HP, Batish DR, Kohli RK (2020) Insights into the tolerance and phytoremediation potential of Coronopus didymus L.(Sm) grown under zinc stress. Chemosphere 244:125350. https://doi.org/10.1016/j.chemosphere.2019.125350
Silber A, Bar-Yosef B, Suryano S, Levkovitch I (2012) Zinc adsorption by perlite: effects of pH, ionic strength, temperature, and pre-use as growth substrate. Geoderma 170:159–167. https://doi.org/10.1016/j.geoderma.2011.11.028
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Sinha S, Mukherjee SK (2008) Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56:55–60. https://doi.org/10.1007/s00284-007-9038-z
Siripornadulsil S, Traina S, Verma DPS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847. https://doi.org/10.1105/tpc.004853
Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377:369–381. https://doi.org/10.1007/s11104-013-2006-4
Song Y, Hudek L, Freestone D, Puhui J, Michalczyk AA, Senlin Z, Ackland ML (2014) Comparative analyses of cadmium and zinc uptake correlated with changes in natural resistance-associated macrophage protein (NRAMP) expression in Solanum nigrum L. and Brassica rapa. Environ Chem 11:653–660. https://doi.org/10.1071/EN14078
Stoknes K, Scholwin F, Jasinska A, Wojciechowska E, Mleczek M, Hanc A, Niedzielski P (2019) Cadmium mobility in a circular food-to-waste-to-food system and the use of a cultivated mushroom (Agaricus subrufescens) as a remediation agent. J Environ Manag 245:48–54. https://doi.org/10.1016/j.jenvman.2019.03.134
Sullivan TS, McBride MB, Thies JE (2013) Soil bacterial and archaeal community composition reflects high spatial heterogeneity of pH, bioavailable Zn, and Cu in a metalliferous peat soil. Soil Biol Biochem 66:102–109. https://doi.org/10.1016/j.soilbio.2013.06.021
Sun RL, Zhou QX, Jin CX (2006) Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil 285:125–134. https://doi.org/10.1007/s11104-006-0064-6
Sun Y, Sun G, Xu Y, Wang L, Liang X, Lin D (2013) Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma 193:149–155. https://doi.org/10.1016/j.geoderma.2012.07.012
Sun J, Li X, Ai X, Liu J, Yin Y, Huang Y, Zhou H, Huang K (2018) Efficient removal of cadmium from soil-washing effluents by garlic peel biosorbent. Environ Sci Pollut Res 25:19001–19011. https://doi.org/10.1007/s11356-018-2109-9
Takanashi K, Shitan N, Yazaki K (2014) The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol 31:417–430. https://doi.org/10.5511/plantbiotechnology.14.0904a
Tan IAW, Chan JC, Hameed BH, Lim LLP (2016) Adsorption behavior of cadmium ions onto phosphoric acid-impregnated microwave-induced mesoporous activated carbon. J Water Process Eng 14:60–70. https://doi.org/10.1016/j.jwpe.2016.10.007
Tang X, Li Q, Wang Z, Hu Y, Hu Y, Li R (2018) In situ electrokinetic isolation of cadmium from paddy soil through pore water drainage: effects of voltage gradient and soil moisture. Chem Eng J 337:210–219. https://doi.org/10.1016/j.cej.2017.12.111
Tang L, Hamid Y, Gurajala HK, He Z, Yang X (2019) Effects of CO2 application and endophytic bacterial inoculation on morphological properties, photosynthetic characteristics and cadmium uptake of two ecotypes of Sedum alfredii Hance. Environ Sci Pollut Res 26:1809–1820. https://doi.org/10.1007/s11356-018-3680-9
Tao Q, Hou D, Yang X, Li T (2016) Oxalate secretion from the root apex of Sedum alfredii contributes to hyperaccumulation of Cd. Plant Soil 398:139–152. https://doi.org/10.1007/s11104-015-2651-x
Tao Q, Jupa R, Luo J, Lux A, Kováč J, Wen Y, Zhou Y, Jan J, Liang Y, Li T (2017) The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii. J Exp Bot 68:739–751. https://doi.org/10.1093/jxb/erw453
Tavangar T, Karimi M, Rezakazemi M, Reddy KR, Aminabhavi TM (2020) Textile waste, dyes/inorganic salts separation of cerium oxide-loaded loose nanofiltration polyethersulfone membranes. Chem Eng J 385:123787. https://doi.org/10.1016/j.cej.2019.123787
