Abstract
Toxic concentrations of monocarboxylic weak acids present in lignocellulosic hydrolyzates affect cell integrity and fermentative performance of Saccharomyces cerevisiae. In this work, we report the deletion of the general catabolite repressor Mig1p as a strategy to improve the tolerance of S. cerevisiae towards inhibitory concentrations of acetic, formic or levulinic acid. In contrast with the wt yeast, where the growth and ethanol production were ceased in presence of acetic acid 5 g/L or formic acid 1.75 g/L (initial pH not adjusted), the m9 strain (Δmig1::kan) produced 4.06 ± 0.14 and 3.87 ± 0.06 g/L of ethanol, respectively. Also, m9 strain tolerated a higher concentration of 12.5 g/L acetic acid (initial pH adjusted to 4.5) without affecting its fermentative performance. Moreover, m9 strain produced 33% less acetic acid and 50–70% less glycerol in presence of weak acids, and consumed acetate and formate as carbon sources under aerobic conditions. Our results show that the deletion of Mig1p provides a single gene deletion target for improving the acid tolerance of yeast strains significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Utilization of lignocellulosic biomass for biofuel and biochemical production offers social, economic and energetic benefits compared to sucrose and starch-based feedstocks. These plant residues can contain up to 75% fermentable sugars in the form of cellulose and hemicellulose, where d-xylose, l-arabinose, d-galactose, d-mannose and d-glucose are the main sugar monomers [51]. Pre-treatment methods are required to disrupt the lignocellulose matrix for improved hydrolytic enzyme accessibility; however, this inevitably causes some degree of degradation of the three major components of lignocellulosic biomass, and generates a broad diversity of toxic compounds such as ketones, aldehydes, phenols and organic acids [20]. These toxic compounds can negatively affect the enzyme hydrolysis and fermentation; first with the reduction of soluble sugar yield in enzyme hydrolysis, and second, with a reduction in the growth rate and ethanol yield in the fermentation stage with Saccharomyces cerevisiae [3, 6, 46].
There are at least 18 different inhibitory compounds released from conventional pre-treatment methods [20], but the type and concentration of the released inhibitory compounds strongly depend on the nature of the lignocellulosic biomass. However, acetic, formic and levulinic acid are the three most abundant monocarboxylic acids found in the lignocellulosic hydrolysates. Acetic acid is formed when amorphous hemicellulose is degraded and releases acetyl side chains, while formic acid and levulinic acid are degradation products from furfural and hydroxymethylfurfural [49]. Concentrations of acetic acid in lignocellulosic hydrolyzates typically range between 1 and 5 g/L [20, 24] and in some cases up to 10 g/L have been reported [57]. Although formic acid can be found in concentrations 10-times lower than acetic acid, its lower pKa of 3.77 compared to acetic acid’s pKa of 4.75, and its smaller size, are responsible for the increased toxicity [26]. The next most commonly found weak acid in hydrolysates is levulinic acid with a pKa of 4.62, and is often found in the range of 1.1–2.6 g/L [20, 26, 47].
When in a pH-environment below to an acid’s pKa, the acid predominantly exists in its undissociated form. Many weak organic acids such as acetic, benzoic or sorbic, have a lipophilic nature, which favours its diffusion across the cell membrane until equilibrium is reached [15]; alternatively it can be transported in its acid form through a plasma membrane channel (Fps1p) or in its anionic form via a proton symporter (Ady2p, Jen1p) in absence of glucose [5]. The pH of the cytosol is typically higher than the extracellular environment in exponentially growing cells, causing the dissociation of the weak acid, raising the concentration of protons and charged anions, and decreasing the cell’s internal pH [47, 48]. S. cerevisiae responds to restore the intracellular pH by activating the plasma membrane ATPase (Pma1p) to pump out protons [10, 61]; however, this defense mechanism demands ATP hydrolysis, and it is known that this H+ translocator enzyme can consume up to 60% of total cellular ATP under some acidic conditions [1, 14]. Thus, higher acid concentrations will lead to less ATP available for cell growth and compromising its development [18, 62]. We have recently characterized this ATP requirement using genome-scale modelling [17]. Moreover, accumulation of high intracellular concentrations of the weak acid’s anion will raise the cytoplasm’s osmolarity [22], causing elevated water inflow to restore homeostasis, and resulting in a potentially lethal increase in the internal pressure of the cell [23]. High acetate concentration (~ 12 g/L) causes elevation in the levels of oxidatively modified proteins and in the activity of antioxidant enzymes in yeast cells, demonstrating its prooxidant effects [24]. As described, acidic stressors have a broad impact of adverse effects, thus in order to improve the overall cell robustness a more comprehensive strategies are required. For example, the manipulation of transcription factors that will results in the modification of the expression patterns of its target genes whose activity might generate an improved tolerance phenotype. For example the overexpression of transcriptional activator HAA1, demonstrated the regulation of a set of genes required for S. cerevisiae tolerance to weak acid stress [58].
Interestingly, in the absence of fermentable sugars, acetate can be assimilated as carbon source by S. cerevisiae under aerobic conditions, requiring the anaplerotic enzymes in the glyoxylate cycle and gluconeogenesis encoded by ACS1, ICL1, MLS1, PCK1, and FBP1 [24,25,26,27]. These genes contain a carbon source-responsive element in their promoters that are activated by Cat8p, which itself is regulated by carbon catabolite repression (CCR) [27, 28]. When glucose is available at high concentrations, the general catabolite repressor Mig1p (YGL035C; a Cys2His2 zinc finger protein) binds to the CAT8 promoter and recruits the repressor complex Ssn1p-Tup1p, blocking its expression [29, 30]. During low glucose levels, Mig1p is phosphorylated by the serine–threonine kinase Snf1p complex, a central component in the CCR signalling pathway, and then is exported to the cytosol, liberating the exerted repression of CAT8 [31, 32]. Also, yeast has two other zinc finger proteins that are closely related to Mig1p, namely, Mig2p and Mig3p. Mig2p seems to be a minor player in glucose repression. Some glucose-repressed genes are synergistically repressed by Mig1p and Mig2p, while others are repressed only by Mig1p. Mig3p does not seem to overlap in function with Mig1p and Mig2p [33]. This transcriptional control, exerted by the CCR network, helps to coordinate the adaptive response towards alternative carbon sources [32, 34, 35]. Interestingly, the Mig1p repressor not only regulates the expression of genes with metabolic functions, but also has been described to repress the expression of genes related with stress tolerance and other diverse functions [37, 38]. For example, the metal toxicity stress-inducible metallothionein Cup1-1p and the salt stress-inducible P-type ATPase sodium pump Ena1p are also overexpressed when Mig1p repressor is deleted or under glucose starvation conditions (Mig1p inactive) [39,40,41]. This cross talk among stress responsive elements (transcription factors and genes) suggests that Mig1p extensively regulates gene expression to cope with the imposed stress, and to improve the tolerance/survival success. This indicates the contribution of Snf1p/Mig1p pathway in cell survival during several types of starvation and environmental stress.
In this work, we report the deletion of the general repressor Mig1p as a strategy to improve the tolerance of S. cerevisiae towards acidic (low pH) stress, imposed by weak organic acids (acetic, formic and levulinic acid) during aerobic, oxygen limiting, and anaerobic growth. We further discuss metabolic causes for improved tolerance and the impact of the initial culture pH on the concentrations tolerated.
Materials and methods
Strains and plasmids used
Laboratory strain S. cerevisiae CEN.PK 113-7D (MATa MAL2-8c SUC2) [42], kindly provided by Prof. Vincent J. J. Martin (Concordia University), was used as reference strain. The MIG1 (YGL035C) gene, encoding the CCR-general repressor Mig1p, was disrupted from parental strain to generate S. cerevisiae CEN.PK 113-7D m9 (MATa MAL2-8c SUC2 mig1::kanMX6). Plasmids pUG6 (carrying loxP-KanMX-loxP, kanr) and pSH65 (Cre-expressing, GAL1 promoter, bler, used to recombine the loxP–marker gene–loxP and remove the marker gene) were purchased from EUROSCARF [43]. Escherichia coli DH5α was used for general cloning and molecular procedures.
