Abstract
Siderophores are small molecular weight metal scavengers which are released by plants, plant growth-promoting bacterial strains and fungi into the rhizosphere. These molecules have been widely reported as Fe3+ carriers under poor iron ion mobilization; however, recently they are being exposed for affinity towards other metal ions such as copper, zinc, etc. highlighting their phytoremedial potential. They are also effective anti-pathogenic agents, important signals towards oxidative stress and new age therapeutics. To understand the mechanism by which these moieties solubilize metal ions at both genetic and protein levels is the crux of our studies as these are extremely versatile molecules having myriad applications in the fields of agriculture, physiology, drug therapy, diagnosis, etc. Additionally, this paper also covers the biosynthesis and classification of microbial siderophores and their roles in plant and animal physiology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
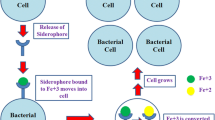
Since the past six decades, various studies have been focused on some 500 small low-molecular-mass (≤10 kDa) molecules called the siderophores which are secreted by both plants and microbes into the rhizosphere (Hider and Kong 2010; Ahmed and Holmström 2014; Johnstone and Nolana 2015). The bacterial siderophores possess higher affinity for metal ions (especially ferric ions or Fe+3) as compared to phytosiderophores (mainly mugineic acid) and are often present in lower concentrations (Kraemer 2004; Kraemer et al. 2006; Glick 2012). Many physiochemical factors such as ligand-binding sites onto metal ion, denticity, pH, redox, etc. govern the metal-binding ability of these molecules (Akafia et al. 2014). Their role as metal scavengers in the rhizosphere is very well known specially for iron ions (Aznar and Dellagi 2015). Other pertinent functions of siderophores include their antibiotic activity against many resistant bacterial strains and potential superbugs as sideromycins (Braun et al. 2009). They possess strong affinity for non-iron metal ions such as copper, manganese, molybdenum, vanadium, zinc, etc. (Hood and Skar 2012). Siderophores from bacterial strains bind to Zn to form zincophores or tsinkophores (Prentice et al. 2007), Pseudomonad strains show affinity for Mn (Harrington et al. 2012; Duckworth et al. 2014), and several other microbial siderophores attract Mo and Vn to form stable complexes (Deicke et al. 2013). Methanobactin is a copper-binding compound (CBC) or chalkophores (Kenney and Rosenzweig 2012), and other well-known copper siderophores include coproporphyrin and yersiniabactin (Chaturvedi et al. 2014). Enterobactin, yersiniabactin and aerobactin possess the capacity to form gold nanoparticles (Wyatt et al. 2014). Their active role as transporters of non-metal moieties such as boron and silicon and signalling molecules in plant defence mechanisms and oxidative stress is also well documented now (Chaturvedi and Henderson 2014; Butler and Theisen 2010; Nadal-Jimenez et al. 2012). Stable non-metal-siderophore complexes exist due to marine siderophores such as vibrioferrein from Marinobacter spp. (Amin et al. 2007). Citrate and catecholate siderophores interact and bind to boron to form strong signalling and sensing molecules (Sandy and Butler 2009). Additionally, they have gained relevance as therapeutic and diagnostic molecules in medical science (Ali and Vidhale 2013). Antioxidant and hormonal signalling cascades indicate the role of iron siderophores in various spheres of biology (Aznar et al. 2015). More recently, siderocalin, a mammalian siderophore-binding protein from the lipocalin family, specifically binding to actinide and lanthanide complexes, has been discovered (Allred et al. 2015). Despite the mind-boggling diversity on display across the microbial, plant and mammalian spheres, this review focuses on the most pertinent and applicative aspects of microbial siderophores in agriculture, therapeutics, etc.
