Abstract
Soil properties affect the bioavailability of cadmium (Cd). In general, soils in southern China are rich in iron (Fe) and contain manganese (Mn), which form ferromanganese nodules through biological or chemical reactions. In this study, it is hypothesized that the formation of Fe/Mn oxides can reduce the bioavailability of Cd and play an important role in reducing the uptake of Cd by rice. In this study, the effects of Fe and Mn on the contents of Fe (II)-oxidizing bacteria (FeOB) communities, Fe oxides, and Cd speciation in Cd-contaminated paddy soils were studied in a pot experiment. Results showed that, compared with the CK treatment, the addition of Mn (II) increased the abundance of FeOB (Thiobacillus sp.) in the soil, thereby increasing the content of amorphous Fe oxides in the rhizosphere. Amorphous Fe oxides effectively immobilized Cd in the soil, that is, with an increasing content of Cd bound to amorphous Fe/Mn oxides in the rhizosphere and non-rhizosphere soils, the mobility and bioavailability of Cd were reduced. After the addition of Mn (II) and Fe (II), the Fe content in the root Fe plaque of rice increased significantly, while the Cd content decreased significantly. The increase of Fe in the root Fe plaque inhibited rice uptake of soil Cd; thus, the Cd content in rice shoots was significantly lower than the Cd content in the CK treatment. Compared with the addition of Fe (II), the addition of Mn (II) effectively reduced the Cd content in rice shoots. These findings suggested that the addition of Mn (II) can promote the formation of Fe oxides in soils and reduce the Cd content in rice. These research results provide support for using Mn-based soil remediation materials to remedy Cd-contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rice is one of the staple foods in Asia. In recent years, incidents of rice cadmium (Cd) pollution have occurred frequently, posing a serious threat to human health. Cd poisoning has been reported to severely damage human tissues and functional systems, such as the kidneys, respiratory system, and reproductive system (Godt et al. 2006). A high background content of Cd in soils due to the discharge of mining wastewater may cause soil Cd to significantly exceed its maximum allowable content. This is especially true for some major rice production areas in Asia, such as Thailand and China, where soils and ground water have been seriously polluted by Cd, resulting in Cd accumulation in rice with adverse impacts on human health (Liu et al. 2015; Robson et al. 2014; Kosolsaksakul et al. 2014; Zhang et al. 2014). Statistics show that approximately 16.1% and 19.4% of soils and farmlands, respectively, are polluted by heavy metals, such as Cd (Zhou et al. 2015). Therefore, determining how to control Cd pollution in rice to ensure healthy rice production has become an important issue in Cd pollution control.
Previous studies have shown that plant uptake of metal ions from soils is affected by the physicochemical properties of the soil, such as pH, organic matter, and mineral content (Pietrzykowski et al. 2014). Cd mobility and bioavailability are controlled by pH. For example, at a low pH, Cd will be released from iron (Fe) or manganese (Mn) oxides, making it more prone to be taken up by plant, thereby increasing the risk of contamination. In the subtropical soils of southern China, Fe oxide contents are appreciable with high activity and play an important role in the migration and immobilization of Cd. For example, Fe oxides effectively immobilize Cd due to their strong adsorption capacity. However, when Fe oxides are dissolved, Cd may be released and easily absorbed by plants (Liu et al. 2014). Therefore, certain forms of metal oxides effectively control the uptake of heavy metals by plants. In soils, various oxides often coexist, such as Fe and Mn oxides. Highly active Mn oxides, which are strong oxidants, can accelerate the oxidation of Fe in soils by forming ferromanganese nodules through strong migration and agglomeration during supergene processes. Due to their poor crystallinity, low point of zero charge (PZC), and high surface negative charge, ferromanganese nodules determine heavy metal speciation and concentration in soils through redox and adsorption/co-precipitation processes (Bartlett 1988). Mn (II/III)/Mn (IV) in soils has similar redox properties to Fe (II)/Fe (III), that is, there is competition for electron acceptors or donors between the Fe and Mn cycles. Too high a Mn content can promote the oxidation of Fe (II) while inhibiting its reduction (Ying et al. 2012). However, the mechanism of how Mn affects the oxidation process of Fe, and in turn the subsequent immobilization of heavy metals, remains unclear.
During paddy field flooding, paddy soils are mainly in an anaerobic state. Due to the radial oxygen loss (ROL) of rice, a micro-aerobic environment is formed in the soil around the rhizosphere. Given the limited oxidant content, the oxidation of Fe and Mn during the flooding period is mainly caused by micro-aerobic or anaerobic microorganisms. It has been reported that microbial-mediated Fe (II) oxidation is the main cause of Fe oxide formation in micro-aerobic/anaerobic environments, such as flooded paddy fields, groundwater, and seafloor sediments (Kappler et al. 2005; Weber et al. 2006). When microbial-mediated Fe (II) oxidation takes place to form Fe oxides, heavy metal ions in the soil or water will be absorbed and immobilized by the Fe oxides (Fabisch et al. 2013; Lack et al. 2002). Neubauer et al. (2007) showed that FeOB can promote the formation of Fe plaque on the root surface of Juncus effusus. Dong et al. (2016) confirmed that FeOB/MnOB (manganese-oxidizing bacteria) can promote the formation of Fe/Mn plaque on rice roots and reduce Cd accumulation in the root Fe plaque and in rice leaves. When oxidizing Fe (II), some neutral FeOB can also oxidize Mn (II), thereby forming a complex mineral rich in Fe and Mn (Tebo et al. 2005), which can eventually develop into ferromanganese nodules in soils through a series of migration and agglomeration processes. Therefore, under neutral micro-aerobic/anaerobic conditions, abundant Mn (II) in soils may affect the microbial-mediated Fe (II) oxidation process, thus forming different types of secondary minerals to affect the adsorptive immobilization, redox, and bioavailability of heavy metal elements.
