Abstract
Soil pollution with heavy metals is a major problem in industrial areas. Here, we explored whether zeolite addition to soil and indigenous arbuscular mycorrhizal fungi (AMF) can reduce cadmium (Cd) uptake from soil by bread wheat. We conducted a pot experiment, in which the effects of indigenous soil AMF, zeolite addition, and Cd spiking to soil [0, 5, 10, and 15 mg (kg soil)−1] were tested. Zeolite addition to soil spiked with 15 mg Cd kg−1 decreased the Cd uptake to grains from 11.8 to 8.3 mg kg−1 and 8.9 to 3.3 mg kg−1 in the absence and presence of indigenous AMF, respectively. Positive growth, nitrogen (N), and phosphorous (P) uptake responses to mycorrhization in Cd-spiked soils were consistently magnified by zeolite addition. Zeolite addition to soil stimulated AMF root colonization. The abundance of AMF taxa changed in response to zeolite addition to soil and soil Cd spiking as measured by quantitative polymerase chain reaction. With increasing Cd spiking, the abundance of Funneliformis increased. However, when less Cd was spiked to soil and/or when zeolite was added, the abundance of Claroideoglomus and Rhizophagus increased. This study showed that soil-indigenous AMF and addition of zeolite to soil can lower Cd uptake to the grains of bread wheat and thereby reduce Cd contamination of the globally most important staple food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil pollution with heavy metals is a major problem in industrial areas that is expected to increase globally (Chibuike and Obiora 2014), due to the extent of industrial activity (Kelly et al. 1996). High concentrations of heavy metals, such as cadmium (Cd), lead (Pb), and arsenic (As), endanger the soil flora and fauna (Garcés-Ruiz et al. 2018) and are toxic for humans. Industrial activities are the main source of toxic levels of heavy metals in soil, such as Cd. Important sources of pollution are mining and smelting, manufacturing, and waste of nickel-Cd batteries and plastics (Bernard 2008).
The use of Cd-contaminated fertilizer in agriculture can also lead to soil Cd pollution (Gruter et al. 2019). Cadmium enters agricultural soils mainly through atmospheric deposition and Cd-contaminated soil amendments, such as manure and sewage sludge (Cui et al. 2005; Gruter et al. 2019; Wang and Zhang 2018). Additionally, depending on the origin of the utilized phosphorus fertilizer, significant amounts of Cd can be unintentionally introduced to agricultural soils (Grant et al. 2002; Imseng et al. 2018). In this regard, it has been reported that phosphorous fertilizers are one of the greatest Cd sources for agricultural soils in Switzerland (0.49 to 0.57 g Cd ha−1 year−1) (Imseng et al. 2018). Afyuni et al. (pers. Com.) found that the Cd concentration in commonly used phosphorus fertilizers in Iran varied from 11.3 to 25.6 mg kg−1.
Crop management can affect the Cd phytoavailability in the soil by changing soil properties (Lair et al. 2006). Gruter et al. (2019) revealed that long-term usage of compost fertilizers can decrease the Cd phytoavailability in soil and Cd concentration in shoot and grain of bread wheat by increasing soil organic carbon, soil pH, and CEC (Gruter et al. 2019).
Human health suffers from exposure to Cd. Cadmium intoxication can lead to kidney failure, cancers, bone and lung damage, and inhibition of vitamin D synthesis (Gao et al. 2010; Nordberg et al. 2018). Therefore, it is necessary to develop strategies to reduce the phytoavailability of Cd in soil. Cadmium immobilization in soil is most promising (Wang et al. 2001), not least by zeolite addition to soil (Zorpas et al. 2000). This natural, but also synthetized, Si/Al-oxide can be used as an effective stabilizer of heavy metals in soil (Zorpas et al. 2000).
Zeolites have a great potential for usage in agriculture and for remediation of heavy metal–contaminated soils due to their high cation-exchange capacity and thus potential to absorb heavy metals and hence reduce their phytoavailability (Jakkula and Wani 2018), by creating pores for water retention while adsorbing heavy metals and hence lowering their phytoavailability (Erdem et al. 2004; Terzano et al. 2005). Additionally, zeolites are known to lower the soil pH and thereby also Cd uptake by plants (Hu et al. 2018). Lija and Kasim (2014) reported that application of formulated fertilizer with clinoptilolite zeolite enhanced the nutrient uptake by maize. Their results also indicated that application of zeolite-amended fertilizers can improve the long-term availability of K in soil due to the ability of zeolite to reversibly adsorb K, thereby, increasing the usage of K from fertilizer (Lija and Kasim 2014). It has been reported that addition of clinoptilolite zeolite to soil can enhance the availability of P and N, through ion exchange and improvement of soil structure and moisture retention (Jakkula and Wani 2018; Mumpton 1999). Improving water and nutrient uptake by the plant can enhance plant growth and thereby dilute the heavy metal concentration in the edible parts of plants contributing to healthier food.
On the contrary, biological methods to reduce heavy metal uptake of plants (bioremediation) have been evaluated because of their lower cost and smaller environmental impact (Council 1993). Today, it is known that some soil microorganisms reduce the toxicity of heavy metals by immobilizing them via absorption or uptake (Akbar 2011; Joner et al. 2000; Joner and Leyval 2000).
The most widespread and abundant plant-fungal nutritional symbiosis is the interaction formed between AMF (phylum: Glomeromycota) and the majority of crop and wild plants (Selosse and Le Tacon 1998). Although, AMF may transfer heavy metals to plants; they may also prevent uptake of toxic amounts of heavy metals by plants (Gao et al. 2010; Zhang et al. 2005). Mechanisms are dilution due to improved uptake of nutrient elements, particularly, phosphorus (P) from the soil, and growth stimulation (Cui et al. 2019; Hetrick et al. 1996), reduced Cd translocation from roots to shoots, and alteration of the pH in the rhizosphere (Cui et al. 2019; Gao et al. 2010). Also, absorption of heavy metals to extraradical hyphae of AMF (Janoušková and Pavlíková 2010; Joner and Leyval 2000) is considered important in reducing Cd uptake by mycorrhizal plants (Chen et al. 2001; Joner et al. 2000). However, root colonization and Cd toxicity to AMF are genotype-specific (Liu et al. 2014; Mani et al. 2015; Zhang et al. 2005).