MIG1 gene deletion
Gene deletion was performed using the standard PCR-mediated gene insertion protocol [43]. Disruption cassette was generated by PCR using primers mig1-F 5′-GAGTATAGTGGAGACGACATACTACCATAGCCatgcaaagCAGCTGAAGCTTCGTACGC-3′ and mig1-R 5′-ATTTATCTGCACCGCCAAAAACTTGTCAGCGTAtcagtccGCATAGGCCACTAGTGGATCTG-3′ and plasmid pUG6 as template. The bolded regions indicate homology to the plasmid pUG6, non-bolded regions to MIG1 gene, and lowercase letters to the MIG1 CDS. Gene deletion was confirmed by PCR analysis and Sanger sequencing using primers mig1-FCK 5′-TCGCGAGAGACTGCGGACTGC -3′ and mig1-RCK 5′- AGAACAATTAATTATCTCTGCGG -3′ and genomic DNA of possible MIG1 disruptant.
Growth media
Yeast peptone dextrose (YPD) was used for regular maintenance of yeast strains. Solid YPD contains, per liter, 10 g yeast extract, 20 g peptone, 20 g agar, and 10 g glucose. For selection of MIG1 disruptants, YPD-agar was supplemented with 200 μg/mL G418 and plates were incubated at 30 °C. For screening acid-tolerant strains, solid YPD was supplemented with 10 g/L of acetic acid using a 20% (V/V) glacial acetic acid stock (filter-sterilized); pH was not adjusted after acid addition. Stain m9 was further analysed in solid YPD and liquid YPD (supplemented with 20 g/L of glucose) containing 1.75 g/L of formic acid (final-pH was not adjusted), or 20 g/L of levulinic acid (final-pH was not adjusted), or in YPD with adjusted pH of 8.0 (using NaOH 1 N) or 3.2 (using HCl 1 N). The evaluated concentrations of acetic and formic acid were selected based on previously reported concentrations found in hydrolysates [20, 26]. For levulinic acid, concentrations were ramped increased, until the growth of the wt strain ceased. Strain wt was used as reference in all the tolerance-screening assays. For experiments in solid YPD, both strains were serially diluted (100–10−4) using an overnight-grown YPD liquid culture, plates were incubated at 30 °C for 3–5 days. For acid-tolerance screening experiments using liquid medium, YPD was supplemented with 20 g/L of glucose, cultures were started with an initial OD620nm of 0.1 (≈ 0.15 gDCW/L biomass), and incubated at 30 °C and 200 rpm. Samples were taken under sterile conditions every 24 h until 5 days, for determination of biomass and ethanol concentration.
Pre-culture preparation
Independent yeast colonies of CEN.PK 113-7D (wt) and CEN.PK 113-7D m9 (m9) strains, isolated from YPD-agar plates, were first cultivated in 50 mL conical tubes containing 15 mL YPD medium and overnight-grown at 30 °C, 200 rpm. Inoculum cultures were started by transferring 500 μL of the tube-grown cultures into 250 mL flasks containing 25 mL YPD medium and incubated for 24 h at 30 °C and 200 rpm. The cells from these precultures were harvested by centrifugation at 18,000g for 5 min at 4 °C, washed twice with sterile YPD media, and then used to inoculate final batch fermentations at an initial optical density at 620 nm (OD620nm) of 0.1 (≈ 0.15 gDCW/L biomass).
Aerobic batch fermentation in presence of weak acids
Aerobic batch fermentations were performed using 250 mL flasks containing 50 mL of YPD supplemented with the correspondent weak acid concentration. For acetic acid, the evaluated concentrations were 0.0, 5.0, and 6.0 g/L. Formic acid was evaluated at 0.0, 1.75, and 2.1 g/L. Levulinic acid was evaluated at concentrations of 0.0, 20 and 25 g/L. The pH media after the acid supplementation was not adjusted. All the flasks cultures were started with an initial OD620nmof 0.1 (≈ 0.15 gDCW/L biomass), and incubated at 30 °C and 200 rpm. Samples for determination of biomass and extracellular metabolite concentration were periodically withdrawn under sterile conditions.
Anaerobic batch fermentation in presence of weak acids
Aerobically precultured wt and m9 cells were transferred into modified Hungate-type tubes containing 10 mL of YPD medium. Medium and headspace were sparged with nitrogen air to purge oxygen; tubes were capped with rubber stoppers and crimped with aluminum seal. These anaerobic precultures were overnight incubated in a rotary shaker at 30 °C and 200 rpm and used to inoculate anaerobic batch cultures. Anaerobic fermentation was carried out in 150 mL serum bottles containing 75 mL of YPD medium supplemented with the correspondent weak acid concentration. Acetic acid was evaluated at 0.0 and 5.0 g/L, and formic acid at 0.0 and 1.75 g/L concentrations. Medium and headspace were sparged with nitrogen air to ensure anaerobic ambience, bottles were capped with rubber stoppers and crimped with aluminum seals. All the anaerobic cultures were started with an initial OD620nm of 0.1 (≈ 0.15 gDCW/L biomass), and incubated at 30 °C and 200 rpm. Samples for determination of biomass and extracellular metabolite concentration were periodically withdrawn under sterile conditions using needle syringes. Anaerobic batch cultivations were also performed using a 1.5 L stirred tank bioreactors (Applikon, The Netherlands), using a working volume of 1 L of YPD medium with a higher concentration of glucose (20 g/L total), supplemented with 5 g/L of acetic acid (pH was not adjusted after acid addition). Cultures were inoculated at an initial OD600nm of 0.5 (≈ 0.75 gDCW/L biomass). pH was monitored but not controlled during the entire cultivation. Temperature was controlled at 30 °C. Nitrogen flow was set to 0.5 vvm. Dissolved oxygen tension was measured with a polarographic oxygen electrode (Applisens, Applikon), the impeller speed was maintained at 150 rpm.
Microaerobic batch fermentation with initial pH adjusted to 4.5
A set of microaerobic batches were carried out using 50 mL conical tubes containing 25 mL of YPD medium supplemented with 20 g/L of glucose and with increasing concentrations of acetic acid: 5.0, 7.5, 10.0, and 12.5 g/L. After acetic acid was added, medium pH was adjusted to 4.5 using KOH 3 M. Once pH was settled, culture medium was filter sterilized. Culture tubes were started with an initial OD620nm of 0.1 (≈ 0.15 gDCW/L biomass), and incubated at 30 °C and 200 rpm. 500 μL samples for determination of biomass and ethanol concentration were periodically withdrawn under sterile conditions.
Calculation of initial ratio of undissociated form (iRUF) of acetic acid
The initial ratio of undissociated form (iRUF) of acetic acid for the different working pH used in this work was calculated using the Henderson-Hasselbalch equation (Eq. 1). pKa = 4.75 was used for acetic acid.
Kinetic parameters calculation
The data plotted were recorded by reading until the maximum concentration of ethanol observed. The specific rates of growth (μ), glucose consumption (qGlc), ethanol production (qEtOH), and yield of ethanol on glucose (YEtOH/Glc), were determined. The μ and qGlc values were calculated during exponential growth phase. Because growth rates and ethanol production kinetics differed among studied strains and culture conditions, qEtOH and YEtOH/Glc were calculated considering only the ethanol production phase, defined as the period from starting one sample before ethanol was detected up to the point when a sharp decrease in ethanol accumulation was observed. Following the same criteria, plots were constructed using only the data corresponding to the ethanol production phase. Cultivations were performed in triplicate. The values reported represent the means of the experiments performed.
Analytical methods
Cell growth was followed as optical density at 620 nm (spectrophotometer GENESYS20, Thermo Fisher Scientific). Biomass was determined as dry-cell weight (DCW) as described previously [43]. Samples taken during cultivation period were centrifuged at 10,000 rpm for 2 min. Supernatant was filtered using 0.45 μm syringe-filter and stored at − 20 °C for subsequent analysis. Glucose, ethanol, acetate, formate, levulinate and glycerol were analysed by high-performance liquid chromatography (HPLC) (Ulti-Mate 3000, Dionex) with refractive index detector (Shodex). Filtered samples were loaded onto an Aminex HPX-87H ion exchange column (Bio-Rad) operated at 42 °C and eluted with 5 mM H2SO4 at a flow rate of 0.4 mL/min.