This study mainly used a field pot experiment with Cd as the target heavy metal to investigate the effect of Mn on Fe (II) oxidation and the migration and transformation of heavy metals. Rhizosphere and non-rhizosphere soils of rice were investigated using a root-bag method to identify how soil Mn (II) affected rice growth, the formation of Fe/Mn plaque on rice roots, Cd uptake, and the composition of the soil microbial community.
2 Materials and Methods
2.1 Pot Experiments
Soils for the pot experiment were collected from the surface (depth 0–20 cm) of a heavy metal-contaminated farmland in Hezhou City (24°22′24.0″ N, 111°37′9.7″ E), Guangxi Province, China. Soil properties were determined according to Lauber et al. (2008); the physicochemical properties are shown in Table 1. The soil samples were air dried and passed through a 2-mm sieve, after which the soil samples were mixed with Cd added at a rate of 1.7 mg (as CdCl2½H2O) per kg of soil. The samples were then allowed to age under moisture for 6 months, followed by air drying. Next, the air-dried samples were passed through a 2-mm sieve and placed in plastic pots (175 mm top diameter, 130 cm bottom diameter, and 132 mm height). Approximately 1.5 kg of air-dried sample was placed in each pot; 0.5 kg of the soil sample was placed in a 300-mesh nylon bag as the rhizosphere soil and the remaining 1.0 kg of the soil sample was placed outside the nylon bag as the non-rhizosphere soil.
The rhizosphere and the non-rhizosphere soils were studied with the root-bag method. Three treatments were set up in this study: (1) no addition of FeSO4 or MnSO4 (CK), (2) addition of 800 mg Fe (as FeSO4·7H2O) per kg of air-dried soil sample (Fe800), and (3) addition of 800 mg Mn (as MnSO4·H2O) per kg of air-dried soil sample (Mn800). Before planting and in addition to FeSO4·7H2O and MnSO4·H2O, the soil samples in each treatment were treated with urea, monoammonium phosphate, and potassium chloride at doses of 75 mg, 1.075 g, and 454 mg per kg of soil sample, respectively, after which the soil samples were allowed to age for 1 week. The test rice was the y-liangyou variety. Seeds were surface-sterilized with 3% NaOCl for 15 min, rinsed with deionized water five times, and then cultivated with vermiculite. Rice seedlings aged for 3 weeks with the same growth state and were transplanted into 300-mesh nylon nets. Two seedlings were placed in a net per pot, with quadruplicates per treatment.
Rice and soil samples were collected during the tillering period. The pH of the rhizosphere and non-rhizosphere soils was determined in situ using an IQ150 pH meter before collection. Some of the rhizosphere and non-rhizosphere soils were collected in a sterile centrifuge tube using a high-temperature-sterilized plastic spoon and stored in a − 18 °C refrigerator for soil microbial analysis. Part of the rhizosphere and non-rhizosphere soils was immediately subjected to Fe (II) and Mn (II) analysis before oxidation of Fe (II) and Mn (II); the remaining rhizosphere and non-rhizosphere soils were air-dried; passed sequentially through 1-mm, 0.3-mm, and 0.15-mm sieves; and analyzed subsequently by soil physicochemical property analysis.
2.2 Sodium DCB Extraction of Root Fe/Mn Plaque
Fe/Mn plaque on fresh rice roots was extracted using the dithionite-citric acid-bicarbonate (DCB) method (Taylor et al. 1984). The extracting agent was made by mixing 40 mL of 0.3 mol L−1 sodium citrate and 5 mL of 1 mol L−1 sodium bicarbonate. The roots were thoroughly washed three times with deionized water. Then, 1 g of fresh roots was placed into a mixture of sodium citrate and sodium bicarbonate; 3 g of sodium dithionite was added, and the mixture was shaken at 25 °C for 3 h. Concentrations of Cd, Mn, and Fe in the extract were analyzed by atomic absorption spectrometry (PinAAcle 900, Perkin Elmer, USA).
After DCB extraction, the rice roots were rinsed with deionized water three times. The roots, stems, and leaves were heated at 105 °C for 0.5 h, and then dried at 65 °C for 72 h to achieve a constant weight. This was followed by HNO3 microwave digestion (CEM Mars 6, Pynnco, USA) and measurement of Cd, Mn, and Fe concentrations by atomic absorption spectrometry (PinAAcle 900, PerkinElmer, USA).
The following procedure was used to determine Fe (II)/Mn (II) in the soil: Fresh soil samples with an equivalent dry weight of 4 g were weighed and added to a 50-mL 0.1 N aluminum sulfate solution (pH 2.50) for 5 min and then filtered. Fe (II) in the filtrate was subjected to colorimetric analysis (UV-2600, Shimadzu, Japan) at a wavelength of 530 nm using phenanthroline colorimetry. Colorimetric analysis of Mn (II) in the filtrate was performed at a wavelength of 540 nm using potassium permanganate colorimetry.