Considering the importance of bread wheat in the Iranian diet and its major production area in central Iran with big industries, it seems necessary to look for a suitable strategy to reduce the concentration of dangerous heavy metals, such as Cd, in wheat grain. As combining chemical and biological soil remediation appeared promising, we investigated the effects of zeolite application and presence of AMF on Cd uptake by bread wheat, grown in Cd-spiked soil. We predicted that (1) zeolite addition to soil improves plant growth and reduces the uptake of Cd from Cd-spiked soil, (2) inoculation of wheat plants with indigenous AMF enhances plant growth and nutrient uptake and reduces Cd uptake, (3) plants grown in zeolite-amended soil become more heavily colonized by AMF, and (4) AMF taxon abundances shift in response to Cd spiking and zeolite addition.
Materials and methods
Experimental design
A complete cross-factorial experiment with the factors Cd spiking to soil [4 levels: none, 5, 10, and 15 mg Cd (kg soil)−1], addition of zeolite (2 levels: none and 1% w/w), and AMF inoculation (2 levels: none, indigenous AMF) was set up with each treatment replicated five times. The resulting 80 pots were randomly arranged in the greenhouse.
Study soil and characterization
The soil used for this experiment was collected from the top 30 cm of a field at Pakal village (33° 49′ 00″ N 49° 20′ 16″ E( located in the Markazi province in central Iran. The soil was a silty clay loam as determined by the hydrometer method (Cambardella and Elliott 1993). The soil pH in water extract was 7.1 and the electrical conductivity (EC) was 0.84 dsm−1, which were measured using a digital pH-meter (Model 691, Metrohm AG, Herisau, Switzerland) and an EC-meter (Model Ohm-644, Metrohm AG, Herisau, Switzerland), respectively (Cambardella and Elliott 1993). The soil organic C content was 0.17% which was measured using the Walkley and Black (1934). The percentage of calcium carbonate was 30%, as measured by neutralization with hydrochloric acid (HCl) and back titration with sodium hydroxide (NaOH) (Black and Hartge 1986). The concentration of DTPA-extractable Cd (Lindsay and Norvell 1978) was below the detection level of atomic absorption spectroscopy (AAS) before Cd spiking to soil.
Zeolite
The chosen zeolite was mined by the Kimiya Pars Shayankar company in Iran. The result of X-ray diffraction (XRD) analysis indicated that the zeolite is mainly clinoptilolite micronizehe, which is a hydrated aluminosilicate of alkaline and alkaline earth metals (Na, K, Ca, and Mg). The chemical composition of the applied zeolite is shown in Table 1. The zeolite added to the soil for this experiment was sterilized by autoclaving at 103 kPa and 121 °C for 2 h.
AMF inoculum
To have a field representative AMF inoculum, we used the soil beneath wheat plants that were growing in the field where we collected the soil. We used this method to have soil-indigenous AMF as it has been reported that soil-indigenous AMF stimulate plant growth and nutrient uptake more than non-indigenous species due to their familiarity with the soil. The edaphic adaptation results from long-term exposure to the soil conditions (Aghilli et al. 2014, Bae et al. 2003, Sylvia and Williams 1992).
Soil sterilization and inoculation with its indigenous microbes
The soil used for this experiment was sterilized, by autoclaving at 103 kPa and 121 °C for 2 h. Thereafter, the autoclaved zeolite was added to the soil at two levels, 0 and 1% w/w. Then, four levels of Cd (0, 5, 10, and 15 mg Cd (kg soil)−1) in the form of CdNO3 were spiked to the soils by spraying a solution on the dry soil while stirring it. Following that, half of the experimental pots were filled with 500 g of Cd-spiked soil and inoculated with AMF in the form of a fresh soil sample. To this end, 20 g field soil was placed in a layer at a depth of 3 cm from the soil surface.
After that, all sterilized soil were re-inoculated with indigenous microbes, except AMF, by applying 50 ml of a microbial filtrate per kilogram of soil. To prepare the microbial filtrate, a 2.5% (w/v) soil suspension was filtered twice through Whatman No. 1 filter papers. The microbial filtrate of the native soil was added back to the sterilized soil; soil was devoid of all bigger soil organisms, such as spores and hyphal fragments of AMF (Aghili et al. 2014a; Aghili et al. 2014b). Comparison between AMF-free soil and soil inoculated with AMF allows testing the effects of AMF on plant Cd uptake. This method was proposed by Thompson (1990) to determine the effect of AMF on Zn uptake and has since been used in many other studies on the effect of AM fungi on the uptake of different nutrients (Nazeri et al. 2013; van de Voorde et al. 2012). By autoclaving all soil, we ensured that our observations are not due to alteration to the soil as a consequence of autoclaving.
Experimental setup and growth conditions
Kernels of Triticum aestivum cv. Backcross, an Iranian wheat cultivar, commonly grown in central Iran (Khoshgoftarmanesh et al. 2009) were surface-sterilized with 15% H2O2 for 15 min thoroughly rinsed in distilled water. After pre-germination on moistened filter paper for 4 days, five seedlings were planted into each pot of 500 g soil and later thinned to the two most vigorous growing plants.
The climate conditions in the greenhouse were adjusted to a 14-h photoperiod, relative air humidity of 40–45%, and day/night temperature of 22/17 °C. Soil moisture was kept at 70% water holding capacity during the experiment by daily watering.
Harvest and nutrient analysis
The wheat plants were harvested at grain maturity, 130 days after planting. Grains and aboveground parts of the plants referred to as straw (i.e., the two plants) were separated from the root fraction, rinsed, dried at 75 °C for 72 h, and weighted to determine the dry biomass.