15.2 Biosynthesis, Classification and Functional Diversity
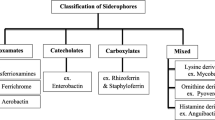
Siderophores are synthesized by several bacteria and show significant variation in structures. These are mainly classified on the basis of characterization of functional or coordinating groups that bind with Fe3+ ions. The most important and commonly occurring groups include catecholates, hydroxamates and carboxylates (Ali and Vidhale 2013; Sah and Singh 2015; Gupta et al. 2015) (Fig. 15.1). A very small group of siderophores include pyoverdines, which are also termed as mixed ligands. They constitute the fourth class of siderophores which have functional groups that are classified in chemically distinct classes. Numerous types of siderophores have been identified employing latest techniques of spectrophotometry, mass spectrometry, acid hydrolysis, electrophoretic mobility, proton NMR (nuclear magnetic resonance) spectroscopy and biological activities (Sah and Singh 2015; Kurth et al. 2016) (Fig. 15.2).
15.2.1 Biosynthesis
Microbial siderophore synthesis takes place through two pathways: non-ribosomal peptide synthetases (NRPSs) multienzyme dependent and NRPS independent (Sah and Singh 2015). NRPS-dependent biosynthesis involves the enzyme ATP pyrophosphate for the formation of hydroxamate siderophores (Lautru and Challis 2004). NRPSs are enzymes with large subunits which catalyse non-ribosomal peptide (NRP) synthesis by incorporating one amino acid per unit into the peptide chain. For instance, NRPSs synthesize the chromophores and the peptide chains of the microbial siderophore pyoverdine (Mossialos et al. 2002; Crosa and Walsh 2002).
Peptide synthetase is a multicomplex enzyme that produces peptide products without RNA template. 2, 3-dihydroxybenzoic acid (DHBA) is one of the precursor compounds of siderophores, which is synthesized from chorismate through the sequential action of a series of enzymes (Farrell et al. 1990). For instance, in anguibactin, the coordinating bonds are synthesized by molecular oxygen from various groups such as diphenoxylate group, hydroxamate group, imidazole group and thiazoline group. The structure of anguibactin is completed by two molecules of anguibactin, metal ion and solvent each. Anguibactin retrobiosynthesis, {(−N-hydroxy-N)- [2-(2,3-dihydroxyphenyl) thiazolin-4-yl] carboxyl} involving histamine, indicates the presence of 2,3-dihydroxybenzoic acid (DHBA), L-cysteine and N-hydroxy-histamine. In retrobiosynthesis of vibriobactin in V.cholerae, N1-(2,3-dihydroxybenzoyl)-N5,N9-bis[2- (2,3-dihydroxyphenyl)-5-methyloxazolinyl-4-carboxamido] norspermidine shows that it is comprised of DHBA, L-threonine and unusual polyamine norspermidine [bis (3-aminopropyl) amine] (Keating and Walsh 1999; Yamamoto et al. 1991).
15.2.2 Catecholate Siderophores
Siderophores belonging to the catecholate category have 2, 3-dihydroxybenzoate (DHB) or phenolate chelating groups as functional moieties (Table 15.1). They are also termed as pyrocatechols or 1, 2-dihydroxybenzene [C6H4(OH)2] (Cornish and Page 1998; Wittmann et al. 2001). Every catecholate group bestows two oxygen atoms to chelate with Fe ions by forming bidentate ligand complexes. As a result of this, a hexadentate octahedral complex is formed (Ali and Vidhale 2013). Catecholates are naturally occurring colourless compounds and are present as trace amounts in environment. They are composed of three isomeric benzenediols which make them an orthoisomeric molecule. One of the most important catecholate widely characterized is enterobactin or enterochelin. It is a prototype of catecholate siderophore and has a cyclic trimester coordinating group (2,3-dyhydroxyserine). It has been reported to be produced by Salmonella typhimurium and Klebsiella pneumoniae (Ali and Vidhale 2013; Achard et al. 2013).