The following procedure was used to extract the free Fe/Mn: A 0.5 g aliquot of the soil sample that passed through a 60-mesh sieve was placed in a 50-mL centrifuge tube to which 20 mL of 0.3 mol L−1 sodium citrate solution and 2.5 mL of 1 mol L−1 sodium bicarbonate solution were added, followed by heating of the tube in a water bath at 80 °C for 5 min. Subsequently, approximately 0.5 g of sodium dithionite was added and the tube was shaken for 15 min and then centrifuged. The supernatant was stored for later analysis (Bascomb 1968).
The following procedure was used to extract amorphous Fe/Mn: A 2 g aliquot of the soil sample that passed through a 60-mesh sieve was weighed in an Erlenmeyer flask. We added 100 mL (soil-to-liquid ratio of 1:50) of a 0.2 mol L−1 ammonium oxalate buffer solution that acted as the extracting agent, after which the flask was covered with a black plastic bag as a light shield. The flask was shaken at a constant temperature for 3 h, after which the mixture was transferred to a centrifuge tube and centrifuged. The supernatant was stored for later analysis (Shuman 1982).
The extraction of organic complexes Fe/Mn was conducted as follows: A 2 g aliquot of the soil sample that passed through a 60-mesh sieve was placed in an Erlenmeyer flask. We added 40 mL of a 0.1-mol-L−1 sodium pyrophosphate solution, which acted as the extracting agent. After an overnight extraction, the resulting mixture was transferred to a centrifuge tube and centrifuged, and the supernatant was stored for later analysis (McKeague 1967).
Relative abundances of different speciation forms of Cd in the soil were determined according to Tessier et al. (1979). Fe, Mn, and Cd in the extract were analyzed by atomic absorption spectrometry (PinAAcle 900, PerkinElmer, USA).
2.3 Soil DNA Extraction, 16S rRNA Gene Amplification, and High-Throughput Sequencing
Total genomic DNA was extracted from 0.25 g of each rhizosphere and non-rhizosphere soil sample using a PowerSoil DNA isolation kit (MO BIO Laboratories Inc., MA, USA) according to the manufacturer’s instructions. The total genomic DNA extracts were submitted for high-throughput amplicon sequencing at Magigen Biotechnology (Shenzhen, China) of the V4 region of 16S ribosomal RNA (rRNA). PCR amplification of 16S rRNA gene fragments was performed using primers F515 (50-GTGCCAGCMGCCGCGGTAA-30) and R806 (50-GGACTACVSGGGTATCTAAT-30), with a sample-specific 12-bp barcode added to the reverse primer (Caporaso et al. 2011). The bioinformatics analysis was processed using Mothur and QIIME (Schloss et al. 2009; Caporaso et al. 2011). Chimeric and low-quality sequences were identified and removed, whereas the 12-bp barcode was examined in order to assign sequences to individual samples. Operational taxonomic units (OTUs) were identified at the 97% sequence similarity level using UCLUST (Edgar 2010), and a representative sequence from each phylotype was selected using PyNAST (DeSantis et al. 2006). The taxonomic classification of each phylotype was determined using the Ribosomal Database Project (RDP) at the 80% threshold. The relative abundance (%) of individual taxa within each community was estimated by comparing the number of sequences assigned to a specific taxon versus the number of total sequences obtained for that sample.
2.4 Statistical Analysis
One-way analysis of variance was performed with SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Statistical differences between two treatments were calculated at a probability level of 5%; a p value < 0.05 indicates a significant difference. The relative abundance of OTUs in each sample was calculated, and the similarity between two samples was calculated using the Bray-Curtis dissimilarity coefficient. The LDA effect size was used to identify the species that differed significantly in abundance between sample treatments.
3 Results
3.1 Changes in the Cd, Fe, and Mn Contents in Rice
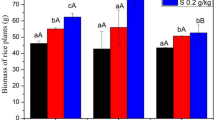
Rice samples were collected during the tillering period, and there was no significant difference in the shoot biomass or plant height between the three treatments (CK, Fe800, and Mn800 (p > 0.05) (Table 2). The Cd content in different rice parts was significantly lower in the Fe800 and Mn800 treatments than in the CK treatment (Fig. 1). The root Cd content in the Fe800 and Mn800 treatments was 49.48% and 47.94% lower (p < 0.05), respectively, with respect to the CK treatment, while the corresponding shoot Cd content was 25.33% and 60.67% lower (p < 0.05), respectively. Compared with the Fe-addition treatment Fe800, the Mn-addition treatment Mn800 led to significantly lower shoot Cd content, i.e., a reduction of 47.32% (p < 0.05).
The root Mn content in the Mn800 treatment was 1.093% higher compared with the CK treatment (p < 0.05). The Mn800 treatment led to a 619% and 773.2% higher root Mn content compared with the CK and Fe800 treatments (p < 0.05), respectively. There was no significant difference in the root Fe content or shoot Fe content among the three treatments (p > 0.05).
3.2 Formation of Root Fe/Mn Plaque and Change in the Cd Content
Measurements of the change in the metal content on the root surface indicated that the Fe800 and Mn800 treatments enhanced the formation of Fe/Mn plaque on the root surface (Table 3) compared with the CK treatment, with the Fe content in the plaque increasing significantly by 75.7% and 20.44%, respectively (p < 0.05). The Fe content in the root Fe plaque was 39.77% higher in the Fe800 treatment than in the Mn800 treatment (p < 0.05). The Mn content in the root Fe/Mn plaque was significantly higher in the Mn800 treatment than in the CK and Fe800 treatments, showing an increase of 1.150% and 1.462%, respectively (p < 0.05).