For the chemical analyses, the plant samples were milled and 100 mg aliquots was incinerated at 550 °C for 6 h, and the ashes were dissolved in 1 ml of 13 M HNO3 at 220 °C for 1 min (Aghili et al. 2014a). The concentrations of Cd in these samples were determined by atomic absorption spectrometry (AAS; PerkinElmer 3030; PerkinElmer, Wellesley, MA, USA). The accuracy of the Cd analyses was checked by analyzing certified standard materials and including blanks in all batches of separately processed samples.
The total P and N contents of the grain fraction were determined by spectrophotometry (model UV-1601, Shimadzu Corp., Kyoto, Japan) (Ohno and Zibilske 1991) and a semi-micro Kjeldahl digestion method (Bremner 1996)
Microscopic and molecular genetic quantification of AMF root colonization
Roots were recovered with a cork-borer at anthesis to quantify the levels of root colonization by AMF. The sampled roots were washed free of soil and cut into 1 cm pieces. Thereafter, one fraction of the roots was stained with 0.05% trypan blue in lactic acid, following alkaline maceration, and the levels of mycorrhizal colonization were assessed according to McGonigle et al. (1990), by evaluating 50 intersections of roots with a line in the eyepiece per sample.
The total DNA of the roots of the plants growing in non-sterile soil was extracted, using the DNeasy Plant MiniKit (QIAGEN, Hilden Germany), following the manufacturer’s procedure. The abundance of four AMF taxa was quantified by real-time PCR (qPCR). The employed qPCR assays (Thonar et al. 2011) targeted the nuclear ribosomal large subunit RNA gene (nrLSU, 28S).
Data and statistical analyses
The growth and nutrient uptake response to mycorrhization by the naturally occurring AMF was calculated as the ratio of measurements of individual mycorrhized experimental units and the mean of the respective non-mycorrhized units of each experimental treatment (Watts-Williams and Cavagnaro 2012).
To statistically test the effects of the experimental factors “presence of AMF,” “zeolite addition to soil,” and “Cd spiking to soil” on plant biomass, grain N, P, and Cd uptake and concentration, three-way fixed factor analyses of variance (ANOVA) were run. To test the effect of zeolite addition and Cd spiking to soil on the root length colonized by AMF, AMF taxon abundance and response to mycorrhization two-way fixed factor analyses of variance were used. In case of significant effects, least square difference (LSD) tests were run for mean comparison.
Normal distribution of residuals and equal variance assumptions were verified by Shapiro-Wilcoxon and Leven’s tests, respectively. If required, the data were logarithm, square-roots, or arcsin-square root-transformed.
All statistical analyses were done using the software package SPSS version17 (SPSS Inc., Chicago, IL, USA). The figures were drawn in SigmaPlot version 12 [Systat Software Inc. (SSI), San Jose, CA, USA].
Results
Plant biomass and grain Cd concentration
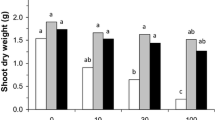
The shoot biomass and grain yield were affected by all experimental factors (Fig. 1A–B, Table 2). The highest shoot biomass [10.6 g (kg soil)−1] was recorded for plants grown in non-Cd-spiked soil to which 1% w/w of zeolite was added and in the presence of AMF (Fig. 1A). In contrast, the lowest shoot biomass (3.6 g (kg soil)−1) was measured for plants grown in soil spiked with 15 mg Cd kg−1 to which zeolite was added (Fig. 1A).
Grain yield (A), shoot (i.e., straw and grain) (B), and grain cadmium (Cd) concentration (C) of bread wheat plants grown in differently treated soil. The soil was differentially spiked with Cd and emended with zeolite and arbuscular mycorrhizal fungi (AMF) or not. Bars show mean values and associated standard errors of five experimental replicates. Different letters indicate statistical differences According to a least significant difference test, following a significant three-way analysis of variance (p < 0.05). For the full statistical details, see Table 2. Note: The Cd concentrations in the grains of the plants grown in soil not spiked with Cd are not shown because they were below the detection limit
The Cd concentration in the grain differed significantly across all treatments (Table 2, Fig. 1C). The lowest grain Cd concentration (0.26 mg (kg DW)−1) was recorded for plants grown in soil spiked with the lowest rate of Cd to which zeolite was added and that contained AMF. The highest concentration of Cd in grain [10.3 mg (kg DW−1)] was observed for plants grown in soil to which most Cd was spiked and to which no zeolite was added and that lacked AMF (Fig. 1C).
Addition of zeolite to soil reduced the differences between the plant biomass and Cd concentrations of those plants that had inoculated with AMF and those that had not, pointing in a statistically significant interaction between zeolite addition to soil and the presence of AMF (Fig. 1A–C).
Cd, P, and N uptake to grain
Addition of zeolite to soil and the presence of AMF in soil significantly reduced Cd allocation to the grain (Fig. 2A, Table 2). Spiking more Cd to soil increased Cd uptake to grain (Fig. 2A). The interactions between the experimental factors significantly changed the Cd allocation to the grain (Fig. 2A). The lowest grain Cd uptake (1.1 mg (kg soil)−1) was recorded for plants grown in soil to which least Cd was spiked and zeolite was added and that contained AMF. The highest Cd uptake (11.8 mg (kg soil)−1) was observed for plants grown in soil to which the highest amount of Cd was spiked and to which no zeolite was added and that lacked AMF (Fig. 2A).