Results
Disruption of MIG1 causes an acetic acid resistance phenotype
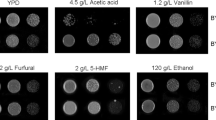
The CCR-general repressor MIG1 gene was deleted from S. cerevisiae CEN.PK 113-7D (wt) strain to evaluate its possible participation in acidic stress response. The MIG1 mutant, m9, and the wt strain were plated onto YPD-agar containing 10 g/L of acetic acid. The wt strain did not show any growth after 5 days of incubation (Fig. 1a); in contrast, the MIG1 disruptant was able to grow by the third day of incubation (Fig. 1a). For further characterization, m9 strain was also cultivated in presence of different stressors such as 1.75 g/L of formic acid, or 20 g/L of levulinic acid, or alkaline (pH of 8.0), or acidic (pH of 3.2) environment. As observed in Fig. 1a, m9 strain showed an improved growth performance towards all the tested stressors in comparison with the wt strain; especially with formic acid (1.75 g/L), where the growth of the wt strain was completely inhibited compared to the robust growth of m9 strain (Fig. 1a). Similar results were observed with acetic acid (Fig. 1a). Liquid YPD cultures of m9 and wt strains under the same concentrations of stressors tested in solid YPD, showed that m9 strain was also fermentative active and ethanol was produced even under high concentrations of the stressors (Fig. 1b). For example, m9 strain showed some growth and ethanol production even in the presence of 10 g/L of acetic acid until the fifth day of cultivation (Fig. 1b), in comparison with the null growth or ethanol production by the wt strain.
Effect of different stressors on the growth and fermentative profiles of the yeast S. cerevisiae CEN.PK 113-7D m9 in a solid and b liquid YPD media. a Overnight cultures of S. cerevisiae CEN.PK 113-7D (wt) and S. cerevisiae CEN.PK 113-7D m9 (m9) were diluted from 100 to 10−4 and spotted on solid YPD media (control) and YPD supplemented with 1.75 g/L of formic acid (final-pH was not adjusted), or 20 g/L of levulinic acid (final-pH was not adjusted), or 10 g/L of acetic acid (final-pH was not adjusted), or in YPD with adjusted pH of 8.0 or 3.2. Growth was recorded after 3 days. b Final concentration of biomass (filled bars) and ethanol (empty bars) from microaerobic cultures of S. cerevisiae CEN.PK 113-7D (wt) and S. cerevisiae CEN.PK 113-7D m9 (m9), in liquid YPD media (control, CTRL) and YPD supplemented with stressors as indicated in a. Each data point represents the mean ± SD from triplicate experiments. Growth and ethanol concentrations were recorded after 3 days, or 5 days for the culture with acetic acid (10 g/L)
Characterization of m9 strain in aerobic batch cultures in presence of toxic concentrations of weak acids
Acetic acid
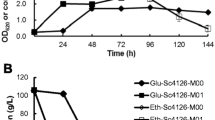
While anaerobic conditions are used for ethanol production in S. cerevisiae, the production of organic acids, such as adipic acid, are favourable under aerobic conditions [2]. Hence, we wanted to evaluate the acid tolerance of m9 strains under aerobic conditions. The performance of strains wt and m9 was characterized in aerobic batch cultures by means of its kinetics of growth, substrate consumption, and ethanol and by-product formation. Cultivations of wt and m9 strains carried out in YPD media containing 10 g/L of glucose and no weak acid addition generated similar profiles of growth, sugar consumption, and ethanol production (Fig. 2a, b). After 12 h of cultivation, glucose was completely consumed and maximum biomass (≈ 6.7 gDCW/L) and ethanol (≈ 3.4 g/L) production were reached for both strains (Table 1) around the 12th hour. A slight decrease in the final concentrations of glycerol and acetate was observed for the m9 strain (0.596 ± 0.047 and 0.472 ± 0.030 g/L, respectively) in comparison with the parental strain (0.663 ± 0.027 and 0.711 ± 0.020 g/L, respectively) (Fig. 2b). Supplementation of 5 g/L of acetic acid caused complete growth inhibition of wt strain, consistent with the previous observations in the low oxygen cultures (Supplementary Fig. 1a and 1b). The presence of 5 g/L of acetic acid in the m9 culture extended the lag phase of growth by 2 h (Fig. 2c), in comparison with the control conditions (without weak acid). Kinetic parameters were also affected by the imposed acidic stress; specific growth rate (μ), glucose consumption (qs), and ethanol formation (qp) were 32.8, 30.4 and 41.25% lower than the obtained in the unstressed cultures (Table 1). Despite a lag phase and decreased growth rate, final biomass and ethanol were comparable to media without weak acids (Table 1). Interestingly, the final concentration of excreted glycerol was 50% lower than the obtained titer when no acid was added in the m9 cultures (Fig. 2c). No acetic acid was co-consumed with glucose during the initial growth phase, but after glucose was almost depleted (cultivation time > 12 h), cells started to co-consume the produced ethanol and the supplemented acetic acid as carbon sources; this caused an increase in the biomass during the subsequent 6 h after glucose exhaustion. With this, final acetate concentration was only 1.9 g/L of the 5 g/L added at the beginning of the cultivation (Fig. 2c). Then, m9 cells were subjected to 6 g/L of acetic acid and kinetic parameters were calculated (Fig. 2d). This high concentration of acetic acid caused a prolonged lag phase; approximately 12 h were needed for m9 strain to show progression into growth phase (Fig. 2d). As expected, a more drastic reduction in kinetic parameters were observed with μ, qs, and qp values being 57.8, 72.0 and 57.9% lower than non-acidified cultures (Table 1). Despite this long lag phase, m9 cells produced around 3.2 g/L of ethanol, quite similar to the production observed in control cultures. The acetate-ethanol co-utilization phase was also observed after glucose exhaustion (Fig. 2d).
Aerobic characterization of S. cerevisiae CEN.PK 113-7D m9 in presence of inhibitory concentrations of different organic weak acids. Growth and fermentation profiles of S. cerevisiae CEN.PK 113-7D m9 during aerobic batch cultivation in glucose (10 g/L), in presence of acetic acid 5 g/L (c) and 6 g/L (d); formic acid 1.75 g/L (e) and 2.15 g/L (f); and levulinic acid 20 g/L (g) and 25 g/L (h). Controls of S. cerevisiae CEN.PK 113-7D (a) and S. cerevisiae CEN.PK 113-7D m9 (b) without acid addition are also included. Biomass (empty square), glucose (empty circle), ethanol (filled diamond), glycerol (empty down triangle), acetate (empty up triangle), formate (filled up triangle) and levulinate (filled down triangle). Each data point represents the mean ± SD from triplicate experiments
Formic acid
Rates of growth, substrate consumption and ethanol formation were also characterized for the m9 strain in presence of toxic concentrations of formic acid in aerobic batch cultures. 1.75 g/L of formic acid proved to be a lethal concentration for the wt strain. In contrast, m9 strain aerobically cultured at this concentration of formic acid suffered a slight inhibitory effect on its growth rate, showing a μ = 0.194/h. This is 37% slower than the non-acidified m9 cultures (Table 1), yet it produced as much biomass (6.6 gDCW/L) and ethanol (3.6 g/L) as the m9 control cultures at 12 h of cultivation. Formic acid was co-consumed with glucose during the exponential growth phase and at the end of the cultivation (16 h) only 38.8% of the added formate remained in the culture (Fig. 2e). The toxic effect of a higher concentration of formic acid, 2.1 g/L, was also evaluated under aerobic batch cultivation. Acidic stress caused a lag phase of 10 h (Fig. 2f), where neither growth nor glucose consumption was observed; however, m9 reached a maximum biomass of 6.5 gDCW/L, similar to the reference cultivations of m9. The maximum ethanol concentration obtained was 3.0 g/L, only 8.5% less than the maximum reported for m9 at non-acidified conditions, although this maximum level was reached after 31 h of cultivation (Fig. 2f), almost 20 h of delay. As observed for acetic cultivations, addition of formic acid resulted in a decreased production of glycerol; 73 and 41% less glycerol were produced at the end of the fermentation when 1.75 or 2.1 g/L was added, respectively (Table 1).