The change in the Cd content in the Fe/Mn plaque on rice roots is shown in Table 3. The Cd content in the Fe800 and Mn800 treatments was 61.01% and 61.37% lower (p < 0.05), respectively, compared with the CK treatment. There was no significant difference in the Cd content between the Fe800 and Mn800 treatments (p > 0.05).
3.3 Changes of the Speciation and Fe, Mn, and Cd Contents in Soils
To evaluate the effect of Mn on the contents of metals in the rhizosphere and non-rhizosphere soils, the contents of different speciation forms of Fe, Mn, and Cd in the two types of soils were detected. Changes in the Fe (II) and Fe oxide contents in the rhizosphere and non-rhizosphere soils are shown in Table 4. In all treatments, the Fe (II) content was significantly higher in the rhizosphere soil than in the non-rhizosphere soil. Compared with the CK treatment, the Fe (II) content in the rhizosphere and non-rhizosphere soils was significantly higher in the Fe800 treatment, by 55.77% and 49.72%, respectively (p < 0.05). Compared with the CK treatment, the Fe (II) content in the rhizosphere and non-rhizosphere soils in the Mn800 treatment was significantly lower, by 18.58% and 9.94%, respectively (p < 0.05). In all treatments, the contents of free Fe oxides, amorphous Fe oxides, and organic complexes Fe in the rhizosphere soil were higher than their counterparts in the non-rhizosphere soil. Compared with the CK treatment, the contents of amorphous Fe oxides in the rhizosphere and non-rhizosphere soils in the Fe800 treatment were significantly higher by 25.18% and 26.96%, respectively (p < 0.05); in addition, the content of organic complexes Fe in the rhizosphere soil was significantly higher in the Fe800 treatment, by 6.76% (p < 0.05). The Mn800 treatment led to a significant increase of 3.72% in the content of amorphous Fe oxides in the rhizosphere soil compared with the CK treatment, but a significant decrease of 12.12% in the content of organic complexes Fe (p < 0.05).
The changes in the contents of Mn (II) and Mn oxides in the rhizosphere and non-rhizosphere soils are shown in Table 5. Compared with the CK treatment, the Mn800 treatment led to a significantly higher Mn (II) content in the rhizosphere and non-rhizosphere soils, respectively. Similarly, a significant increase of 1.522% and 1.585% for free Mn oxides, by 2.431% and 2.788%, a significant increase of 2.349% and 2.441% for amorphous Mn oxides, and a significant increase of 256% and 272% for organic complexes Mn (p < 0.05 for all) occurred in the rhizosphere and non-rhizosphere soils, respectively, by Mn800 treatments. In the Fe800 treatment, the contents of organic complexes Mn in the rhizosphere and non-rhizosphere soils increased by 35.81% and 34.18%, respectively (p < 0.05).
The relative abundances of different speciation forms of Cd in the rhizosphere and non-rhizosphere soils are shown in Fig. 2. In all treatments, the content of exchangeable Cd in the rhizosphere soil was significantly higher than that in the non-rhizosphere soil (p < 0.05); the content of Cd bound to Fe/Mn oxides in the rhizosphere soil was significantly lower than that in the non-rhizosphere soil (p < 0.05). Compared with the CK treatment, the contents of carbonate-bound Cd in the rhizosphere and non-rhizosphere soils was significantly lower in the Fe800 treatment, by 9.04% and 10.16%, respectively. Similarly, the contents of carbonate-bound Cd in the rhizosphere and non-rhizosphere soils were significantly lower in the Mn800 treatments, by 11.26% and 13.92%, respectively (p < 0.05). In contrast, the content of Cd bound to Fe/Mn oxides in the rhizosphere soil increased significantly by 9.34% and 7.27% in the Fe800 and Mn800 treatments, respectively (p < 0.05), relative to the CK treatment. Compared with the CK treatment, the content of Cd bound to Fe/Mn oxides in the non-rhizosphere soil was significantly higher, by 6.20% (p < 0.05), in the Fe800 treatment.
Figure 3 shows the content of Cd bound to amorphous Fe/Mn oxides in the soil. Compared with the CK treatment, the content of Cd bound to amorphous Fe/Mn oxides was 7.61% and 7.73% higher in the rhizosphere soil of the Fe800 and Mn800 treatments, respectively (p < 0.05), and 8.55% and 8.66% higher in the non-rhizosphere soil (p < 0.05). There was no significant difference in the content of each form of Cd between the Fe800 and Mn800 treatments (Figs. 1 and 2).
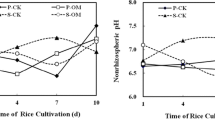
The rhizosphere and non-rhizosphere soil pH values are shown in Table 6, which indicates that the former soil had significantly lower pH than the latter (p < 0.05). Compared with the CK treatment, the pH of the rhizosphere soil in the Fe800 and Mn800 treatments was significantly lower, by 0.22 and 0.27, respectively (p < 0.05), while the pH in the non-rhizosphere soils was 0.12 and 0.26 lower, respectively (p < 0.05).