15.2.3 Hydroxamate Siderophores
The most commonly occurring group of siderophores is the hydroxamate type, which is made up of C(=O) N-(OH) R. Here, R is an amino acid or its derivative that is primarily released by bacteria (Renshaw et al. 2002). Hydroxamate siderophores contain a fixed constancy ratio of 1:1 with Fe (III), which is in close proximity to that of the Fe (III)-EDTA complex (Mosa et al. 2016). On the basis of the side chain of the hydroxamate functional group, the hydroxamate siderophores are divided into three categories, i.e. ferrioxamines, ferrichrome and aerobactin (Winkelmann 2007). Ferrioxamines is linear in structure and its molecular formula is C25H48N6O. The ferrichromes are cyclic in structure, made of two unpredictable amino acids (alanine, glycine or serine), three N-acyl-N-hydroxyl-L-ornithine and a glycine connected by peptide bonds (Ali et al. 2011). Aerobactin is the third type of hydroxamate siderophore with a molecular formula of C22H36N4O13 (Neilands 1995). It is found in E. coli, Pseudomonas, K. pneumoniae, A. aerogenes and other bacteria (Buyer et al. 1991).
15.2.4 Carboxylate Siderophores
A recent group of siderophores have been identified, whose members neither exhibit hydromate nor 2, 3-dihydroxybenzoate (DHB) chelating groups (Table 15.1.). The chelation in this category of siderophores is done by carboxylate or hydroxyl carboxylate groups (Sah and Singh 2015; Schwyn and Neilands 1987). One of the most important carboxylate siderophore was also isolated from Rhizobium meliloti strain DM4. Rhizobactin is an aminopoly (carboxylic acid) which has hydroxycarboxyl and ethylenediamine dicarboxyl moieties or coordinating groups (Bergeron et al. 2014). Another imperative member of carboxylate siderophores is staphyloferrin A which is synthesized by Staphylococcus hyicus DSM20459. This siderophore consists of two citric acid residues and one D-ornithine residue, which bind by two amide bonds (Ali and Vidhale 2013). Rhizoferrin is another carboxylate siderophore synthesized by fungi belonging to zygomycetes family (Holinsworth and Martin 2009; Al-Fakih 2014).
15.2.5 Mixed Siderophores
Mixed siderophores possess a minimum of two different Fe-binding ligands (Aznar and Dellagi 2015). Mixed ligands are those siderophores which are derived from ornithine (pyoverdines), lysine (mycobactin) and histamine (anguibactin) (Sah and Singh 2015). Pyoverdine is the ornithine derivative type of mixed siderophore which is also known as pseudobactin. It is actively produced by Pseudomonas species (Meneely and Lamb 2007). Mycobactin is the lysine derivative type of mixed siderophores. It is produced by Mycobacterium tuberculosis and Mycobacterium smegmatis (Varma and Podila 2005). Anguibactin is the histamine derivative type of mixed siderophore. It is synthesized by marine pathogen Vibrio anguillarum (Naka et al. 2013). These diverse classes have been elaborated with their applications in plants in the table given below.
15.3 Siderophore-Mediated Responses Against Various Abiotic Stresses
Phytoremediation is today acknowledged as the most accepted green technology which is an effective in situ method for removal/treatment of heavy metals (Gratão et al. 2005). Rhizosphere is the region of soil and root interface and has an important role in the phytoremediation of various pollutants most importantly the heavy metals. This rhizospheric region is an extremely microbial active region due to the presence of siderophore-producing bacteria (SPB) (Rajkumar et al. 2010). These bacteria are reported to improve the phytoremediation process by increasing the mobility and bioavailability of heavy metals through their various secretions such as chelating compounds, phosphate-solubilizing complexes, production of phytohormones, changing redox state, etc. (Ma et al. 2011). Most common metals like Cd, Ni, Cu, Pb and Zn and actinides like U(IV), Th(IV) and Pu(IV) are found to be highly solubilized and bioavailable in the presence of siderophores (Schalk et al. 2011). But, the ability of siderophores in increasing the phytoremediation mainly depends upon their ligand specificity or selectivity to form a stable metal-siderophore complex (Braud et al. 2006, 2007). The siderophore-producing bacteria which are resistant to metal play a vital role in growth and endurance of plants by providing necessary nutrients (e.g. iron) to plants which grow in contaminated soils. Increased growth in presence of siderophore-producing bacteria will further improve the efficiency of phytoremediation process (Braud et al. 2009; He and Yang 2007; Rajkumar et al. 2010).