Levulinic acid
A final set of aerobic batches was done in presence of toxic concentrations of levulinic acid. Neither growth nor ethanol production were observed in the wt strain, after 19 h of cultivation in YPD medium supplemented with 10 g/L of glucose and 20 g/L of levulinic acid. Although m9 strain grew 43 and 69% slower than the growth rate of m9 under control conditions (Table 1), was able to tolerate concentrations of 20 and 25 g/L of the acid (Fig. 2g, h), respectively. A striking difference was observed for the production of ethanol by m9 strain when levulinic acid was added, 4.3 g/L and 4.2 g/L of ethanol (Fig. 2g, h) were accumulated; 1.3- and 1.28-times higher than the ethanol produced in m9 control conditions (Fig. 2b) and the highest obtained from all aerobic cultivations performed (Table 1). This high ethanol concentration was accompanied by high ethanol production rates, and high values of ethanol yield on glucose; the highest obtained by far (Table 1). As observed for the acetic and formic acid cultivations, final concentration of glycerol was reduced by 60% when levulinic acid was added to the culture medium (Fig. 2g, h). These results indicate that addition of high concentrations of levulinic acid caused a positive effect in the fermentative performance of m9 strain. However, these high concentrations of levulinic acid (20-25 g/L) never have been described as part of lignocellulosic hydrolysates, thus no further characterization was done for the toxic effects of this acid.
Characterization of m9 strain in anaerobic batch cultures in presence of toxic concentrations of weak acids
The effect of acidic stress on the fermentative performance of wt and m9 strains was also characterized. Thus batch cultures under fully anaerobic conditions were performed, using YPD with 10 g/L of glucose. Reference cultures of wt and m9 strains were carried out with no weak acid supplementation (Fig. 3a, b). After 12 h of cultivation both strains consumed completely 10 g/L of glucose at similar rate of consumption (Table 2), producing ≈ 3.5 gDCW/L of biomass and a maximum of ≈ 4.5 g/L of ethanol. As expected, in comparison with aerobic fermentations, anaerobic cultivation of wt and m9 strains produced less biomass and high ethanol concentrations at the end of fermentation (Table 2).
Anaerobic characterization of S. cerevisiae CEN.PK 113-7D m9 in presence of inhibitory concentrations of different organic weak acids. Growth and fermentation profiles of S. cerevisiae CEN.PK 113-7D m9 during anaerobic batch cultivation in glucose (10 g/L), in presence of 1.75 g/L of formic acid (c), or 5 g/L of acetic acid (d). Controls of S. cerevisiae CEN.PK 113-7D (a) and S. cerevisiae CEN.PK 113-7D m9 (b) without acid addition are also included. Biomass (empty square), glucose (empty circle), ethanol (filled diamond), glycerol (empty down triangle), acetate (empty up triangle), and formate (filled up triangle). Each data point represents the mean ± SD from triplicate experiments
Formic acid
Anaerobic fermentation of wt strain in presence of 1.75 g/L of formic acid confirmed the high acidic stress exerted at this concentration, causing total inhibition of growth and fermentative capabilities of wt strain (data not shown). In contrast, the same formic acid concentration caused a minor toxic effect on the fermentative performance of m9 strain. Although growth performance of m9 strain was highly affected (Fig. 3c), with a final biomass concentration of 1.098 gDCW/L, a decrease of almost 66%, this was the lowest biomass concentration obtained from all the anaerobic characterizations of m9 strain (Table 2). Despite the reduced biomass yield, m9 strain was able to produce 3.87 g/L of ethanol; a slight 17% decrease compared to the levels produced by m9 in the control conditions (Table 1).
Acetic acid
Either growth or glucose consumption were observed after 20 h of cultivation of wt strain in presence of a concentration of 5 g/L of acetic acid. Interestingly, m9 strain was able to tolerate the toxicity of 5 g/L of the acetic acid under anaerobic conditions (Fig. 3d). After 6 h of cultivation, growth and ethanol production showed progression and maximum levels of biomass and ethanol were reached at 20 h of fermentation (Fig. 3d); 8 h delayed from the reference m9 cultivations without acetic acid (Fig. 3b). Acidic stress caused m9 strain to produce 59% less biomass at the end of fermentation (Table 2), with a μ = 0.141/h, 51% slower than the growth rate from non-acidified m9 cultures. Despite this low biomass production, m9 strain produced 4.06 g/L of ethanol (Fig. 3d), only 13% less than m9 under control anaerobic conditions. As observed in aerobic m9 cultivations, supplementation of the culture medium with acetic acid caused a drastic reduction of 72% in the final levels of produced glycerol (Table 2) under anaerobic environment. Consumption of acetate or ethanol after glucose exhaustion was not observed in anaerobic experiments (Fig. 3d).
Characterization of m9 strain in anaerobic fermenter batch cultures in presence of toxic concentration of acetic acid
A set of anaerobic batch bioreactor cultivations, with 1.0 L of YPD and 20 g/L of glucose, were carried out in presence of 5 g/L of acetic acid to evaluate the performance of m9 strain in a controlled anaerobic environment. As observed in Fig. 4, after 4 h of cultivation, biomass and ethanol production showed progression, reaching its maximum value at 15 h. After 32 h of cultivation, m9 strain produced 4.17 ± 0.16 gDCW/L of biomass with a μ = 0.135/h. Glucose was completely consumed at 18 h with a qs= 0.345gGLC/gDCW h. Whereas the final ethanol concentration was 9.488 g/L with a qp = 0.144 gEtOH/gDCW h, and a YEtOH= 0.422 gEtOH/gGLC, that is 83% close to the theoretical ethanol yield on glucose. The pH of the YPD medium decreased from 6.5 to 4.2 after the addition of acetic acid (5 g/L), and it remained at the same value for the entire cultivation, indicating the tolerance of m9 strain to acidic environments. Consumption of acetate or ethanol after glucose exhaustion was not observed in anaerobic experiments (Fig. 4).
Fermenter anaerobic characterization of S. cerevisiae CEN.PK 113-7D m9 in presence of inhibitory concentration of acetic acid. Growth and fermentation profiles of S. cerevisiae CEN.PK 113-7D m9 during fermenter anaerobic batch cultivation in glucose (20 g/L), in presence of acetic acid (5 g/L). Biomass (empty square), glucose (empty circle), ethanol (filled diamond), glycerol (empty down triangle), acetate (empty up triangle), % dissolved oxygen (dotted line), pH (dashed line). Each data point represents the mean ± SD from triplicate experiments
Results from these batch culture characterizations of m9 strain confirmed that the increased robustness towards acidic stress of the Mig1p disrupted-yeast is still maintained under fully anaerobic conditions, indicating that m9 strain conserved its fermentative characteristics despite varying oxygen levels.
Initial pH cultivation is determinant for acidic stress response
As the results from previous experiments indicated, 4.0 g/L of acetic acid exerted a high acidic stress causing full growth inhibition of wt strain (Supplementary Fig. 1a). Initial pH from those experiments was not adjusted. The pH of the medium added with 4.0 g/L of acetic acid was 4.27, favouring the undissociated form of the weak acid to be transported across the membrane by lipophilic diffusion or via a channel (e.g., Fps1p), since the media pH is lower than the acid’s pKa (4.75). We carried out a set of microaerobic (oxygen-limiting) cultures in which the medium-pH was adjusted to 4.5 after weak acid addition. Acetic acid was evaluated at 5.0, 7.5, 10.0 and 12.5 g/L concentrations; the medium-pH decreased from 6.5 to 4.2, 4.12, 4.02, and 3.85, respectively. After the pH of the medium was adjusted to 4.5, cultivations of wt and m9 strains were carried out and their growth and ethanol production were monitored. In contrast with the previous experiments, an initial pH cultivation of 4.5 allowed wt strain to grow and produce ethanol even in presence of 7.5 g/L of acetic acid (Fig. 5a–c). Specific growth rate of wt strain decreased in a stepwise fashion as the concentration of acetic acid increased (Fig. 5a); however, even in a medium with a pH of 4.5, the toxicity exerted by acetic acid at 12.5 g/L caused full inhibition of wt growth (Fig. 5a), showing no progression after 50 h of cultivation. Values of final concentration of maximum produced ethanol and ethanol yield followed a similar trend than growth profile (Fig. 5c, d), remaining undetermined in the cultures added with 12.5 g/L of the acid.