3.4 Changes in the Abundance of Soil FeOB and MnOB
High-throughput analysis revealed the microbial community compositions after each treatment, as shown in Fig. 4 (relative abundance > 1%). Principal coordinate analysis (PCoA) was used to analyze the similarities and dissimilarities in microbial community compositions among the CK, Fe800, and Mn800 treatments, revealing that the soil microbial community compositions in the treatments with Fe (II) and Mn (II) were significantly different from the communities in the CK treatment. However, there was no significant difference in the microbial community composition between the rhizosphere and non-rhizosphere soils for a particular treatment (Fig. 5). Compared with the CK treatment, the abundance of Thiobacillus bacteria, which are 99% similar to the FeOB Thiobacillus denitrificans (NC007404) in sequences, was significantly higher in the non-rhizosphere soil in the Mn800 treatment. The abundance of Oxalobacteraceae bacteria, which is 99% similar to the MnOB Oxalobacteraceae bacterium AB_7 (JQ033387) in sequences, was significantly higher in the non-rhizosphere soil in the Mn800 treatment. Lastly, the abundance of Pedomicrobium bacteria, which are 97% similar to the MnOB Pedomicrobium manganicum ATCC33121(GU269549) in sequences, was significantly higher in the rhizosphere soil in this treatment. There was no significant increase in FeOB abundance in the soil in the Fe800 treatment.
4 Discussion
Fe oxides are important active minerals in soils and can adsorb heavy metals due to their large surface area and chemical activity (Hall et al. 1996; Bolton and Evans 1996). Fe oxides in paddy soils are activated under flooding conditions and undergo a transformation from crystalline Fe to amorphous Fe, and further to ionic Fe (Tack et al. 2006). The trace metal system cycles that are required by most plants are related to the activity of rhizosphere soil microbes (Hawkes et al. 2007). Under flooding conditions, micro-aerobic/anaerobic FeOB dominate the Fe oxidation process in soils (Byrne et al. 2015). We attempted to identify the potential FeOB and MnOB based on comparing sequences of soil samples with sequences of known FeOB and MnOB reported in previous studies (Emerson et al. 2010; Hedrich et al. 2011; Liu et al. 2018). This study showed that the addition of Mn (II) reduced the Fe (II) content in soils and increased the abundance of FeOB in the non-rhizosphere soil, thereby accelerating the Fe oxidation process in soils and forming more amorphous Fe oxides (Table 4). However, the addition of Fe (II) had no significant effect on the abundance of FeOB in soils. An increase in the Fe oxide content may provide more adsorption sites and accelerate the immobilization of heavy metal ions. The addition of Mn (II) increased the abundance of FeOB in the non-rhizosphere soil but had no significant effect on the abundance of FeOB in the rhizosphere soil, which may be attributed to the fact that ROL had a greater effect than Mn (II) on FeOB abundance. The addition of Mn (II) activates MnOB in soils to generate different speciation forms of Mn oxides, which have a strong adsorption capability for heavy metals such as Cd, Cu, and Pb (Xu et al. 2015).
The migration and transformation of Cd in soils mainly refer to the transfer of spatial position and the change of speciation forms. Specifically, Cd is bound to soil colloids through ion-exchange adsorption or complexation-chelation or undergoes dissolution and precipitation reactions. The impact or toxic effect of heavy metals on the environment and organisms is largely determined by the speciation forms of heavy metals (Kot and Namiesnik 2000). Tessier et al. (1979) classified the speciation forms of heavy metals in soils as exchangeable metals, carbonate-bound metals, Fe/Mn oxide-bound metals, organic matter/sulfide-bound metals, and residual metals, with the former two forms having a poor stability and being prone to release into the environment. Amorphous Fe oxides have a larger specific surface area than crystalline Fe oxides and therefore adsorb more Cd (Randall et al. 1999; Tack et al. 2006). Li et al. (2004) pointed out that Fe/Mn oxides undergo complexation reactions and selective ion-exchange reactions with Cd, which promotes the transformation of weakly acidic, soluble Cd to reducible, oxidizable, and residual Cd, thereby controlling the migration, transformation, and enrichment of Cd in soils. In this study, the addition of Fe (II) or Mn (II) to the soils increased the content of amorphous Fe oxides (the addition of Mn (II) also promoted the formation of Mn oxides) (Tables 4 and 5), which promoted the transformation of carbonate-bound Cd to Fe/Mn oxide-bound Cd in the soil (Fig. 2), thus increasing the content of Cd bound to amorphous Fe/Mn oxides (Fig. 3). In addition, the content of Fe/Mn oxide-bound Cd in the rhizosphere soil increased significantly. Fe/Mn oxide-bound heavy metals are considered negatively correlated with metal assimilation efficiency (AE = ingestion-excretion-egestion); thus, the bioavailability of Cd was greatly reduced (Baumann and Fisher 2011). The change of Cd speciation forms leads to a significant reduction in the mobility and bioavailability of Cd, which inhibits the uptake of soil Cd by rice, thereby reducing the accumulation of Cd in rice roots.
The roots of rice and other aquatic plants are typically covered with a layer of Fe oxides called root Fe plaque (Otte et al. 1989; Fu et al. 2016). Root Fe plaque is mainly composed of ferrihydrite, goethite, and lepidocrokite (Kuo 1986). The micro-aerobic environment formed by ROL provides favorable conditions for micro-aerobic FeOB, and as a result, the Fe (II) generated under anaerobic conditions forms the root Fe plaque around the rhizosphere, with Fe as the major plaque component (Wu et al. 2012; Yang et al. 2014; Xu and Yu 2013; Hansel et al. 2002). This study showed that Fe (II) and Mn (II) promoted the oxidative precipitation of Fe and Mn on the root surface of rice. The addition of Mn (II) increased both Fe and Mn contents in the root Fe plaque, while the addition of Fe (II) increased the Fe content in the root Fe plaque (Table 3). The content of Cd in the root Fe/Mn plaque in the Mn800 and Fe800 treatments was significantly lower than that in the CK treatment (Fig. 1). This was likely because the addition of Fe (II) and Mn (II) accelerated the formation of Fe/Mn oxides in the soil, resulting in more Cd being adsorbed by Fe/Mn oxides in the soil and less Cd being able to reach the surface of the root Fe plaque (Fig. 3).