Grain cadmium (Cd, A), grain phosphorous (P, B), and grain nitrogen (N, C) uptake of bread wheat plants grown in differently treated soil. The soil was differentially spiked with Cd and emended with zeolite and arbuscular mycorrhizal fungi (AMF) or not. Bars show mean values and associated standard errors of five experimental replicates. Different letters indicate statistical differences according to a least significant difference test, following a significant three-way analysis of variance (p < 0.05). For the full statistical details, see Table 2. Note: The Cd concentrations in the grains of the plants grown in soil not spiked with Cd are not shown because they were below the detection limit
Nitrogen uptake to the grain was significantly higher in soil containing AMF and to which zeolite was added, compared with the AMF-free soil without application of zeolite (Fig. 2B). Spiking Cd to soil reduced N uptake to grain (Fig. 2B). Phosphorus uptake to grain was stimulated by AMF inoculation and addition of zeolite to soil, but reduced by spiking the soil with Cd (Fig. 2C). Highest P uptake to grain was recorded for plants grown in soil containing AMF and after addition of zeolite and no Cd spiking (Fig. 2C). In contrast, the lowest amount of P uptake to grain was observed in plants grown in AMF-free soil which was spiked with the highest amount of Cd and to which no zeolite was added (Fig. 2C). Also, both N and P uptake to grain were significantly affected by the interaction effect of the experimental factors (Fig. 2B–C).
Root-to-shoot Cd translocation and Cd:P and Cd:N ratios in grain
The Cd translocation from root to shoot (i.e., straw and grain) ranged between 34 and 72%. It differed among all treatments (Fig. 3A, Table 2). Addition of zeolite to soil lowered the root-to-shoot Cd translocation, as did the presence of indigenous AMF (Fig. 3A).
Root:shoot (i.e., straw and grain) translocation of cadmium (Cd A), grain cadmium (Cd), phosphorus (P) ratio (B), and grain Cd:nitrogen (N) ratio (C) of bread wheat plants grown in differently treated soil. The soil was differentially spiked with Cd and emended with zeolite and arbuscular mycorrhizal fungi (AMF) or not. Bars show mean values and associated standard errors of five experimental replicates. Different letters indicate statistical differences according to a least significant difference test, following a significant three-way analysis of variance (p < 0.05). For the full statistical details, see Table 2. Note: The Cd concentrations in the grains of the plants grown in soil not spiked with Cd are not shown because they were below the detection limit
Addition of zeolite to soil and the presence of indigenous AMF reduced the Cd:P and Cd:N ratios in the grain (Table 2, Fig. 3B–C). The highest Cd:P and Cd:N ratios in the grain (5.4 × 10−3 and 9.5 × 10−4 respectively) were recorded for plants grown on AMF-free soil spiked with the highest amount of Cd and when no zeolite was added to the soil (Fig. 3B–C).
Root colonization by AMF
Wheat plants raised in sterilized soil showed no sign of AMF root colonization. The percentage of wheat root length colonized by AMF increased when 5 mg Cd kg−1 was spiked to soil and zeolite was added, but dropped when more Cd was spiked to the soil (Fig. 4, Table 3). Addition of zeolite to soil increased AMF root colonization (Fig. 4).
Arbuscular mycorrhizal fungal (AMF) root colonization of bread wheat plants grown in differently treated soil. The soil was differentially spiked with Cd and emended with zeolite and arbuscular mycorrhizal fungi (AMF) or not. Bars show mean values and associated standard errors of five experimental replicates. Different letters indicate statistical differences According to a least significant difference test, following a significant two-way analysis of variance (p < 0.05). For the full statistical details, see Table 3
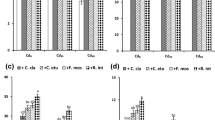
Spiking Cd to soil increased the abundance of Funneliformis particularly when no zeolite was added to the soil (Fig. 5A). On the contrary, Cd spiking to soil strongly reduced the abundance of Claroideoglomus, Diversispora, and Rhizophagus in the roots and zeolite addition to soil did not alleviate this detrimental effect much (Fig. 5B–D), although it increased the overall root colonization by AMF (Fig. 4).
Quantitative abundance of arbuscular mycorrhizal fungi of the genera Funneliformis (A), Rhizophagus (B), Claroideoglomus (C), and Diversispora (D) in the roots of bread wheat plants grown in differently treated soil as quantified by real-time PCR with taxon-specific assays. The soil was differentially spiked with Cd and emended with zeolite or not. Bars show mean values and associated standard errors of five experimental replicates. Different letters indicate statistical differences according to a least significant difference test, following a significant two-way analysis of variance (p < 0.05). For the full statistical details, see Table 3
Response to mycorrhization
The response of grain biomass and grain P and N uptake to mycorrhization was all positive and stimulated by zeolite addition and Cd spiking to soil (Fig. 6A, C, D, Table 4). The response in grain biomass to mycorrhization was consistently positive with 16 to > 75% increases when no zeolite was added to the soil and > 185% increases when zeolite was added to the soil (Fig. 6A). Zeolite addition to soil also led to a positive response to mycorrhization in N uptake to grain of wheat plants (Fig. 6C).
Percentage response of grain yield (A), shoot (i.e., straw and grain) biomass (B), grain cadmium Cd concentration (C) and grain Cd (D), grain P (E), and grain N (F) uptake of bread wheat plants grown in differently treated soil to mycorrhization by naturally occurring arbuscular mycorrhizal fungi. The soil was differentially spiked with Cd and emended with zeolite or not. Bars show mean values and associated standard errors of five experimental replicates. Different letters indicate statistical differences according to a least significant difference test, following a significant two-way analysis of variance (p < 0.05). For the full statistical details, see Table 4. Note: The Cd concentrations in the grains of the plants grown in soil not spiked with Cd are not shown because they were below the detection limit
The response in P uptake to mycorrhization was most prominent and positive with 140 to 200% increases when zeolite was added to the soil, and between 91 and 125% increases when no zeolite was added to the soil (Fig. 6D).
Mycorrhization significantly reduced Cd uptake to grain, particularly after zeolite addition to soil and at the high amounts of spiked Cd (Fig. 6E). After zeolite addition to soil, there was a 58–65% mycorrhiza-dependent reduction in grain Cd uptake, but when no zeolite was added to soil, there was only a 24–33% mycorrhiza-dependent reduction (Fig. 6E).