Effect of initial pH (adjusted to 4.5) on the growth, fermentative capacities and tolerance to acetic acid of the yeast CEN.PK 113-7D m9. Specific growth rate; µ (a), final biomass concentration (b), final ethanol concentration (c), and ethanol yield; YETOH (d), from microaerobic cultures of S. cerevisiaeCEN.PK 113-7D (filled bars) and S. cerevisiaeCEN.PK 113-7D m9 (empty bars), in presence of different concentrations of acetic acid (0, 5, 7.5, 10 and 12.5 g/L). Medium initial pH was adjusted to 4.5 after acid addition. Each data point represents the mean ± SD from triplicate experiments
With an initial pH cultivation of 4.5, increasing the acetic acid concentration had a less drastic effect on the specific growth rate of m9 compared to wt strain (Fig. 5a). The presence of 10 g/L of acetic acid caused a lag phase of 18 h in the growth of wt strain; whereas, the lag for m9 strain was only 6 h. In contrast to the lethal effect observed on wt growth, m9 strain grew in presence of acetic acid at 12.5 g/L with a growth rate of 0.12/h (Fig. 5a). Although the decrease in the final biomass levels produced by m9 strain was minor (Fig. 5b), m9 produced similar concentrations of ethanol (≈ 5.5 g/L) in presence of all the evaluated concentrations of acetic acid (Fig. 5c).
Discussion
Toxic concentrations of monocarboxylic weak acids will affect cell integrity of yeast at multiple levels of organization including membrane structure destabilization, inactivation of key metabolic enzymes, cytosol acidification, and energetic drain by ATP depletion, intracellular accumulation of weak acid anion, increased intracellular turgor pressure by weak acid anion accumulation, oxidative stress, and apoptosis [39, 52]. Various mechanisms have been found that play a role in its tolerance to weak acids but they can either be summarized as preventing weak acids from entering the cell or mitigating their effects once inside the cell [11]. Interestingly, few of the approaches focused to improve tolerance to acetic acid have shown improvement in tolerance to other weak organic acids, especially those found in lignocellulosic biomass such as formic or levulinic acid [13, 27, 43, 53]. Among these strategies is the manipulation of transcription factors in order to modify the expression of sets of genes whose activity possibly result involved in an improved tolerance. For example the elimination of RIM101 (Cys2His2 zinc-finger transcriptional repressor) proved to be responsible for an increasing sensitivity in S. cerevisiae BY4741 towards 3.6 g/L acetic acid, but also revealed a set of 22 new Rim101p-regulated genes that might be involved in a robust adaptive response and resistance to the imposed stress by propionic acid [37]. S. cerevisiae cells treated with weak organic acids, rapidly accumulated the transcription factors Msn2p and Msn4p in the nucleus and activated a relative large regulon of common stress responsible genes [54]. In another example, S. cerevisiae transformed with an artificial zinc finger protein transcription factor (ZFP-TFp) library helped to screen strains with improved tolerance towards 5 g/L of acetic acid, and identify novel functional genes QDR3 (multidrug transporter of the major facilitator superfamily) and IKS1 (protein kinase of unknown cellular role) whose elimination improved stress tolerance [32]. In this work, elimination of general repressor Mig1p resulted in a phenotype with tolerance against the three main toxic acids found in lignocellulosic biomass; acetic, formic and levulinic acid. m9 strain robustness was attested under aerobic and anaerobic conditions; tolerating 5 g/L of acetic acid, or 2.15 g/L of formic acid or 25 g/L of levulinic acid; and even 12.5 g/L of acetic acid when initial pH was raised to 4.5. Also, under anaerobic conditions, m9 strain produced 4.058 ± 0.138 and 3.871 ± 0.058 g/L of ethanol in presence of lethal concentrations of acetic acid (5 g/L) or formic acid (1.75 g/L), respectively. This is the first report that shows the participation of the CCR-general repressor Mig1p in the tolerance of S. cerevisiae to acidic stress imposed by monocarboxylic weak acids. The deletion of MIG1 rescued an almost 100% the defects in the growth of the yeast, that was completely repressed in the parental strain under toxic concentrations of acetic, formic and levulinic acids. Besides the high tolerance to acidic stress showed by m9 strain as observed by the kinetic parameters reported, m9 strain maintained its respiro-fermentative capabilities in presence of the tested acid concentrations.
The general catabolite repressor Mig1p is responsible for the regulation of approximately 153 genes, most of them related to metabolic activities for the consumption of alternative carbon sources [21, 44]. However, Mig1p also interacts with other genes and transcription factors that are involved in response to other types of stresses, such as DNA replication, osmotic, hyperosmotic and oxidative [44]. In addition, the Snf1p-Mig1p signalling pathway is involved in the regulation of genes related to other types of stressors, such as oxidative stress, heat shock, alkaline pH and NaCl [55]. Thus in this work, the approach to eliminate the general catabolite repressor Mig1p was motivated by the idea that the modification of their regulation activities would generate a strain with a supple genetic background; in terms of removing repression of target genes that might help in the tolerance towards the stress imposed by weak organic acids. Our results suggest that the Mig1p, as part of the SNF1/AMPK signalling pathway, might be involved in the tolerance response of S. cerevisiae to weak acid stress. Mira et al. [38] genome-wide identified approximately 490 determinants that are required for tolerance to acetic acid. Among these, a set of 25 genes that confers tolerance to acetic acid was clustered by being regulated by Mig1p; genes that are related to mitochondrial and cell wall integrity, DNA replication stress, redox balance maintenance, alkaline pH response, including others. Also, in the same report Snf1p was induced in response to acetic acid stress and this activation is apparently non-dependent of the acetic acid-inhibition of glucose uptake. Also, a higher Snf1p phosphorylation level was observed in cells incubated for 30 min with 4.2 g/L acetic acid (at pH 4.0), compared to control cells. These results are in agreement with our experimental findings that elimination of MIG1 is responsible for tolerance to weak monocarboxylic acids in S. cerevisiae, since the reported higher activity of Snf1p in response to acetic acid stress would cause the phosphorylation of Mig1p targeting it to exit the nucleus and release its inhibitory regulation on potential stress responsive genes [38]. Moreover, Mig1p was found to be a negative regulator of lifespan of yeast cells via the proteasome. Cells with increased proteasome activity exhibit reduced Mig1p levels, increased expression of genes required for the induction of respiratory metabolism, enhanced oxidative stress response and elevated respiratory capacity [64]. The SNF1/AMPK signalling pathway is highly conserved, representing a key sensor of the cellular energy level that regulates metabolic adaptation and oxidative stress response. Thus, since weak monocarboxylic acid stress results in a high AMP/ATP ratio, especially for acetic acid stress, this would induce Snf1p with the concomitant phosphorylation of Mig1p, in order to trigger a vast transcriptional and metabolic reprograming that restores energy homeostasis and promotes tolerance to adverse conditions [7]. However, in order to identify the exact genes that were activated by the elimination of Mig1p and that are responsible for the tolerance towards weak organic acids more comprehensive analyses are required.
Besides improved tolerance to formic acid, strain m9 was able to co-consume this acid with glucose during aerobic conditions (Fig. 2e) and anaerobic conditions (Fig. 3c). In the case of formic acid, cytosolic formate dehydrogenase (Fdh1p) yields CO2 and cytosolic NADH [45]; then NADH can be oxidized by external NADH dehydrogenase (Nde1p) and generate additional ATP via oxidative phosphorylation [31] under aerobic conditions. This dissimilation pathway is independent of the Tri-Carboxylic Acid (TCA) cycle but requires electron transport chain capacity. In the case of absence of oxygen, formate in addition to provide an auxiliary energy source [promoting NAD(P)H formation] for cell anabolism, it also contributes with carbon backbones via folate-mediated C1 pathways [50]. On the other hand, acetate co-consumption with glucose would require an increase in TCA flux, generation of additional matrix NADH, and demand electron transport chain capacity starting with internal NADH dehydrogenase, assuming NADH is not shuttled across the mitochondrial membrane [33, 34, 63]. Also, will require to cope with the stronger Crabtree-effect phenotype with glucose [36, 60]. Zygosaccharomyces bailii exposed to acetic acid in the presence of glucose has been shown to increase its expression of enzymes involved in TCA (Aco1p, Cit1p, Idh2p) and energy generation (Atp1p and Atp2p) [12]. This response has the benefit for eliminating intracellular acetate and supplying ATP to restore pHi via proton-pumping ATPase (Pma1p).