Root Fe plaque of rice plays an important role in Cd migration, transformation, and bioavailability (Chen et al. 2008; Chen et al. 2014). Studies have shown that root Fe plaque can decrease the activity of the heavy metals Cd, Zn, and As by adsorbing and immobilizing these metals in the rhizosphere of rice, which inhibits rice uptake of these metals (Yang et al. 2014; Sebastian and Prasad 2016; Fu et al. 2018). Cheng et al. (2014) found that root Fe plaque inhibited the migration of heavy metals Pb and Cd in rice plant tissues. Liu et al. (2008) found that the Fe content in roots and the root Fe plaque increased with an increase in the concentration of exogenous Fe, and that the increase in root Fe plaque inhibited the uptake of Cd by rice and decreased the Cd content in rice roots. After the addition of Mn (II) and Fe (II) in this study, most of the Cd was bound to soil Fe/Mn oxides and the root Fe/Mn plaque increased, which reduced the migration of Cd from the roots to the shoots, thereby reducing the shoot Cd content (Fig. 1). When plants absorb trace elements, Cd2+ can be transported through the low-specificity carrier proteins and ion channels that are located on the plant cell membranes, resulting in the transport of Mn2+ and Ca2+ (Tong and Guo 2007; Pittman 2005; Pinto and Ferreira 2015; Yue et al. 2017). This study showed that the addition of Mn (II) was more effective than the addition of Fe (II) in reducing the Cd content in rice roots (Table 2). This occurs because the addition of exogenous Mn (II) increases the root Mn content (Fig. 1), and Mn2+ antagonizes Cd2+ by preferentially binding carrier proteins and channel proteins on cell membranes. This process inhibits the transmembrane transport of Cd2+, reducing the Cd2+ content in the root cell cytosol and the amount of Cd2+ transported to the shoots, which alleviates the physiological toxicity of Cd and reduces its accumulation in the plants.
5 Conclusion
The amount of soil oxides affects the content of Cd in rice. By controlling the forms and contents of metal oxides in soils, it is possible to effectively inhibit the absorption and accumulation of Cd in rice. In this study, Mn (II) was added to soil to promote the growth of FeOB and the oxidative precipitation of Fe in order to accelerate the formation of amorphous Fe oxides in soils as well as the formation of Fe/Mn oxide plaque on the root surface of rice. The increase in the Fe oxide content effectively reduced the mobility of Cd in the soil, and the majority of the Cd was bound in the soil. Further, the formation of root Fe/Mn oxide plaque in the rhizosphere effectively inhibited rice uptake of soil Cd and reduced the Cd content in the rice roots, thereby effectively inhibiting Cd transport to the rice shoots.
References
Bartlett, R. J. (1988). Manganese redox reactions and organic interactions in soils. Manganese in Soils and Plants., 59–73.
Bascomb, C. L. (1968). Distribution of pyrophosphate extractable iron and organic carbon in soils of various groups. European Journal of Soil Science, 19, 251–268.
Baumann, Z., & Fisher, N. S. (2011). Relating the sediment phase speciation of arsenic, cadmium, and chromium with their bioavailability for the deposit-feeding polychaete Nereis succinea. Environmental Toxicology and Chemistry, 30(3), 747–756.
Bolton, K. A., & Evans, L. J. (1996). Cadmium adsorption capacity of selected Ontario soils. Canadian Journal of Soil Science, 76(2), 183–189.
Byrne, J. M., Klueglein, N., Pearce, C., Rosso, K. M., Appel, E., & Kappler, A. (2015). Redox cycling of Fe (II) and Fe (III) in magnetite by Fe-metabolizing bacteria. Science, 347(6229), 1473–1476.
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., Fierer, N., & Knight, R. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 108, 4516–4522.
Chen, X. P., Kong, W. D., He, J. Z., Liu, W. J., & Zhu, Y. G. (2008). Do water regimes affect iron-plaque formation and microbial communities in the rhizosphere of paddy rice? Journal of Plant Nutrition and Soil Science, 171(2), 193–199.
Chen, M. X., Cao, L., Song, X. Z., Wang, X. Y., Qian, Q. P., & Liu, W. (2014). Effect of iron plaque and selenium on cadmium uptake and translocation in rice seedlings (Oryza sativa) grown in solution culture. International Journal of Agriculture and Biology, 16(6), 1159–1164.
Cheng, H., Wang, M. Y., Wong, M. H., & Ye, Z. H. (2014). Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant and Soil, 375, 137–148.
Desantis, T. Z., Hugenholtz, P., Keller, K., Brodie, E. L., Larsen, N., Piceno, Y. M., Phan, R., & Andersen, G. L. (2006). Nast: a multiple sequence alignment server for comparative analysis of 16s rRNA genes. Nucleic Acids Research, 34, W394–W399.
Dong, M. F., Feng, R. W., Wang, R. G., Sun, Y., Ding, Y. Z., Xu, Y. M., Fan, Z. L., & Guo, J. K. (2016). Inoculation of Fe/Mn-oxidizing bacteria enhances Fe/Mn plaque formation and reduces Cd and As accumulation in rice plant tissues. Plant and Soil, 404(1–2), 75–83.