The response in grain Cd concentration to mycorrhization was significantly affected by zeolite addition and Cd spiking to the soil (Fig. 6F). In zeolite-amended soil, there was a 64–87% reduction in grain Cd concentration, but in soil to which no zeolite was added, the reduction in the grain Cd concentration was only 41–62% (Fig. 6F).
Discussion
The results confirmed our prediction that zeolite addition to Cd-spiked soil together with AMF root colonization promotes wheat grain yield and reduces grain Cd concentration. Zeolite was reported to promote plant growth by retaining water and mineral nutrients in plant- and AMF-accessible forms and by absorbing Cd and thereby reducing Cd toxicity (Erdem et al. 2004; Lija and Kasim 2014).
The study further showed that the roots of plants grown in zeolite-amended soil become more heavily colonized by AMF, but only in Cd-spiked soil. The abundance of different AMF taxa depended on zeolite addition to soil and Cd spiking. Our experiment shows that zeolite addition to soil spiked with Cd can promote N and P uptake to grain especially when plants are mycorrhized.
The reduced grain Cd concentration and Cd uptake and increased plant biomass and nutrient uptake from Cd-spiked and zeolite-amended soil containing AMF (Figs. 1, 2, and 3) must be explained by different chemical soil conditions, increased root growth, and/or an overall improved plant nutrition.
Addition of zeolite to soil and root colonization by AMF reduced the Cd concentration in grains of bread wheat
AMF root colonization and zeolite addition to soil reduced grain Cd concentration and Cd uptake (Figs. 1C and 2A). According to the Codex Alimentarius of the FAO and WHO, the maximum allowable Cd concentration in wheat grain is 0.2 mg (kg FW)−1. FAO/WHO (2012) AMF root colonization and addition of zeolite to soil spiked with 5 mg Cd (kg soil)−1 reduced the grain Cd concentration from 0.75 mg (kg FW)−1 to 0.24 mg (kg FW)−1, thus close to the tolerance level for safe human consumption. Safari et al. (2018) reported that 19 of 65 harvested wheat grain samples from the Zanjan plain in Iran had a higher Cd concentration than the maximally allowed value of 0.2 mg kg−1 because they were grown on Cd-polluted land [> 5 mg (kg soil)−1].
The arbuscular mycorrhizal symbiosis thus appears to alleviate heavy metal toxicity. The mechanisms of detoxification may, however, differ under different conditions (Chen et al. 2004; Cui et al. 2019). Arbuscular mycorrhizal fungi reduce plant Cd levels through growth or Cd retention/immobilization (Huang et al. 2017; Cui et al. 2019). The present study showed that AMF can increase shoot biomass and grain yield and that zeolite addition to soil is promoting it (Fig. 1A–B). The better growth of mycorrhized plants, compared with non-mycorrhized plants, translated into higher grain yield (Fig. 6A–B). This seems due to better plant P and N nutrition, especially after the soil was amended with zeolite (Fig. 6C–D). Some studies on heavy metal–polluted soils showed that root colonization by AMF can protect plants against heavy metal toxicity by supporting the plants in mineral nutrient and water uptake (Joner and Leyval 2000), something that is particularly important in dry calcareous soil, because of the low bioavailability of P and micronutrients, such as Zn (Akbar 2011).
Another mechanism of AMF reducing the toxicity of heavy metals for plants is their ability to selectively transport elements. Arbuscular mycorrhizal fungus-mediated nutrient acquisition occurs in different steps, namely uptake, translocation, and transfer. Reduced root-to-shoot translocation may occur when there is intravacuolar precipitation of metallic cations with polyphosphate in the fungus (Joner et al. 2000); heavy metals may further bind to the chitin of fungal cell walls (Zhou 1999). Furthermore, AMF may alter the mobility of heavy metals indirectly via changes to rhizosphere pH (LI et al. 1991), root exudation (Laheurte et al. 1990), and/or soil microbial communities (Olsson et al. 1998).
Our analyses showed that shoot, i.e., straw and grain, Cd translocation was reduced in mycorrhized, compared with not mycorrhized plants, a difference that was larger in zeolite-amended soil (Fig. 3A). Li and Christie (2001) suggested that AMF decrease root-to-shoot Cd translocation by immobilizing heavy metals in and on fungal biomass (Li and Christie 2001). Reduced Cd:P and Cd:N ratios in mycorrhized, compared with not mycorrhized plants (Fig. 3B–C), point at element fractionation by AMF (Joner and Leyval 2000). Since addition of zeolite to soil additionally decreased the ratio of Cd:P and Cd:N, it seems that zeolite also alters the availability of P, N, and Cd in the soil (Jakkula and Wani 2018), and reduces Cd toxicity to the benefit of AMF.
Furthermore, the significant interaction between experimental factors (Tables 2, 3, and 4 and Figs. 1, 2, 3, 4, 5, and 6) pointing at triple synergistic (i.e., more than additive/positive interactive) effects of soil-indigenous AMF and zeolite addition to soil on N and P nutrition of bread wheat as well as a reduction of root-to-shoot Cd translocation as a consequence of mycorrhization are responsible for the increased grain yield and reduced grain Cd concentration and uptake.
Changes to root length colonized by AMF and AMF taxon abundance
Some previous studies showed that AMF root colonization can be reduced or even prevented by high concentrations of heavy metals (Miransari 2011; Mozafar et al. 2002; Weissenhorn and Leyval 1995). But other studies reported that high Cd concentrations in soil do not affect AMF root colonization (Chen et al. 2004; de Andrade et al. 2008).
The results of our study indicated that Cd spiking to soil at a rate of > 10 mg (kg soil)−1 reduced colonization by AMF and that zeolite addition to soil could ameliorate this toxicity effect (Fig. 4). Del Val et al. (1999) revealed that as the heavy metal concentrations increase in soil, the number of AMF spores decreases. Biró (2005) quantified AMF root colonization in barley growing in soils amended with different heavy metals including Al, As, Ba, Cd, Cr, Cu, Hg, Ni, Pb, Se, Sr, and Zn at four levels, 0, 30, 90, and 270 mg (kg soil)−1 after 12 years of exposure and found that their sporulation was most sensitive to heavy metal toxicity, as it declined with increasing concentrations of the heavy metals, except Ni (Biró 2005).