Tudoreanu L, Phillips CJC (2004) Modeling cadmium uptake and accumulation in plants. Adv Agron 84:121
Ueno D, Yamaji N, Kono I, Huang CFT, Yano M, Ma JF (2010) Gene limiting cadmium accumulation in rice. Proc Natl Acad Sci USA 107:16500–16505. https://doi.org/10.1073/pnas.1005396107
Uraguchi S, Fujiwara T (2012) Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice 5:5. https://doi.org/10.1186/1939-8433-5-5
Uraguchi S, Watanabe I, Yoshitomi A, Kiyono M, Kuno K (2006) Characteristics of cadmium accumulation and tolerance in novel Cd-accumulating crops, Avena strigosa and Crotalaria juncea. J Exp Bot 57:2955–2965. https://doi.org/10.1093/jxb/erl056
Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T (2011) Low affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA 108:20959–20964. https://doi.org/10.1073/pnas.1116531109
Vives-Peris V, de Ollas C, Gómez-Cadenas A, Pérez-Clemente RM (2019) Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep 39:3–17. https://doi.org/10.1007/s00299-019-02447-5
Wang K, Zhang J, Zhu Z, Huang H, Li T, He Z, Yang X, Alva A (2012) Pig manure vermicompost (PMVC) can improve phytoremediation of Cd and PAHs cocontaminated soil by Sedum alfredii. J Soil Sediment 12:1089–1099. https://doi.org/10.1007/s11368-012-0539-4
Wang X, Shi Y, Chen X, Huang B (2015) Screening of Cd-safe genotypes of Chinese cabbage in field condition and Cd accumulation in relation to organic acids in two typical genotypes under long-term Cd stress. Environ Sci Pollut Res 22:16590–16599. https://doi.org/10.1007/s11356-015-4838-3
Wang R, Wei S, Jia P, Liu T, Hou D, Xie R, Lin Z, Ge J, Qiao Y, Chang X, Lu L, Tian S (2019) Biochar significantly alters rhizobacterial communities and reduces Cd concentration in rice grains grown on Cd-contaminated soils. Sci Total Environ 676:627–638. https://doi.org/10.1016/j.scitotenv.2019.04.133
Wang Q, Ge C, Wu Y, Sahito ZA, Ma L, Pan F, Zhou Q, Huang L, Feng Y, Yang X (2020) The endophytic bacterium Sphingomonas SaMR12 alleviates Cd stress in oilseed rape through regulation of the GSH-AsA cycle and antioxidative enzymes. BMC Plant Biol 20:1–14. https://doi.org/10.1186/s12870-020-2273-1
Weston LA, Ryan PR, Watt M (2012) Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J Exp Bot 63:3445–3454. https://doi.org/10.1093/jxb/ers054
WHO (2007) Health risks of heavy metals from long-range transboundary air pollution. World Health Organization 2007. WHO Regional Office for Europe, Copenhagen
Wong CKE, Cobbett CS (2009) HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol 181:71–78. https://doi.org/10.1111/j.1469-8137.2008.02638.x
Wu B, He T, Wang Z, Qiao S, Wang Y, Xu F, Xu H (2020) Insight into the mechanisms of plant growth promoting strain SNB6 on enhancing the phytoextraction in cadmium contaminated soil. J Hazard Mater 385:121587. https://doi.org/10.1016/j.jhazmat.2019.121587
Xiao R, Wang P, Mi S, Ali A, Liu X, Li Y, Zhang Z (2019) Effects of crop straw and its derived biochar on the mobility and bioavailability in Cd and Zn in two smelter-contaminated alkaline soils. Ecotoxicol Environ Saf 181:155–163. https://doi.org/10.1016/j.ecoenv.2019.06.005
Xie X, Weiss DJ, Weng B, Liu J, Lu H, Yan C (2013) The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata (S., L.) Yong) roots. Environ Sci Pollut Res 20:997–1008. https://doi.org/10.1007/s11356-012-1031-9
Xin J, Huang B, Dai H, Zhou W, Yi Y, Peng L (2015) Roles of rhizosphere and root-derived organic acids in Cd accumulation by two hot pepper cultivars. Environ Sci Pollut Res 22:6254–6261. https://doi.org/10.1007/s11356-014-3854-z
Xu J, Zhu YY, Ge Q, Li YL, Sun JH, Zhang Y, Liu XJ (2012) Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol 196:125–138. https://doi.org/10.1111/j.1469-8137.2012.04236.x
Xu S, Xing Y, Liu S, Huang Q, Chen W (2019) Role of novel bacterial Raoultella sp. strain X13 in plant growth promotion and cadmium bioremediation in soil. Appl Microbiol Biotechnol 103:3887–3897. https://doi.org/10.1007/s00253-019-09700-7