Recent advances indicate incorporating the siderophore-producing genes from bacterial and fungal genomes into the plant genomes or direct application of isolated siderophores onto the plant. Many studies have come forward to support the active production of siderophores by root-dwelling bacterial strains to overcome metal stress. Iron-phytosiderophore complexes and their transporters were found to be present in high concentrations in the root extracts of transgenic Petunia hybrida plants grown in iron deficient highly alkaline soils through the electrospray ionization-Fourier transform-ion cyclotron resonance mass spectrometry (Murata et al. 2015). Plants possess the ability to produce multiple siderophores such as enterobactin, which further facilitates E. coli colonization and commensalism in inducing stress tolerance (Searle et al. 2015). cDNA of ferritin siderophores from chickpea plants exposed to extreme dehydration and high salt stress showed immense induction of stress signals, which suggested a strong iron buffering role in the soil medium (Parveen et al. 2016). Systematic DNA analysis of siderophore producing bacteria Klebsiella sp. D5A genome and identification of its genes contributing to plant growth and stress management resulted in an increase in salt tolerance and wide pH adaptability. It became evident that they had well-defined roles to play under extreme environmental conditions (Liu et al. 2016). Soil- borne Cd-resistant bacterium Enterobacter sp. strain EG16 was found to produce multiple siderophores and plant hormone indole-3-acetic acid (IAA), both of which promote plant growth. The isolated extracts from bacteria were applied to plants which showed 31% Cd accumulation as compared to controls which made the bacterial strain a very apt instrument for inducing assisted phytoremediation through (Chen et al. 2016).
15.4 Diagnostic and Therapeutic Values
Most of the siderophores are reported to have major role in virulence by acting as iron scavengers, and these ferrisiderophores reenter the bacterial cells by means of specific cell surface receptors (Lamont et al. 2002). Convergence of sensitive technologies leads to siderophore neutralization by mammals and their re-consumption by bacterial pathogens (Aznar et al. 2015). Similarly, the hosts have also developed certain important cell conversion and siderophore-based iron delivery methods which are of great interest for diagnostic and therapeutic studies. There are different possible methods for exploitation of iron requirement which ultimately effect multiplication of pathogens and development of virulence (Aznr and Dellagi 2015). In recent past, the usage of various natural and synthetic compounds for effective treatment of iron-dependent infections and others had become popular. However, the use of bacterial siderophores against pathogen inhibition, removal of transuranic elements and against malaria has also emerged as a potential strategy (Beneduzi et al. 2012). These siderophores can adopt different mechanisms by which they can cut the supply of iron which effects the pathogen development and multiplication by either acting as “Trojan horse” toxins or by inhibiting siderophore synthesis pathway through the formation of siderophore-antibiotic conjugates. Application of siderophores in conception of “Trojan horse” makes them to act as intermediates which assist the uptake of antibiotics in the cells. The other ways include either the depletion of iron by application of siderophores which cannot be consumed as a source of iron by the pathogens or inhibition of siderophore utilization endogenously (Miethke and Marahiel 2007; Ahmed and Holmström 2014). However, all of the three mechanisms act differently for different pathogens. Different studies on the role of siderophores in biocontrol methods of pathogen development are available, e.g. siderophore secreted by Bacillus subtilis effectively controls the growth in Fusarium oxysporum, which causes the Fusarium wilting of pepper (Yu et al. 2011). Similarly, siderophores produced from Azadirachta indica effectively chelates Fe (III) from the soil which later affects the growth of various fungal pathogens negatively (Verma et al. 2011). The siderophore-triggered immunity is regulated by MYB72 gene, which imbalances the metal homeostasis and is required along with MYB10 to combat with deficiency of iron (Palmer et al. 2013). It was also reported that the dual function of NAGL (neutrophil gelatinase-associated lipocalin) can be used to kill cancer cells, by declining the supply of iron and increased efflux of iron leading to cell death due to inactivation of major oxidative enzymes (Tang et al. 2016). Similarly, the main causative agent of tuberculosis, i.e. Mycobacterium tuberculosis, secretes siderophores like mycobactin and carboxymycobactin. It was reported by Jones et al. (2014) that M. tuberculosis reuses its siderophores to effectively use the iron source. When this process is disordered, accumulation of siderophores in intracellular spaces was observed which later harms and detoxifies M. tuberculosis. These siderophores are poisonous and hamper the capacity of recycling of iron and the use of haeme as iron source. Thus, the enhancement of siderophore recycling can be used for development of one of the major pathogenic bacteria causing tuberculosis. The antibacterial property of siderophores was observed with the use of gallium to quench iron-scavenging siderophores in pathogenic bacteria Pseudomonas aeruginosa. It was observed that in gallium-mediated siderophore quenching is able to resist the bacterial growth and restrict virulence development (Ross-Gillespie et al. 2014).