We found that increased Cd spiking to soil in the absence of zeolite increased the abundance of Funneliformis (Fig. 6A). This could be explained by Cd immobilization by this species (Joner et al. 2000). The abundance of Rhizophagus increased only in response to addition of 5 mg Cd kg−1 soil, but declined when more Cd was spiked to soil (Fig. 5B), suggesting that this AMF taxon is only moderately tolerant to soil Cd contamination.
Zarei et al. (2010) studied the AMF diversity in roots under heavy metal contamination and revealed that differences in soil properties and concentration of heavy metals relate with differences in AMF community composition and structure in roots of several dominant plant species in the Anguran mining region (Zarei et al. 2010). Funneliformis and Rhizophagus were reported as highly and moderately tolerant taxa to heavy metal contamination, respectively (Zarei et al. 2010). Heavy metal tolerance of AMF and their effects on plant growth, nutrient, and heavy metal uptake is known to vary among plants (Liao et al. 2003; Wang et al. 2007; Zhang et al. 2005). It was reported that Funneliformis can promote maize plant growth and reduce the Cd translocation from root to shoot via Cd immobilization in roots (Chen et al. 2004; Liang et al. 2009). Claroideoglomus, on the contrary, was reported to even reduce the biomass of maize and increase root-to-shoot Cd translocation (Liao et al. 2003; Wang et al. 2007). Diaz et al. (1996) who studied the uptake of Zn and Pb by Lygeum spartum L. and Anthyllis cytisoides in response to inoculation of Funneliformis mosseae and Glomus microcarpum did not find any difference in Pb and Zn concentration between mycorrhized and not mycorrhized control plants at low heavy metal concentrations. However, at higher heavy metal contamination, the plants inoculated with Funneliformis mosseae took up less heavy metals than the not inoculated control. In contrast, the same or even higher concentrations of the heavy metals were found in plants inoculated with G. microcarpum than such not inoculated.
These contradictory pieces of information suggest that AMF need to be carefully selected for inoculation, accounting for soil conditions, type and concentration of the pollutant, and the availability of mineral nutrients to the fungi and plants. Zeolite was found as an optimal soil conditioner to reduce plant Cd uptake.
Implications for agronomic usage of AMF and zeolite
Agronomic approaches to immobilize and precipitate heavy metals aim at reducing the solubility and mobility of heavy metals in soil and thereby at reducing the heavy metal uptake to edible parts of crop plants (Abbaspour and Golchin 2010; Lair et al. 2006). Heavy metals can be immobilized via organic soil amendments (Brazauskiene et al. 2008; Sánchez-Martín et al. 2007), or addition of chemicals (Erdem et al. 2004; Hu et al. 2018). However, in spite of promising reports that AMF reduce the Cd concentration in crop plants (Chen et al. 2004, Liang et al. 2009), our study clearly showed that there are physiological limits to when and the degree to which AMF reduce Cd concentration in bread wheat. A strong reduction in grain Cd concentration of plants grown in Cd-spiked soil was only observed after additional zeolite addition to soil. This points at either Cd toxicity for AMF or growth stimulation of AMF by zeolite.
The AMF taxon abundance was differentially affected by Cd spiking and zeolite addition to soil, pointing at taxon-specific Cd toxicity and/or stimulation by zeolite addition. Whether our findings relate to field conditions remains to be verified, since conditions in pots in the glasshouse are different in respect to lighting, irrigation, and the space for root growth. Furthermore, contamination with just one heavy metal is not representative for heavy metal contamination in the field. Lastly, it remains unclear what effect the autoclaving and microbial re-inoculation had on the interaction of AMF with the soil.
Therefore, the meaning of the findings of this study for those under actual field conditions for application remains unclear, although it showed that naturally occurring AMF are capable to reduce Cd toxicity for crop plants, particularly when the soil conditions are improved by zeolite addition.
Conclusion
The results of the present study suggest that zeolite addition to soil with indigenous AMF can improve grain yield of bread wheat produced on Cd-polluted soil. The efficiency of AMF to reduce Cd uptake and promote growth and nutrient uptake by bread wheat increased with the addition of zeolite to soil.
The total root colonization by AMF declined when more Cd was spiked to the soil and the abundance of Funneliformis increased, while Rhizophagus seemed to benefit particularly from the addition of zeolite to the soil. This shows that soil-indigenous AMF and the addition of zeolite to soil generally show synergistic effects, reducing the Cd load of bread wheat, a major quality impairment to the most important staple food. The logical next step will be to verify the findings of this pot study in the field.