Xue W, Peng Z, Huang D, Zeng G, Wan J, Xu R, Cheng M, Zhang C, Jiang D, Hu Z (2018) Nanoremediation of cadmium contaminated river sediments: microbial response and organic carbon changes. J Hazard Mater 359:290–299. https://doi.org/10.1016/j.jhazmat.2018.07.062
Yang H, Yang ZM, Zhou LX, Wong JW (2001) Ability of Agrogyron elongatum to accumulate the single metal of cadmium, copper, nickel and lead and root exudation of organic acids. J Environ Sci 13:368–375
Yokosho K, Yamaji N, Ma JF (2011) An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J 68:1061–1069. https://doi.org/10.1111/j.1365-313X.2011.04757.x
Yousaf B, Liu G, Wang R, Zia-ur-Rehman M, Rizwan MS, Imtiaz M, Shakoor A (2016) Investigating the potential influence of biochar and traditional organic amendments on the bioavailability and transfer of Cd in the soil–plant system. Environ Earth Sci 75:374. https://doi.org/10.1007/s12665-016-5285-2
Yu HY, Liu C, Zhu J, Li F, Deng DM, Wang Q, Liu C (2016) Cadmium availability in rice paddy fields from a mining area: the effects of soil properties highlighting iron fractions and pH value. Environ Pollut 209:38–45. https://doi.org/10.1016/j.envpol.2015.11.021
Zeng X, Xu H, Jijie L, Chen Q, Li W, Wu L, Tang J, Ma L (2020) The immobilization of soil cadmium by the combined amendment of bacteria and hydroxyapatite. Sci Rep 10:2189. https://doi.org/10.1038/s41598-020-58259-1
Zhang L, Wang H (2002) Changes of root exudates to Cadmium stress in wheat (Triticum aestivum L.). Acta Ecol Sin 22:496–502
Zhang M, Liu X, Yuan L, Wu K, Duan J, Wang X, Yang L (2012) Transcriptional profiling in cadmium-treated rice seedling roots using suppressive subtractive hybridization. Plant Physiol Biochem 50:79–86. https://doi.org/10.1016/j.plaphy.2011.07.015
Zhang Y, He Q, Xia L, Li Y, Song S (2018) Algae cathode microbial fuel cells for cadmium removal with simultaneous electricity production using nickel foam/graphene electrode. Biochem Eng J 138:179–187. https://doi.org/10.1016/j.bej.2018.07.021
Zhang F, Liu M, Li Y, Che Y, Xiao Y (2019) Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci Total Environ 655:1150–1158. https://doi.org/10.1016/j.scitotenv.2018.11.317
Zhang XF, Hu ZH, Yan TX, Lu RR, Peng CL, Li SS, Jing YX (2019) Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol Environ Saf 171:352–360. https://doi.org/10.1016/j.ecoenv.2018.12.097
Zhou G, Delhaize E, Zhou M, Ryan PR (2013) The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Ann Bot 112:603–612. https://doi.org/10.1093/aob/mct135
Zhu XF, Zheng C, Hu YT, Jiang TAO, Liu YU, Dong NY, Yang JL, Zheng SJ (2011) Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant, Cell Environ 34:1055–1064. https://doi.org/10.1111/j.1365-3040.2011.02304.x
Zhu Y, Hu X, Duan Y, Li S, Wang Y, Rehman AU, He J, Zhang J, Hua D, Yang L, Wang L (2020) The Arabidopsis nodulin homeobox factor atndx interacts with AtRING1A/B and negatively regulates abscisic acid signaling. Plant Cell 32:703–721. https://doi.org/10.1105/tpc.19.00604
Zieliński J, Huculak-Mączka M, Kaniewski M, Nieweś D, Hoffmann K, Hoffmann J (2019) Kinetic modelling of cadmium removal from wet phosphoric acid by precipitation method. Hydrometallurgy 190:105157. https://doi.org/10.1016/j.hydromet.2019.105157
Zoghlami LB, Djebali W, Abbes Z, Hediji H, Maucourt M, Moing A, Brouquisse R, Chaïbi W (2011) Metabolite modifications in Solanum lycopersicum roots and leaves under cadmium stress. Afr J Biotechnol 10:567–579. https://doi.org/10.5897/AJB10.1275
Acknowledgement
GPS and ASB designed the review. GPS, ASB and VK wrote the manuscript. All authors read and approved the review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bali, A.S., Sidhu, G.P.S. & Kumar, V. Root exudates ameliorate cadmium tolerance in plants: A review. Environ Chem Lett 18, 1243–1275 (2020). https://doi.org/10.1007/s10311-020-01012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-020-01012-x