15.5 Current Relevance and Future Prospects
Microbial siderophores synthesized and secreted by bacterial pathogenic strains such as Aerobacter, E. coli, Enterobacter, Pseudomonas sp. Salmonella, Vibrio, Yersinia, etc. acquire metal ions from the surrounding plant rhizospheric environment and end up generating several defence responses against fungal and bacterial pathogens and oxidative damage as well. However, more investigation is needed for getting a clear idea for the metal-siderophore interaction phenomenon. Metal scavenging is a competitive soil phenomenon for the diverse class of compounds, and many side benefits of prime agricultural and plant stress physiology regulation emerge. Not only this, these versatile agents have been studied as a special case of coordination chemistry in the living systems using techniques like NMR and X-ray crystallography. Advanced investigation has substantiated their role in phytoremediation, therapy against many contagious human diseases and improvising agents in imaging techniques such as MRI. In the coming times, the use of siderophores in immediate sensor-based technologies to curb spread of epidemics holds a lot of promise. Hence, microbial siderophores could be the next “wonder drugs” and “new age agricultural wizards” of our era. But, despite seeing siderophores in this new light of information and facts, we need to find out better ways of isolating them, applying them to living systems, inducing them in transgenic organisms and making all this cost-effective as well. A very interesting fact being that unlike other signalling molecules, siderophores are short-lived and don’t persist after triggering plant immunity. It will be amazing to discover the involvement of the lipocalin family in siderophore activation and a siderocalin-like response system in plants as in the case of mammals. Thus, the question of the role of such proteins in siderophore-mediated immunity remains to be addressed. If we are able to ace up research at the genetic level and crack the molecular mechanisms that bestow precision and versatility to siderophores, this could lead to better crop management strategies and extensive bio-patenting of siderophores to be used as novel therapeutic agents for fortifying both plant and human health care.
References
Achard ME, Chen KW, Sweet MJ, Watts RE, Schroder K, Schembri MA (2013) An antioxidant role for catecholate siderophores in Salmonella. Biochem J 454(3):543–549
Ahemad M (2014) Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.11.020
Ahmed E, Holmström SJ (2014) Siderophores in environmental research: roles and applications. Micro Biotechnol 7(3):196–208
Akafia MM, Harrington JM, Bargar JR, Duckworth OW (2014) Metal oxyhydroxide dissolution as promoted by structurally diverse siderophores and oxalate. Geochim Cosmochim Acta 141:258–269
Al-Fakih AA (2014) Overview on the fungal metabolites involved in mycopathy. Open J Med Microbiol 4:38–63
Ali SS, Vidhale NN (2013) Bacterial siderophores and their application: a review. Int J Curr Microbiol App Sci 2(12):303–312
Ali T, Bylund D, Essén SA, Lundström US (2011) Liquid extraction of low molecular mass organic acids and hydroxamate siderophores from boreal forest soil. Soil Biol Biochem 43:2417–2422
Allred BE, Rupertb PB, Gaunya SS, Ana DD, Ralstonc CY, Hoehnea MS, Strongb RK, Abergela RJ (2015) Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides. Proc Natl Acad Sci 112(33):10342–10347
Amin SA, Küpper FC, Green DH, Harris WR, Carrano CJ (2007) Boron binding by a siderophore isolated from marine bacteria associated with the toxic dinoflagellate Gymnodinium catenatum. J Am Chem Soc 129(3):478–479
Aznar A, Dellagi A (2015) New insights into the role of siderophores as triggers of plant immunity: what can we learn from animals? J Exp Bot 66(11):3001–3010
Aznar A, Chen NW, Thomine S et al (2015) Immunity to plant pathogens and iron homeostasis. Plant Sci 240:90–97