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than blast. Bioinformatics, 26(19), 2460–2461.
Emerson, D., Fleming, E. J., & Mcbeth, J. M. (2010). Iron-oxidizing bacteria: an environmental and genomic perspective. Annual Review of Microbiology, 64(1), 561–583.
Fabisch, M., Beulig, F., Akob, D. M., & Kusel, K. (2013). Surprising abundance of Gallionella-related iron oxidizers in creek sediments at pH 4.4 or at high heavy metal concentrations. Frontiers in Microbiology, 4, 390. https://doi.org/10.3389/fmicb.2013.00390.
Fu, Y. Q., Yang, X. J., Ye, Z. H., & Shen, H. (2016). Identification, separation and component analysis of reddish brown and non-reddish brown iron plaque on rice (Oryza sativa) root surface. Plant and Soil, 402(1–2), 277–290.
Fu, Y. Q., Yang, X. J., & Shen, H. (2018). Root iron plaque alleviates cadmium toxicity to rice (Qryza sativa) seedlings. Ecotoxicology and Environmental Safety, 161, 534–541.
Godt, J., Scheidig, F., Grosse-Siestrup, C., Esche, V., Brandenburg, P., Reich, A., & Groneberg, D. A. (2006). The toxicity of cadmium and resulting hazards for human health. Journal of Occupational Medicine and Toxicology, 1, 1–6.
Hall, G. E. M., Gauthier, G., Pelchat, J. C., Pelchat, P., & Vaive, J. E. (1996). Application of a sequential extraction scheme to ten geological certified reference materials for the determination of 20 elements. Journal of Analytical Atomic Spectrometry, 11(9), 787–796.
Hansel, C. M., Fendorf, S., Sutton, S., & Newville, M. (2002). Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environmental Science and Technology, 35(19), 3863–3868.
Hawkes, C. V., Deangelis, K. M., & Firestone, M. K. (2007). Root interactions with soil microbial communities and processes (pp. 1–29). Rhizosphere: An Ecological Perspective.
Hedrich, S., Schlömann, M., & Johnson, D. B. (2011). The iron-oxidizing proteobacteria. Microbiology, 157(6), 1551–1564.
Kappler, A., Schink, B., & Newman, D. K. (2005). Fe (III) mineral formation and cell encrustation by the nitrate-dependent Fe (II)-oxidizer strain BoFeN1. Geobiology, 3, 235–245.
Kosolsaksakul, P., Farmer, J. G., Oliver, I. W., & Graham, M. C. (2014). Geochemical associations and availability of cadmium (Cd) in a paddy field system, northwestern Thailand. Environmental Pollution, 187, 153–161.
Kot, A., & Namiesnik, J. (2000). The role of speciation in analytical chemistry. Trends in Analytical Chemistry, 19(2–3), 69–79.
Kuo, S. (1986). Concurrent sorption of phosphate and zinc, cadmium, or calcium by a hydrous ferric oxide. Soil Science Society of America Journal, 50, 1412–1419.
Lack, J. G., Chaudhuri, S. K., Kelly, S. D., Kemner, K. M., & Coates, J. D. (2002). Immobilization of radionuclides and heavy metals through anaerobic bio-oxidation of Fe (II). Applied and Environmental Microbiology, 68(6), 2704–2710.
Lauber, C. L., Strickland, M. S., Bradford, M. A., & Fierer, N. (2008). The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology & Biochemistry, 40(9), 2407–2415.
Li, X. L., Pan, G., Qin, Y. W., Hu, T. D., Wu, Z. Y., & Xie, Y. N. (2004). EXAFS studies on adsorption-desorption reversibility at manganese oxide-water interfaces II. Reversible adsorption. Journal of Colloid and Interface Science, 271(1), 28–34.
Liu, H. J., Zhang, J. L., Christie, P., & Zhang, F. S. (2008). Influence of iron plaque on uptake and accumulation of cd by rice (Oryza sativa L.) seedlings grown in soil. Science of the Total Environment, 394(2–3), 361–368.
Liu, R., Altschul, E. B., Hedin, R. S., Nakles, D. V., & Dzombak, D. A. (2014). Sequestration enhancement of metals in soils by addition of iron oxides recovered from coal mine drainage sites. Journal of Soil Contamination, 23, 374–388.
Liu, Y. Z., Xiao, T. F., Baveye, P. C., Zhu, J. M., Ning, Z. P., & Li, H. J. (2015). Potential health risk in areas with high naturally-occurring cadmium background in southwestern China. Ecotoxicology and Environmental Safety, 112, 122–131.
Liu, J. C., Wang, O. M., Li, J. J., & Liu, F. H. (2018). Mechanisms of extracellular electron transfer in the biogeochemical manganese cycle. Acta Microbiologica Sinica, 58(4), 546–559 (in Chinese).
McKeague, J. A. (1967). An evaluation of 0.1 M pyrophosphate and pyrophosphate-dithionate in comparison with oxalate as extractants of the accumulation products in Podzols and some other soils. Canadian Journal of Soil Science, 47, 95–99.
Neubauer, S. C., Toledo-Duran, G. E., Emerson, D., & Megonigal, J. P. (2007). Returning to their roots: iron-oxidizing bacteria enhance short-term plaque formation in the wetland-plant rhizosphere. Geomicrobiology, 24(1), 65–73.