In all control experiments (without weak acid), m9 strain showed no significant reduction in growth rate, or biomass or ethanol yields, under aerobic or anaerobic conditions, suggesting no futile cycling between glycolytic and gluconeogenic enzymes, and a possible Mig2p-compensated repression of CAT8 [59], as previously observed [9]. Under aerobic conditions, m9 strain was able to start consuming acetate almost immediately after glucose was completely consumed, indicating the possible de-repressed state of ACS1 brought about by Mig1p inactivation, as previously described [66]. Also, in order to improve the ethanol yields it is important to consider the redirection of the carbon flow that goes in the synthesis of glycerol, which can be accumulated as a non-desirable by-product. Several strategies have been considered in order to decrease the amount of glycerol accumulated by S. cerevisiae [25, 42, 65]. In this work, elimination of MIG1 in S. cerevisiae caused a 10% reduction in the glycerol production under aerobic conditions. Interestingly, addition of weak acids in m9 strain cultivations, especially for levulinic acid, caused a reduction of glycerol accumulation in the range of 50–70%, under aerobic and anaerobic conditions. Reduced glycerol excretion was a common outcome in these experiments raising several possible mechanisms: repression or degradation of Fps1p [40, 56]; a change in redox levels from increased ATP hydrolysis [60]; inhibition of NADH utilization by the electron transport chain in the mitochondria so that the cells are forced to consume NADH through glycerol production [16], a change in plasma membrane composition requiring glycerol in glycolipids and sphingolipids, or decreasing glycerol permeability [28, 29]. Along with the observed decrease of glycerol production, m9 strain produced 33.6% less acetate in comparison with the wt strain, under aerobic conditions. Elimination of the Mig1p repressor as a strategy to reduce the carbon flow through acetate has been previously described, with decreases of 26% [23], 42.7% [4] and 71.4% [22].Still, with the decrease in glycerol and acetate accumulation, a slight increase in the ethanol final concentration and ethanol yield by m9 fermentation was observed, compared with the wt strain.
In this work, the importance of initial pH cultivation and its impact on acidic stress tolerance was also proven under conditions of non-adjusted initial pH. The growth of wt strain was fully arrested in presence of 5 g/L of acetic acid with no initial pH adjustment (initial pH 4.2), but when initial pH was adjusted from 4.2 to 4.5, wt yeast was able to growth and produced ethanol at the same concentration when no acid was added. Correction in the initial pH cultivation allowed wt strain to growth even in presence of 7.5 and 10 g/L of acetic acid. Using the Henderson–Hasselbalch equation, we determined the initial ratio of the undissociated form (iRUF) of acetic acid at different working pH used. When 5 g/L of acetic acid was supplemented to batch cultures, the medium pH decreased to 4.2, at this working pH, acetic acid will have an iRUF of 0.28, indicating that 72% of the acid will be undissociated and able to be transported by lipophilic diffusion or by facilitated diffusion through channels (FPS1) or permeases (ADY2, JEN2). Contrastingly, for the case, when medium pH was adjusted to 4.5, the iRUF of acetic acid increased to 0.56, meaning that only 44% of the acid will be in its lipophilic form and cross the yeast cell wall. These differences in the values of iRUF for the same concentration of weak acid might explain why wt strain grew in presence of 5 g/L of acetic acid, or even higher concentrations, when pH was adjusted to 4.5. Thus, a pH of 4.5 represents a more permissive condition for yeast growth since decreases the concentration of the toxic undissociated form of acetic acid. These results show the impact that initial pH has over the availability of acetic acid to S. cerevisiae, since a lower pH increases the undissociated form of acetic acid that will be available to enter the cells and cause their inhibitory effects [41]. With this observation, especial attention must be taken since some of previous reports have used an adjustment of initial pH, to working pH’s equal or higher than acetic acid’s pKa; even higher than 5.0 (Fig. 6), to report tolerant phenotypes of S. cerevisiae, but the observed tolerance might be an effect of the lower availability of acetic acid. In this context, several industrial S. saccharomyces strains have been reported as acetic acid tolerant, for example strain ER HAA1-OP (constructed from the industrial strain ER) tolerates 5 g/L of acetate (pH 4.5) [19]. Strain GSE16-T18-HAA1 (which contains the HAA1 allele of industrial strain Ethanol Red) is reported to tolerate 20 g/L of acetic acid (pH 5.2) [35]. Strain YZ2 (derived by drug resistance marker-aided genome shuffling from industrial strain 308) is capable to grow in presence of 5 g/L of acetic acid (pH 4.5) [67]. Strain R32 (obtained from the industrial strain CE25 by diethyl sulphate treatment and genome shuffling) showed tolerance to 6 g/L of acetic acid (YPD plates, pH 4.5) [30]. Another example of reported tolerance is for the strain GSE16 (a hybrid from industrial strain Ethanol Red) showed tolerance to acetic acid 6 g/L (pH4.5) and inhibitors in spruce hydrolysate (80% of the liquid portion of spruce hydrolysate, pH5.0) [8]. In comparison with our results, m9 strain showed similar tolerance towards acetic acid than the reported for industrial strains, since it can tolerate 5 g/L (at pH 4.2) or even 12.5 g/L (at pH 4.5). As observed in Fig. 6, comparing the values of the iRUF for the different concentrations reported for acidic-tolerance and their related working pHs at which the experiments were done, the fraction of undissociated acid that is readily to enter the cells decreases as the pH of the medium increases. Also, we can observe that m9 strain was exposed to a higher concentration of undissociated acetic acid than the industrial tolerant strains, confirming the significance of the deletion of MIG1 as a strategy for tolerance to acetic acid. Additionally, the mentioned industrial strains, in the best of our knowledge, are not described as tolerant for other weak monocarboxylic acids, such as formic or levulinic acid, as m9strain is. These results indicate that Mig1p plays a central role in the tolerance of S. cerevisiae to acidic stress imposed by different types of weak organic acids, and that m9 strain has the potential to increase tolerance to weak acids in lignocellulosic hydrolysates.
Comparison of the initial ratio of the undissociated form (iRUF) of acetic acid for the concentrations and correspondent pH values reported for acetic tolerant S. cerevisiae industrial strains and CEN.PK 113-7D m9 strain. Concentration of dissociated (white bars) and undissociated (black bars) forms of acetic acid were calculated using the Henderson-Hasselbalch equation and a pKa = 4.75 for acetic acid. Strain ER HAA1-OP (tolerates 5 g/L of acetic acid, pH 4.5) [19]. Strain GSE16-T18-HAA1 (tolerates 20 g/L of acetic acid, pH 5.2) [35]. Strain YZ2 (tolerates 5 g/L of acetic acid, pH 4.5) [66]. Strain R32 (tolerates 6 g/L of acetic acid, pH 4.5) [30]. Strain GSE16 (tolerates 6 g/L of acetic acid, pH4.5) [8]. Strain m9 4.2 (tolerates 5 g/L, pH 4.2), and strain m9 4.5 (tolerates 12.5 g/L of acetic acid, pH 4.5)
Conclusions
In this work, the manipulation of yeast Snf1p/Mig1p transcriptional regulation machinery was found to be a successful novel approach to improve the tolerance and fermentative performance of S. cerevisiae at toxic concentrations of acetic, formic and levulinic acids. The change in downstream targets of Mig1p could generate a more permissive genetic background in m9 strain that caused the resistance to acidic stress; however, further comprehensive analysis, such as transcriptomics and metabolomics approaches, is required in order to gain a deeper knowledge of the molecular traits responsible of the tolerance phenotype.