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35(4):1044–1051
Bergeron RJ, Wiegand J, McManis JS, Bharti N (2014) Desferrithiocin: a search for clinically effective iron chelators. J Med Chem 57(22):9259–9291
Braud A, Jézéquel K, Vieille E, Tritter A, Lebeau T (2006) Changes in extractability of Cr and Pb in a polycontaminated soil after bioaugmentation with microbial producers of biosurfactants, organic acids and siderophores. Water Air Soil Pollut 6:261–279
Braud A, Jézéquel K, Lebeau T (2007) Impact of substrates and cell immobilization on siderophore activity by Pseudomonads in a Fe and/or Cr, Hg, Pb containing-medium. J Hazard Mater 144(1–2):229–239
Braud A, Jézéquel K, Bazot S et al (2009) Enhanced phytoextraction of an agricultural Cr-and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74(2):280–286
Braun V, Pramanik A, Gwinner T, Köberle M, Bohn E (2009) Sideromycins: tools and antibiotics. Biometals 22(1):3–13
Butler A, Theisen RM (2010) Iron(III)-siderophore coordination chemistry: reactivity of marine siderophores. Coord Chem 254(3–4):288–296
Buyer JS, Lorenzo VDE, Neilands JB (1991) Production of the siderophore aerobactin by a halophilic Pseudomonas. Appl Environ Microbiol 57(8):2246–2250
Chaiharn M, Chunhaleuchanon S, Kozo A, Lumyong S (2008) Screening of rhizobacteria for their plant growth promoting activities. J KMITL Sci Tech 8:18–23
Chaturvedi KS, Henderson JP (2014) Pathogenic adaptations to host-derived antibacterial copper. Front Cell Infect Microbiol 4:3. https://doi.org/10.3389/fcimb.2014.00003
Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP (2014) ACS Chem Biol 9:551–561. [PubMed: 24283977]
Chen Y, Chao Y, Li Y, Lin Q, Bai J, Tang L, Wang S, Ying R, Qiua R (2016) Survival strategies of the plant-associated bacterium Enterobacter sp. Strain EG16 under cadmium stress. Appl Environ Microbiol 82(6):1734–1744
Cornish AS, Page WJ (1998) The catecholate siderophores of Azotobacter vinelandii: their affinity for iron and role in oxygen stress management. Microbiology 144(7):1747–1754
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249
Deicke M, Bellenger JP, Wichard TJ (2013) Direct quantification of bacterial molybdenum and iron metallophores with ultra-high-performance liquid chromatography coupled to time-of-flight mass spectrometry. Chromatogr A 1298:50–60
Duckworth OW, Akafia MM, Andrews MY, Bargar JR (2014) Siderophore-promoted dissolution of chromium from hydroxide minerals. Environ Sci Process Impact 16:1348–1359
Farrell DH, Mikesell P, Actis LA, Crosa JH (1990) A regulatory gene, angR, of the iron uptake system of Vibrio anguillarum: similarity with phage P 22 cro and regulation by iron. Gene 86:45–51
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Hindawi Sci. https://doi.org/10.6064/2012/963401
Gratão PL, Prasad MNV, Cardoso PF et al (2005) Phytoremediation: green technology for the cleanup of toxic metals in the environment. Braz J Plant Physiol 17(1):53–64
Gregory JA, Li F, Tomosada LM, Cox CJ (2012) Topol AB, algae – produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7(5):371–379
Gupta G, Parihar SS, Ahirwar NK, Snehi SK, Singh V (2015) Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J Microb Biochem Technol 7:096–102
Harrington JM, Parker DL, Bargar JR et al (2012) Structural dependence of Mn complexation by siderophores: donor group dependence on complex stability and reactivity. Geochim Cosmochim Acta 88:106–119
He ZL, Yang XE (2007) Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J Zhejiang Univ Sci B 8(3):192–207
Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27(5):637–657
Höfte M (1993) Classes of microbial siderophores. In: Barton LL, Hemming BC (eds) Iron chelation in plants and soil microorganisms. Academic, San Diego, pp 3–26
Holinsworth B, Martin JD (2009) Siderophore production by marine-derived fungi. Biometals 22(4):625–632
Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10(8):525–537
Husen E (2003) Screening of soil bacteria for plant growth promotion activities in vitro. Indones J Agric Sci 4(1):27–31
Johnstone TC, Nolana EM (2015) Beyond iron: non-classical biological functions of bacterial siderophores. Dalton Trans 44(14):6320–6339
Jones CM, Wells RM, Madduri AV et al (2014) Self-poisoning of mycobacterium tuberculosis by interrupting siderophore recycling. Proc Natl Acad Sci U S A 111(5):1945–1950
Joshi H, Dave R, Venugopalan VP (2014) Pumping iron to keep fit: modulation of siderophore secretion helps efficient aromatic utilization in Pseudomonas putida KT2440. Microbiology 160:1393–1400
Keating T, Walsh C (1999) Initiation, elongation, and termination strategies in polyketide and polypeptide antibiotic biosynthesis. Curr Opin Chem Biol 3:598–606
Kenney GE, Rosenzweig AC (2012) Chemistry and biology of the copper chelator methanobactin. ACS Chem Biol 7:260–268
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Kraemer SM, Crowley D, Kretzschmar R (2006) Siderophores in plant iron acquisition: geochemical aspects. Adv Agron 91:1–46
Kurth C, Kage H, Nett (2016) Siderophores as molecular tools in medical and environmental applications. Org Biomol Chem 14:8212–8227
Lamont IL, Beare PA, Ochsner U et al (2002) Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 99(10):7072–7077
Lautru S, Challis GL (2004) Substrate recognition by non-ribosomal peptide synthetase multi-enzymes. Microbiology 150:1629–1636
Leong SA, Neilands JB (1982) Siderophore production by phytopathogenic microbial species. Arch Biochem Biophys 281:351–359
Liu W, Wang Q, Hou J, Tu C, Luo Y, Christie P (2016) Whole genome analysis of halotolerant and alkalotolerant plant growth-promoting rhizobacterium Klebsiella sp.D5A. Sci Rep 6:26710. https://doi.org/10.1038/srep26710
Ma Y, Prasad MN, Rajkumar M et al (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258
Manwar AV, Khandelwal SR, Chaudhari BL, Kothari RM, Chincholkar SB (2001) Generic technology for assured biocontrol of groundnut infections leading to its yield improvement. Chem Weekly 6(26):157–158
Meneely KM, Lamb AL (2007) Biochemical characterization of an FAD-dependent monooxygenase, the ornithine hydroxylase from Pseudomonas aeruginosa, suggests a novel reaction mechanism. Biochemistry 46:11930–11937
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 3:413–451
Mosa KA, Saadoun I, Kumar K, Helmy M, Dhankher OP (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci doi. https://doi.org/10.3389/fpls.2016.00303
Mossialos D, Ochsner U, Baysse C, Chablain P, Pirnay JP, Koedam N (2002) Identification of new, conserved, non-ribosomal peptide synthetases from fluorescent pseudomonads involved in the biosynthesis of the siderophore pyoverdine. Mol Microbiol 45(6):1673–1685
Munzinger M, Taraz K, Budzikiewicz H (1999) SS-rhizoferrin (enantio-rhizoferrin) – a siderophore of Ralstonia (Pseudomonas) pickettii DSM 6297 – the optical antipode of R, R-rhizoferrin isolated from fungi. Biometals 12:189–193
Murata Y, Itoh Y, Iwashita T, Namba K (2015) Transgenic petunia with the iron(III)-phytosiderophore transporter gene acquires tolerance to iron deficiency in alkaline environments. PLoS One 10(3):e0120227. https://doi.org/10.1371/journal.pone.0120227
Nadal-Jimenez P, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol 76(1):46–65
Naka H, Liu M, Actis LA, Crosa JH (2013) Plasmid- and chromosome-encoded siderophore anguibactin systems found in marine vibrios: biosynthesis, transport and evolution. Biometals 26(4):537–547
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 70(45):26723–26726
Palmer CM, Hindt MN, Schmidt H et al (2013) MYB10 and MYB72 are required for growth under iron-limiting conditions. PLoS Genet 9:e1003953