References
Abbaspour A, Golchin A (2010) Immobilization of heavy metals in a contaminated soil in Iran using di-ammonium phosphate, vermicompost and zeolite. Environ Earth Sci 63:935–943
Aghili F, Gamper HA, Eikenberg J, Khoshgoftarmanesh AH, Afyuni M, Schulin R, Jansa J, Frossard E (2014a) Green manure addition to soil increases grain zinc concentration in bread wheat. PLoS One 9:1–3
Aghili F, Jansa J, Khoshgoftarmanesh AH, Afyuni M, Schulin R, Frossard E, Gamper HA (2014b) Wheat plants invest more in mycorrhizae and receive more benefits from them under adverse than favorable soil conditions. Appl Soil Ecol 84:93–111
Akbar K (2011) Arbuscular mycorrhizal fungi and heavy metal contaminated soils. Afr J Microbiol Res 5:1571–1576
Bae Y, Fukushima S, Harada A, Kataoka K (2003) Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem 115:4788–4791
Bernard A (2008) Cadmium and its adverse effects on human health. Indian J Med Res 128:557
Biró B (2005) Mycorrhizal functioning as part of the survival mechanisms of barley (Hordeum vulgare L.) at long-term heavy metal stress. Acta Biol Szeged 49:65–67
Black G, Hartge K (1986) Bulk density. Methods of soil analysis part I: physical and mineralogical methods. 2nd edn. American Society of Agronomy, Inc. Soil Science Society of America, Inc., Madison
Brazauskiene D-M, Paulauskas V, Sabiene N (2008) Speciation of Zn, Cu, and Pb in the soil depending on soil texture and fertilization with sewage sludge compost. J Soils Sediments 8:184–192
Bremner J (1996) Nitrogen-total. Methods of soil analysis part 3—chemical methods, pp 1085–1121
Cambardella C, Elliott E (1993) Methods for physical separation and characterization of soil organic matter fractions, soil structure/soil biota interrelationships. Geoderm 56:449–457
Chen B, Christie P, Li X (2001) A modified glass bead compartment cultivation system for studies on nutrient and trace metal uptake by arbuscular mycorrhiza. Chemospher 42:185–192
Chen B, Liu Y, Shen H, Li X, Christie P (2004) Uptake of cadmium from an experimentally contaminated calcareous soil by arbuscular mycorrhizal maize (Zea mays L.). Mycorrhiza 14:347–354
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:1–12
Council NR (1993) In situ bioremediation: when does it work? National Academies Press
Cui Y, Zhu Y-G, Zhai R, Huang Y, Qiu Y, Liang J (2005) Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ Int 31:784–790
Cui G, Ai S, Chen K, Wang X (2019) Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol Environ Saf 171:231–239
De Andrade SAL, da Silveira APD, Jorge RA, de Abreu MF (2008) Cadmium accumulation in sunflower plants influenced by arbuscular mycorrhiza. Int J Phytoremediat 10:1–13
Del Val C, Barea J, Azcon-Aguilar C (1999) Diversity of arbuscular mycorrhizal fungus populations in heavy-metal-contaminated soils. Appl Environ Microbiol 65:718–723
Diaz G, Azcón-Aguilar C, Honrubia M (1996) Influence of arbuscular mycorrhizae on heavy metal (Zn and Pb) uptake and growth of Lygeum spartum and Anthyllis cytisoides. Plant Soil 180:241–249
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
FAO/WHO (2012) Joint FAO/WHO food standards programme, Codex committee on contaminants in foods. FAO, Maastricht
Gao X, Akhter F, Tenuta M, Flaten DN, Gawalko EJ, Grant CA (2010) Mycorrhizal colonization and grain Cd concentration of field-grown durum wheat in response to tillage, preceding crop and phosphorus fertilization. J Sci Food Agric 90:750–758
Garcés-Ruiz M, Senés-Guerrero C, Declerck S, Cranenbrouck S (2018) Community composition of arbuscular mycorrhizal fungi associated with native plants growing in a petroleum-polluted soil of the Amazon region of Ecuador. Microbiol Open 2018:e703. https://doi.org/10.1002/mbo3.703
Grant CA, Bailey LD, Harapiak JT, Flore NA (2002) Effect of phosphate source, rate and cadmium content and use of Penicillium bilaii on phosphorus, zinc and cadmium concentration in durum wheat grain. J Sci Food Agric 82:301–308
Gruter R, Costerousse B, Mayer J, Mader P, Thonar C, Frossard E, Schulin R, Tandy S (2019) Long-term organic matter application reduces cadmium but not zinc concentrations in wheat. Sci Total Environ 669:608–620
Hetrick B, Wilson G, Todd T (1996) Mycorrhizal response in wheat cultivars: relationship to phosphorus. Can J Bot 74:19–25
Hu Q, Zeng W-A, Li F, Huang Y, Gu S, Cai H, Zeng M, Li Q, Tan L (2018) Effect of nano zeolite on the transformation of cadmium speciation and Its uptake by tobacco in cadmium-contaminated soil. Open Chem 16:667–673
Huang X, Ho S-H, Zhu S, Ma F, Wu J, Yang J, Wang L (2017) Adaptive response of arbuscular mycorrhizal symbiosis to accumulation of elements and translocation in Phragmites australis affected by cadmium stress. J. Environ Manag 197:448–455
Imseng M, Wiggenhauser M, Keller A, Muller M, Rehkamper M, Murphy K, Kreissig K, Frossard E, Wilcke W, Bigalke M (2018) Fate of Cd in agricultural soils: a stable isotope approach to anthropogenic impact, soil formation, and soil-plant cycling. Environ Sci Technol 52:1919–1928
Jakkula V, Wani S (2018) Zeolites: potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci Rev Chem Commun 8:1–15
Janoušková M, Pavlíková D (2010) Cadmium immobilization in the rhizosphere of arbuscular mycorrhizal plants by the fungal extraradical mycelium. Plant Soil 332:511–520
Joner EJ, Leyval C (2000) Bioavailability of heavy metals in the mycorrhizosphere, trace elements in the rhizosphere. CRC Press, pp 173–193
Joner EJ, Briones R, Leyval C (2000) Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 226:227–234
Kelly J, Thornton I, Simpson P (1996) Urban geochemistry: a study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and non-industrial areas of Britain. Appl Geochem 11:363–370
Khoshgoftarmanesh AH, Sadrarhami A, Sharifi HR, Afiuni D, Schulin R (2009) Selecting zinc-efficient wheat genotypes with high grain yield using a stress tolerance index. Agron J 101:1409–1416
Laheurte F, Leyval C, Berthelin J (1990) Root exudates of maize, pine and beech seedlings influenced by mycorrhizal and bacterial inoculation. Symbiosis (Rehovot) 9:111–116