References
Bracey D, Holyoak C, Nebe-von Caron G, Coote P (1998) Determination of the intracellular pH (pHi) of growing cells of Saccharomyces cerevisiae: the effect of reduced-expression of the membrane H+-ATPase. J Microbiol Methods 31:113–125. https://doi.org/10.1016/S0167-7012(97)00095-X
Burgard A, Pharkya P, Osterhout R (2011) Microorganisms for the production of adipic acid and other compounds. US Patent Number: US7799545B2
Cantarella M, Cantarella L, Alberto Gallifuoco AS, Alfani F (2004) Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol Prog 20:200–206. https://doi.org/10.1021/bp0257978
Cao H, Yue M, Li S, Bai X, Zhao X, Du Y (2011) The impact of MIG1 and/or MIG2 disruption on aerobic metabolism of succinate dehydrogenase negative Saccharomyces cerevisiae. Appl Microbiol Biotechnol 89:733–738. https://doi.org/10.1007/s00253-010-2894-7
Casal M, Paiva S, Queirós O, Soares-Silva I (2008) Transport of carboxylic acids in yeasts. FEMS Microbiol Rev 32:974–994. https://doi.org/10.1111/j.1574-6976.2008.00128.x
Casey E, Sedlak M, Ho NWY, Mosier NS (2010) Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res 10:385–393. https://doi.org/10.1111/j.1567-1364.2010.00623.x
Crozet P, Margalha L, Confraria A, Rodrigues A, Martinho C, Adamo M, Elias CA, Baena-GonzÃlez E (2014) Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front Plant Sci 5:1–17. https://doi.org/10.3389/fpls.2014.00190
Demeke MM, Dumortier F, Li Y, Broeckx T, Foulquié-Moreno MR, Thevelein JM (2013) Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production. Biotechnol Biofuels 6:120. https://doi.org/10.1186/1754-6834-6-120
Dikicioglu D, Pir P, Onsan ZI, Ulgen KO, Kirdar B, Oliver SG (2008) Integration of metabolic modeling and phenotypic data in evaluation and improvement of ethanol production using respiration-deficient mutants of Saccharomyces cerevisiae. Appl Environ Microbiol 74:5809–5816. https://doi.org/10.1128/AEM.00009-08
Eraso P, Gancedo C (1987) Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett 224:187–192. https://doi.org/10.1016/0014-5793(87)80445-3
Fleet G (1992) Spoilage yeasts. Crit Rev Biotechnol. https://doi.org/10.3109/07388559209069186
Guerreiro JF, Mira NP, Sá-Correia I (2012) Adaptive response to acetic acid in the highly resistant yeast species Zygosaccharomyces bailii revealed by quantitative proteomics. Proteomics 12:2303–2318. https://doi.org/10.1002/pmic.201100457
Hasunuma T, Sanda T, Yamada R, Yoshimura K, Ishii J, Kondo A (2011) Metabolic pathway engineering based on metabolomics confers acetic and formic acid tolerance to a recombinant xylose-fermenting strain of Saccharomyces cerevisiae. Microb Cell Fact 10:2. https://doi.org/10.1186/1475-2859-10-2
Holyoak C, Stratford M, McMullin Z, Cole M, Crimmins K, Brown A, Coote P (1996) Activity of the plasma membrane H(+)-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl Envir Microbiol 62:3158–3164
Holyoak CD, Bracey D, Piper PW, Kuchler K, Coote PJ (1999) The Saccharomyces cerevisiae weak-acid-inducible ABC transporter Pdr12 transports fluorescein and preservative anions from the cytosol by an energy-dependent mechanism. J Bacteriol 181:4644–4652
Hou J, Scalcinati G, Oldiges M, Vemuri GN (2010) Metabolic impact of increased NADH availability in Saccharomyces cerevisiae. Appl Environ Microbiol 76:851–859. https://doi.org/10.1128/AEM.02040-09
Hyland PB, Mun SLS, Mahadevan R (2013) Prediction of weak acid toxicity in Saccharomyces cerevisiae using genome-scale metabolic models. Ind Biotechnol 9:229–235. https://doi.org/10.1089/ind.2013.0004
Imai T, Ohno T (1995) The relationship between viability and intracellular pH in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 61:3604–3608
Inaba T, Watanabe D, Yoshiyama Y, Tanaka K, Ogawa J, Takagi H, Shimoi H, Shima J (2013) An organic acid-tolerant HAA1-overexpression mutant of an industrial bioethanol strain of Saccharomyces cerevisiae and its application to the production of bioethanol from sugarcane molasses. AMB Express 3:74. https://doi.org/10.1186/2191-0855-3-74
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Klein CJL, Olsson L, Rønnow B, Mikkelsen JD, Nielsen J (1996) Alleviation of glucose repression of maltose metabolism by MIG1 disruption in Saccharomyces cerevisiae. Appl Environ Microbiol 62:4441–4449
Klein CJL, Olsson L, Rønnow B, Mikkelsen JD, Nielsen J (1997) Glucose and maltose metabolism in MIG1-disrupted and MAL-constitutive strains of Saccharomyces cerevisiae. Food Technol Biotechnol 35:287–292
Klein CJL, Rasmussen JJ, Rønnow B, Olsson L, Nielsen J (1999) Investigation of the impact of MIG1 and MIG2 on the physiology of Saccharomyces cerevisiae. J Biotechnol 68:197–212. https://doi.org/10.1016/S0168-1656(98)00205-3
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26. https://doi.org/10.1007/s00253-004-1642-2
Kong QX, Gu JG, Cao LM, Zhang AL, Chen X, Zhao XM (2006) Improved production of ethanol by deleting FPS1 and over-expressing GLT1 in Saccharomyces cerevisiae. Biotechnol Lett 28:2033–2038. https://doi.org/10.1007/s10529-006-9185-5
Larsson S, Palmqvist E, Hahn-Hgerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant NO (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159. https://doi.org/10.1016/s0141-0229(98)00101-x
Li YC, Gou ZX, Liu ZS, Tang YQ, Akamatsu T, Kida K (2014) Synergistic effects of TAL1 over-expression and PHO13 deletion on the weak acid inhibition of xylose fermentation by industrial Saccharomyces cerevisiae strain. Biotechnol Lett 36:2011–2021. https://doi.org/10.1007/s10529-014-1581-7
Lindahl L, Genheden S, Eriksson LA, Olsson L, Bettiga M (2016) Sphingolipids contribute to acetic acid resistance in Zygosaccharomyces bailii. Biotechnol Bioeng 113:744–753. https://doi.org/10.1002/bit.25845
Lindberg L, Santos AXS, Riezman H, Olsson L, Bettiga M (2013) Lipidomic profiling of Saccharomyces cerevisiae and Zygosaccharomyces bailii reveals critical changes in lipid composition in response to acetic acid stress. PLoS One. https://doi.org/10.1371/journal.pone.0073936
Lu Y, Cheng YF, He XP, Guo XN, Zhang BR (2012) Improvement of robustness and ethanol production of ethanologenic Saccharomyces cerevisiae under co-stress of heat and inhibitors. J Ind Microbiol Biotechnol 39:73–80. https://doi.org/10.1007/s10295-011-1001-0
Luttik MAH, Overkamp KM, Kötter P, De Vries S, Van Dijken JP, Pronk JT (1998) The Saccharomyces cerevisiae NDE1 and NDE2 genes encode separate mitochondrial NADH dehydrogenases catalyzing the oxidation of cytosolic NADH. J Biol Chem 273:24529–24534. https://doi.org/10.1074/jbc.273.38.24529
Ma C, Wei X, Sun C, Zhang F, Xu J, Zhao X, Bai F (2015) Improvement of acetic acid tolerance of Saccharomyces cerevisiae using a zinc-finger-based artificial transcription factor and identification of novel genes involved in acetic acid tolerance. Appl Microbiol Biotechnol 99:2441–2449. https://doi.org/10.1007/s00253-014-6343-x
Van Maris AJA, Geertman JMA, Vermeulen A, Groothuizen MK, Winkler AA, Piper MDW, Van Dijken JP, Pronk JT (2004) directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast. Appl Environ Microbiol 70:159–166. https://doi.org/10.1128/AEM.70.1.159-166.2004
Marres CAM, Vries S, Grivell LA (1991) Isolation and inactivation of the nuclear gene encoding the rotenone-insensitive internal NADH: ubiquinone oxidoreductase of mitochondria from Saccharomyces cerevisiae. Eur J Biochem 195:857–862. https://doi.org/10.1111/j.1432-1033.1991.tb15775.x