Parveen S, Gupta DB, Dass S, Kumar A, Pandey A, Chakraborty S, Chakraborty N (2016) Chickpea Ferritin CaFer1 participates in oxidative stress response, and promotes growth and development. Sci Rep 6:31218
Prentice AM, Ghattas H, Cox SE (2007) Host-pathogen interactions: can micronutrients tip the balance? J Nutr 137(5):1334–1337
Rajkumar M, Ae N, Prasad MN et al (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biochem Sci 28(3):142–149
Raymond KN, Emily AD, Sanggoo SK (2003) Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100(7):3584–3588
Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison D, Taylor RJ (2002) Fungal siderophores: structures, functions and applications. Mycol Res 106:1123–1142
Ross-Gillespie A, Weigert M, Brown SP et al (2014) Gallium-mediated siderophore quenching as an evolutionarily robust antibacterial treatment. Evol Med Public Health 1:18–29
Sah S, Singh R (2015) Siderophore: structural and functional characterisation – a comprehensive review. Agriculture (Poľnohospodárstvo) 61(3):97–114
Sandy M, Butler A (2009) Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 109(10):4580–4595
Schalk IJ, Hannauer M, Braud A (2011) New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol 13(11):2844–2854
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Searle LJ, Méric G, Porcelli I, Sheppard SK, Lucchini S (2015) Variation in siderophore biosynthetic gene distribution and production across environmental and faecal populations of Escherichia coli. PLoS One 10(3):e0117906. https://doi.org/10.1371/journal.pone.0117906
Sharma A, Johri BN, Sharma AK, Glick BR (2003) Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck). Soil Biol Biochem 35(7):887–894
Singh A, Singh SS, Pandey PC, Mishra AK (2010) Attenuation of metal toxicity by frankial siderophores. Toxicol Environ Chem 92(7):1339–1346
Tang HC, Chang PC, Chen YC (2016) Iron depletion strategy for targeted cancer therapy: utilizing the dual roles of neutrophil gelatinase-associated lipocalin protein. J Mol Model 22(1):1–5
Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P (2007) Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol Plant-Microbe Interact 20(4):441–447
Varma A, Podila GK (2005) Siderophore their biotechnological application. Biotech Appl Microbes:177–199
Verma VC, Singh SK, Prakash S (2011) Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A Juss. J Basic Microbiol 51(5):550–556
Ward TR, Reas L, Serge P, Parel JE, Philipp G, Peter B, Chris O (1999) An iron-based molecular redox switch as a model for iron release from enterobactin via the salicylate binding mode. Inorg Chem 38(22):5007–5017
Winkelmann G (2007) Ecology of siderophores with special reference to the fungi. Biometals 20:379–392
Wittmann S, Heinisch L, Scherlitz-Hofmann INA, Stoiber T, Dorothe AF, Möllmann U (2001) Catecholates and mixed catecholate hydroxamates as artificial siderophores for mycobacteria. Biometals 17:53–64
Wyatt MA, Johnston CW, Magarvey NA (2014) Gold nanoparticle formation via microbial metallophore chemistries. J Nanopart 16:2212. https://doi.org/10.1007/s11051-013-2212-2
Yamamoto S, Chowdhury MAR, Kuroda M, Nakano T, Koumoto Y, Shinoda S (1991) Further study on polyamine compositions in Vibrionaceae. Can J Microbiol 37:148–153
Yu X, Ai C, Xin L, Zhou G (2011) The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur J Soil Biol 47(2):138–145
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sharma, R. et al. (2018). Microbial Siderophores in Metal Detoxification and Therapeutics: Recent Prospective and Applications. In: Egamberdieva, D., Ahmad, P. (eds) Plant Microbiome: Stress Response. Microorganisms for Sustainability, vol 5. Springer, Singapore. https://doi.org/10.1007/978-981-10-5514-0_15
Download citation
DOI: https://doi.org/10.1007/978-981-10-5514-0_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5513-3
Online ISBN: 978-981-10-5514-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)