Lair GJ, Gerzabek MH, Haberhauer G, Jakusch M, Kirchmann H (2006) Response of the sorption behavior of Cu, Cd, and Zn to different soil management. J. Plant Nut. Soil Sci 169:60–68
Li X, Christie P (2001) Changes in soil solution Zn and pH and uptake of Zn by arbuscular mycorrhizal red clover in Zn-contaminated soil. Chemosphere 42:201–207
Li XL, George E, Marschner H (1991) Phosphorus depletion and pH decrease at the root–soil and hyphae–soil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytol 119:397–404
Liang C-C, Li T, Xiao Y-P, Liu M-J, Zhang H-B, Zhao Z-W (2009) Effects of inoculation with arbuscular mycorrhizal fungi on maize grown in multi-metal contaminated soils. Int J Phytoremediation 11:692–703
Liao J, Lin X, Cao Z, Shi Y, Wong M (2003) Interactions between arbuscular mycorrhizae and heavy metals under sand culture experiment. Chemosphere 50:847–853
Lija M, Kasim A (2014) Maize (Zea mays L.) nutrient use efficiency as affected by formulated fertilizer with Clinoptilolite Zeolite. Emir J Food Agric 26:284–292
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper 1. Soil Sci Soc Am J 42:421–428
Liu L, Gong Z, Zhang Y, Li P (2014) Growth, cadmium uptake and accumulation of maize (Zea mays L.) under the effects of arbuscular mycorrhizal fungi. Ecotox 23:1979–1986
Mani D, Kumar C, Patel NK (2015) Integrated micro-biochemical approach for phytoremediation of cadmium and zinc contaminated soils. Ecotoxicol Environ Saf 111:86–95
McGonigle T, Miller M, Evans D, Fairchild G, Swan J (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Miransari M (2011) Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol Adv 29:645–653
Mozafar A, Ruh R, Klingel P, Gamper H, Egli S, Frossard E (2002) Effect of heavy metal contaminated shooting range soils on mycorrhizal colonization of roots and metal uptake by leek. Environ Monit Assess 79:177–191
Mumpton FA (1999) La roca magica: uses of natural zeolites in agriculture and industry. Proc Natl Acad Sci 96:3463–3470
Nazeri NK, Lambers H, Tibbett M, Ryan MH (2013) Do arbuscular mycorrhizas or heterotrophic soil microbes contribute toward plant acquisition of a pulse of mineral phosphate? Plant Soil 373:699–710
Nordberg GF, Bernard A, Diamond GL, Duffus JH, Illing P, Nordberg M, Bergdahl IA, Jin T, Skerfving S (2018) Risk assessment of effects of cadmium on human health (IUPAC technical report). Pure Appl Chem 90:755–808
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. SSSAJ 55:892–895
Olsson P, Francis R, Read D, Söderström B (1998) Growth of arbuscular mycorrhizal mycelium in calcareous dune sand and its interaction with other soil microorganisms as estimated by measurement of specific fatty acids. Plant Soil 201:9–16
Safari Y, Delavar MA, Zhang C, Noori Z, Rahmanian M (2018) Assessing cadmium risk in wheat grain using soil threshold values. Int J Environ Sci Technol 15:887–894
Sánchez-Martín M, García-Delgado M, Lorenzo L, Rodríguez-Cruz M, Arienzo M (2007) Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 142:262–273
Selosse M-A, Le Tacon F (1998) The land flora: a phototroph-fungus partnership? Trends Ecol Evol 13:15–20
Sylvia DM, Williams SE (1992) Vesicular-arbuscular mycorrhizae and environmental stress. Mycorrhizae Sustain Agric 25:101–124
Terzano R, Spagnuolo M, Medici L, Tateo F, Ruggiero P (2005) Zeolite synthesis from pre-treated coal fly ash in presence of soil as a tool for soil remediation. Appl Clay Sci 29:99–110
Thompson J (1990) Soil sterilization methods to show VA-mycorrhizae aid P and Zn nutrition of wheat in vertisols. Soil Biol Biochem 22:229–240
Thonar C, Schnepf A, Frossard E, Roose T, Jansa J (2011) Traits related to differences in function among three arbuscular mycorrhizal fungi. Plant Soil 339:231–245
van de Voorde TF, van der Putten WH, Bezemer TM (2012) Soil inoculation method determines the strength of plant–soil interactions. Soil Biol Biochem 55:1–6
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wang M, Zhang H (2018) Accumulation of heavy metals in roadside soil in urban area and the related impacting factors. Int J Environ Res Public Health 15:1064–1078
Wang Y, Chen T, Yeh K, Shue M (2001) Stabilization of an elevated heavy metal contaminated site. J. Hazard Mater 88:63–74
Wang FY, Lin XG, Yin R (2007) Effect of arbuscular mycorrhizal fungal inoculation on heavy metal accumulation of maize grown in a naturally contaminated soil. Int J Environ Res Public Health 15:1–11
Watts-Williams SJ, Cavagnaro TR (2012) Arbuscular mycorrhizas modify tomato responses to soil zinc and phosphorus addition. Biol Fertil Soils 48:285–294
Weissenhorn I, Leyval C (1995) Root colonization of maize by a Cd-sensitive and a Cd-tolerant Glomus mosseae and cadmium uptake in sand culture. Plant Soil 175:233–238
Zarei M, Hempel S, Wubet T, Schafer T, Savaghebi G, Jouzani GS, Nekouei MK, Buscot F (2010) Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environ Pollut 158:2757–2765
Zhang X, Zhu Y-G, Chen B, Lin A, Smith S, Smith F (2005) Arbuscular mycorrhizal fungi contribute to resistance of upland rice to combined metal contamination of soil. J Plant Nutr 28:2065–2077
Zhou J (1999) Zn biosorption by Rhizopus arrhizus and other fungi. Appl Microbiol Biotechnol l51:686–693
Zorpas A, Constantinides T, Vlyssides A, Haralambous I, Loizidou M (2000) Heavy metal uptake by natural zeolite and metals partitioning in sewage sludge compost. Bioresour Technol 72:113–119
Acknowledgments
The authors would like to thank the Arak Brach, Islamic Azad University, for its support. We appreciate the critical comments by two anonymous reviewers that helped us a lot to improve and clarify the main message of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baghaie, A.H., Aghili, F. & Jafarinia, R. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ Sci Pollut Res 26, 30794–30807 (2019). https://doi.org/10.1007/s11356-019-06237-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06237-0