Meijnen J-P, Randazzo P, Foulquié-Moreno MR, van den Brink J, Vandecruys P, Stojiljkovic M, Dumortier F, Zalar P, Boekhout T, Gunde-Cimerman N, Kokošar J, Štajdohar M, Curk T, Petrovič U, Thevelein JM (2016) Polygenic analysis and targeted improvement of the complex trait of high acetic acid tolerance in the yeast Saccharomyces cerevisiae. Biotechnol Biofuels 9:5. https://doi.org/10.1186/s13068-015-0421-x
Merico A, Capitanio D, Viigenti I, Ranzi B, Compagno C (2003) Aerobic sugar metabolism in the spoilage yeast. FEMS Yeast Res 4:277–283. https://doi.org/10.1016/s1567-1356(03)00167-3
Mira NP, Lourenço AB, Fernandes AR, Becker JD, Sá-Correia I (2009) The RIM101 pathway has a role in Saccharomyces cerevisiae adaptive response and resistance to propionic acid and other weak acids. FEMS Yeast Res 9:202–216. https://doi.org/10.1111/j.1567-1364.2008.00473.x
Mira NP, Palma M, Guerreiro JF, Sá-Correia I (2010) Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb Cell Fact 9:79. https://doi.org/10.1186/1475-2859-9-79
Mira NP, Teixeira MC, Sá-Correia I (2010) Adaptive response and tolerance to weak acids in Saccharomyces cerevisiae: a genome-wide view. OMICS 14:525–540. https://doi.org/10.1089/omi.2010.0072
Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27:6446–6456. https://doi.org/10.1128/MCB.02205-06
Mollapour M, Shepherd A, Piper PW (2008) Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 25:169–177. https://doi.org/10.1002/yea.1576
Nissen TL, Kielland-Brandt MC, Nielsen J, Villadsen J (2000) Optimization of ethanol production in Saccharomyces cerevisiae by metabolic engineering of the ammonium assimilation. Metab Eng 2:69–77. https://doi.org/10.1006/mben.1999.0140
Nygård Y, Mojzita D, Toivari M, Penttilä M, Wiebe MG, Ruohonen L (2014) The diverse role of Pdr12 in resistance to weak organic acids. Yeast 31:219–232. https://doi.org/10.1002/yea.3011
Oughtred R, Chatr-Aryamontri A, Breitkreutz BJ, Chang CS, Rust JM, Theesfeld CL, Heinicke S, Breitkreutz A, Chen D, Hirschman J, Kolas N, Livstone MS, Nixon J, O’Donnell L, Ramage L, Winter A, Reguly T, Sellam A, Stark C, Boucher L, Dolinski K, Tyers M (2016) Biogrid: a resource for studying biological interactions in yeast. Cold Spring Harb Protoc 2016:29–34. https://doi.org/10.1101/pdb.top080754
Overkamp KM, Kötter P, van der Hoek R, Schoondermark-Stolk S, Luttik MAH, van Dijken JP, Pronk JT (2002) Functional analysis of structural genes for NAD(+)-dependent formate dehydrogenase in Saccharomyces cerevisiae. Yeast 19:509–520. https://doi.org/10.1002/yea.856
Palmqvist E, Grage H, Meinander NQ, Hahn-Hägerdal B (1999) Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol Bioeng 63:46–55. https://doi.org/10.1002/(sici)1097-0290(19990405)63:1<46::aid-bit5>3.0.co;2-j
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Pampulha ME, Loureiro-Dias MC (1989) Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl Microbiol Biotechnol 31:547–550. https://doi.org/10.1007/BF00270792
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: review. Crit Rev Biotechnol 31:20–31. https://doi.org/10.3109/07388551003757816
Piper MD, Hong S-P, Ball GE, Dawes IW (2000) Regulation of the Balance of One-carbon Metabolism inSaccharomyces cerevisiae. J Biol Chem 275:30987–30995. https://doi.org/10.1074/jbc.M004248200
Ravindran R, Jaiswal AK (2016) A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102. https://doi.org/10.1016/j.biortech.2015.07.106
Russell JB (1992) Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J Appl Bacteriol 73:363–370. https://doi.org/10.1111/j.1365-2672.1992.tb04990.x
Sanda T, Hasunuma T, Matsuda F, Kondo A (2011) Repeated-batch fermentation of lignocellulosic hydrolysate to ethanol using a hybrid Saccharomyces cerevisiae strain metabolically engineered for tolerance to acetic and formic acids. Bioresour Technol 102:7917–7924. https://doi.org/10.1016/j.biortech.2011.06.028
Schuller C, Mamnun YM, Mollapour M, Krapf G, Schuster M, Bauer BE, Piper PW, Kuchler K (2004) Global phenotypic analysis and transcriptional profiling defines the weak acid stress response regulon in Saccharomyces cerevisiae. Mol Biol Cell 15:706–720
Shashkova S, Welkenhuysen N, Hohmann S (2015) Molecular communication: crosstalk between the Snf1 and other signaling pathways. FEMS Yeast Res. https://doi.org/10.1093/femsyr/fov026
Sutherland FC, Lages F, Lucas C, Luyten K, Albertyn J, Hohmann S, Prior BA, Kilian SG (1997) Characteristics of Fps1-dependent and -independent glycerol transport in Saccharomyces cerevisiae. J Bacteriol 179:7790–7795. https://doi.org/10.1128/jb.179.24.7790-7795.1997
Taherzadeh MJ, Eklund R, Gustafsson L, Niklasson C, Lidén G (1997) Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind Eng Chem Res 36:4659–4665. https://doi.org/10.1021/ie9700831
Tanaka K, Ishii Y, Ogawa J, Shima J (2012) Enhancement of acetic acid tolerance in Saccharomyces cerevisiae by overexpression of the HAA1 gene, encoding a transcriptional activator. Appl Environ Microbiol 78:8161–8163. https://doi.org/10.1128/AEM.02356-12
Turcotte B, Liang XB, Robert F, Soontorngun N (2010) Transcriptional regulation of nonfermentable carbon utilization in budding yeast. FEMS Yeast Res 10:2–13. https://doi.org/10.1111/j.1567-1364.2009.00555.x
Vemuri GN, Eiteman MA, McEwen JE, Olsson L, Nielsen J (2007) Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 104:2402–2407. https://doi.org/10.1073/pnas.0607469104
Viegas CA, Almeida PF, Cavaco M, Sa-Correia I (1998) The H + -ATPase in the plasma membrane of Saccharomyces cerevisiae is activated during growth latency in octanoic acid-supplemented medium accompanying the decrease in intracellular pH and cell viability. Appl Envir Microbiol 64:779–783
Viegas CA, Sá-Correia I (1991) Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J Gen Microbiol 137:645–651. https://doi.org/10.1099/00221287-137-3-645
Wei N, Quarterman J, Kim SR, Cate JHD, Jin Y-S (2013) Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat Commun 4:2580. https://doi.org/10.1038/ncomms3580
Yao Y, Tsuchiyama S, Yang C, Bulteau AL, He C, Robison B, Tsuchiya M, Miller D, Briones V, Tar K, Potrero A, Friguet B, Kennedy BK, Schmidt M (2015) Proteasomes, Sir2, and Hxk2 form an interconnected aging network that impinges on the AMPK/Snf1-regulated transcriptional repressor Mig1. PLoS Genet. https://doi.org/10.1371/journal.pgen.1004968
Zhang JG, Liu XY, He XP, Guo XN, Lu Y, Zhang BR (2011) Improvement of acetic acid tolerance and fermentation performance of Saccharomyces cerevisiae by disruption of the FPS1 aquaglyceroporin gene. Biotechnol Lett 33:277–284. https://doi.org/10.1007/s10529-010-0433-3
Zhang M, Galdieri L, Vancura A (2013) The yeast AMPK homolog SNF1 regulates acetyl coenzyme A homeostasis and histone acetylation. Mol Cell Biol 33:4701–4717. https://doi.org/10.1128/MCB.00198-13
Zheng DQ, Wu XC, Wang PM, Chi XQ, Tao XL, Li P, Jiang XH, Zhao YH (2011) Drug resistance marker-aided genome shuffling to improve acetic acid tolerance in Saccharomyces cerevisiae. J Ind Microbiol Biotechnol 38:415–422. https://doi.org/10.1007/s10295-010-0784-8
Acknowledgements
Authors would like to thank Prof. Vince Martin for S. cerevisiae CEN.PK 113-7D strain. Authors would like to acknowledge funding and postdoctoral scholarship (VEBH) from Agricultural Bioproducts Innovation Program, NSERC Bioconversion Network and BioFuelNet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balderas-Hernández, V.E., Correia, K. & Mahadevan, R. Inactivation of the transcription factor mig1 (YGL035C) in Saccharomyces cerevisiae improves tolerance towards monocarboxylic weak acids: acetic, formic and levulinic acid. J Ind Microbiol Biotechnol 45, 735–751 (2018). https://doi.org/10.1007/s10295-018-2053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2053-1