Otte, M. L., Rozema, J., Koster, L., Haarsma, M. S., & Broekman, R. A. (1989). Iron plaque on roots of Aster tripolium L.: interaction with zinc uptake. New Phytologist, 111(2), 309–317.
Pietrzykowski, M., Socha, J., & van Doorn, N. S. (2014). Linking heavy metal bioavailability (Cd, Cu, Zn and Pb) in Scots pine needles to soil properties in reclaimed mine areas. Science of the Total Environment, 470-471C, 501–510.
Pinto, E., & Ferreira, I. (2015). Cation transporters/channels in plants: tools for nutrient biofortification. Journal of Plant Physiology, 179, 64–82.
Pittman, J. K. (2005). Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytologist, 167(3), 733–742.
Randall, S. R., Sherman, D. M., Ragnarsdottir, K. V., & Collins, C. R. (1999). The mechanism of cadmium surface complexation on iron oxyhydroxide minerals. Geochimica et Cosmochimica Acta, 63, 2971–2987.
Robson, T. C., Braungardt, C. B., Rieuwerts, J., & Worsfold, P. (2014). Cadmium contamination of agricultural soils and crops resulting from sphalerite weathering. Environmental Pollution, 184, 283–289.
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., Lesniewski, R. A., Oakley, B. B., Parks, D. H., & Robinson, C. J. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 75(23), 7537–7541.
Sebastian, A., & Prasad, M. N. V. (2016). Iron plaque decreases cadmium accumulation in Oryza sativa L. and serves as a source of iron. Plant Biology, 18(6), 1008–1015.
Shuman, L. M. (1982). Separating soil iron and manganese-oxide fractions for microelement analysis. Soil Science Society of America Journal., 46(5), 1099–1102.
Tack, F. M. G., Ranst, E. V., Lievens, C., & Vandenberghe, R. E. (2006). Soil solution cd, cu and Zn concentrations as affected by short-time drying or wetting: the role of hydrous oxides of Fe and Mn. Geoderma, 137(1–2), 0–89.
Taylor, G. J., Crowder, A. A., & Rodden, R. (1984). Formation and morphology of an iron plaque on the roots of Typha latifolia L. grown in solution culture. American Journal of Botany, 71(5), 666–675.
Tebo, B. M., Johnson, H. A., McCarthy, J. K., & Templeton, A. S. (2005). Geomicrobiology of manganese (II) oxidation. Trends in Microbiology, 13, 421–428.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51, 844–851.
Tong, Y., & Guo, M. L. (2007). Cloning and characterization of a novel periplasmic heme-transport protein from the human pathogenpseudomonas aeruginosa. Journal of Biological Inorganic Chemistry, 12(6), 735–750.
Weber, K. A., Achenbach, L. A., Coates, J. D. (2006). Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nature Reviews Microbiology, 4(10), 752–764.
Wu, C., Ye, Z. H., Li, H., Wu, S. C., Dan, D., Zhu, Y. G., & Wong, M. (2012). Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? Journal of Experimental Botany, 63(8), 2961–2970.
Xu, B., & Yu, S. (2013). Root iron plaque formation and characteristics under N2 flushing and its effects on translocation of Zn and Cd in paddy rice seedlings (Oryza sativa). Annals of Botany, 111(6), 1189–1195.
Xu, W., Lan, H. C., Wang, H. J., Liu, H. M., & Qu, J. H. (2015). Comparing the adsorption behaviors of cd, cu and Pb from water onto Fe-Mn binary oxide, MnO2 and FeOOH. Frontiers of Environmental Science and Engineering, 9, 385–393.
Yang, J. X., Tam, F. Y., & Ye, Z. H. (2014). Root porosity, radial oxygen loss and iron plaque on roots of wetland plants in relation to zinc tolerance and accumulation. Plant and Soil, 374(1–2), 815–828.
Ying, S. C., Kocar, B. D., & Fendorf, S. (2012). Oxidation and competitive retention of arsenic between iron- and manganese oxides. Geochimica et Cosmochimica Acta, 96, 294–303.
Yue, J. Y., Zhang, X., & Liu, N. (2017). Cadmium permeates through calcium channels and activates transcriptomic complexity in wheat roots in response to cadmium stress. Genes and Genomics, 39(2), 1–14.
Zhang, W. L., Du, Y., Zhai, M. M., & Shang, Q. (2014). Cadmium exposure and its health effects: A 19-year follow-up study of a polluted area in China. Science of the Total Environment, 470-471, 224–228.
Zhou, H., Zeng, M., Zhou, X., Liao, B. H., Peng, P. Q., Hu, M., Zhu, W., Wu, Y. J., & Zou, Z. J. (2015). Heavy metal translocation and accumulation in iron plaques and plant tissues for 32 hybrid rice (Oryza sativa L.) cultivars. Plant and Soil, 386(1–2), 317–329.
Funding
This research was financially supported by the National Natural Science Foundation of China (nos. 41201517, 41561092, 41603127, and U1612442), GDAS’ Project of Science and Technology Development (2019GDASYL-0401003 and 2018GDASCX-0928), the Science and Technology Project of Guangdong, China (2017BT01Z176, 2016TX03Z086, and 2017B030314092), and National High-tech Research and Development Projects (863 Torch Program) (nos. 2013AA06A209).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Lei, J., Tong, H. et al. Effects of Mn(II) on the Oxidation of Fe in Soils and the Uptake of Cadmium by Rice (Oryza sativa). Water Air Soil Pollut 230, 190 (2019). https://doi.org/10.1007/s11270-019-4237-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-019-4237-3