Abstract
An overview of the different inhibitors formed by pre-treatment of lignocellulosic materials and their inhibition of ethanol production in yeast and bacteria is given. Different high temperature physical pre-treatment methods are available to render the carbohydrates in lignocellulose accessible for ethanol fermentation. The resulting hydrolyzsates contain substances inhibitory to fermentation—depending on both the raw material (biomass) and the pre-treatment applied. An overview of the inhibitory effect on ethanol production by yeast and bacteria is presented. Apart from furans formed by sugar degradation, phenol monomers from lignin degradation are important co-factors in hydrolysate inhibition, and inhibitory effects of these aromatic compounds on different ethanol producing microorganisms is reviewed. The furans and phenols generally inhibited growth and ethanol production rate (QEtOH) but not the ethanol yields (YEtOH) in Saccharomyces cerevisiae. Within the same phenol functional group (aldehyde, ketone, and acid) the inhibition of volumetric ethanol productivity was found to depend on the amount of methoxyl substituents and hence hydrophobicity (log P). Many pentose-utilizing strains Escherichia coli, Pichia stipititis, and Zymomonas mobilis produce ethanol in concentrated hemicellulose liquors but detoxification by overliming is needed. Thermoanaerobacter mathranii A3M3 can grow on pentoses and produce ethanol in hydrolysate without any need for detoxification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fuel ethanol from biomass has been the subject of intensive research in the US, where it is widely used in gasoline blends up to 10%. European legislation has now stipulated increased use of biomass for electricity and fuel, and a new European directive has mandated the use of biofuels in the transportation sector corresponding to 2% in 2005 and 5.75% by 2010. Growing crops for bioethanol production will be expensive and have only limited energy benefits. Lignocellulosic plant residues containing up to 70% carbohydrates (as cellulose and hemicellulose) are prominent substrates for cheap ethanol production, however, due to the close association to lignin in the plant cell wall, pre-treatment is necessary to make the carbohydrates available for enzymatic hydrolysis and fermentation. Aqueous pre-treatment at elevated temperature result in an insoluble cellulose-rich fraction and a soluble fraction, containing hemicellulose sugars and degradation products (Klinke et al. 2002). The degradation products formed (e.g., phenols, furans and carboxylic acids) can be considered potential fermentation inhibitors (Fig. 1). Due to their inhibitory effects on productivity and end-product formation, the inhibitors can be a limiting factor in the feasibility of biotechnological conversions of lignocellulosics to ethanol.
In order to obtain an economically feasible conversion process, reduction in the content and/or inhibitory effect of the degradation products are necessary. Recirculation of the stillage water in the process will minimize water consumption, but detoxicification prior to recirculation is required (Larsson et al. 1997; Wilkie et al. 2000) unless the inhibitory compounds are removed (e.g., by anaerobic purification (Torry-Smith et al. 2003)). Also detoxification of pre-treated hydrolysates have been shown to improve their fermentability, however, the cost is often higher than the benefits achieved (Palmqvist and Hahn-Hägerdal 2000b; von Sivers and Zacchi 1996; von Sivers et al. 1994). Furthermore, separating the liquid hemicellulose fraction containing the inhibiting compounds from the cellulose fraction prior to enzymatic hydrolysis and fermentation can reduce the content of inhibitory compounds. In this review, we present an overview of the different potential inhibitors formed by pre-treatment of lignocellulosics and their inhibition of ethanol production.
Degradation products from pre-treatment of lignocellulose
Biomass composition
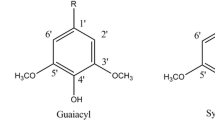
Plants consisting of lignocellulose can be divided in three types according to plant taxonomy: softwood (gymnosperms), hardwood (woody angiosperms) and annual plants, e.g., crops (herbaceous angiosperms). The main component in lignocellulose is holocellulose approx. 60–70%, and is composed of the polysaccharides cellulose and hemicellulose. Cellulose is a high molecular weight glucose polymer, while hemicellulose is composed of various sugars, such as xylose, arabinose, mannose, galactose, and glucose, dependent on the plant material (Bobleter 1994; Fan et al. 1982). Lignin is a co-polycondensate of dehydrogenated products obtained from the lignin monomers (p-coumaryl alcohol, coniferyl alcohol, sinapyl alcohol). The terms p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S), respectively, are used to denote the three types of aromatic rings in monomer residues (Fig. 2), and the ratio of H/G/S units in the lignin is highly dependent on plant taxonomy (Table 1). Softwood lignins are mainly formed from coniferyl alcohol (G), together with small proportions of p-coumaryl alcohol. Hardwood lignin generally results from coniferyl (G) and sinapyl alcohols (S) in roughly equal amounts as well as small quantities of p-coumaryl alcohol (H). Apart from the monolignols (H, G, and S), herbaceous plants (e.g., grasses) also contain p-hydroxycinnamic acids (p-coumaric acid, ferulic acid, and sinapic acids) integrated into their lignin (Campbell and Sederoff 1996; Lawther et al. 1996b). Wood materials have similar cellulose content, but the lignin content in softwood is generally higher than in hardwoods.
The three main phenol building blocks in lignin, p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S), differs in the methoxy-groups ortho to the phenol group. The R-function indicates that cross-linking via ester or ether bonds can occur at these positions. R=H represents the free phenol. At the polymer site, many possible types of bonding on the propanoic sidechain are possible
In addition to holocellulose and lignin, plant materials are composed of extractive (soluble in water or organic solvent) and non-extractive non-cell wall materials (NCWM) (Fan et al. 1982). The non-extractives are mainly inorganic ash components such as silica and alkali salts, but also includes pectin, proteins, and starch. Herbaceous material, especially straw has high non-extractive NCWM with ash contents up to 10%. Ca, Mg, and K are the major inorganic constituents in wood (Saka 1997). In addition, Si, Cl, and Na are abundant in herbaceous materials (Fan et al. 1982). Wood materials have low ash contents (<1%) and contain variable amounts of extractives or secondary metabolites such as resins, terpenes, phenols, quinones, and tannins (Umezawa and Higuchi 1991). The extractives often have protective biological and anti-microbial activities and aid in the chemotaxonomic division of plant species by their specific biosynthetic pathways (Torssell 1997).
Pre-treatment
Different pre-treatment methods are available to render the carbohydrates in lignocellulose accessible for enzymatic hydrolysis and ethanol fermentation, including acid hydrolysis, steaming or steam explosion (STEX), ammonia freeze explosion (AFEX) and wet oxidation (WO). Acid or alkaline catalysts are often applied in pre-treatments at 121–200°C, and when no catalysts are added, autohydrolysis occurs by the release of carboxylic acids (primarily acetic acid) from the lignocellulose during pre-treatment. Hydrosulphuric acid (added as H2SO4 or SO2) and NaOH are widely used catalysts, however also NH3 (Holtzapple et al. 1991), Na2CO3 (Bjerre et al. 1996), and other catalysts have been applied (Bobleter 1994; Fan 1992). Comparison of the pre-treatments and the mechanisms of lignocellulose fractionation and degradation have been reviewed recently (Bobleter 1994; Galbe and Zacchi 2002; Garrote et al. 1999; McMillan 1994b; Sun and Cheng 2002). Lignocellulose degradation is a heterogeneous process with initial solution and hydrolysis reactions followed by high temperature chemical reactions of the soluble components. During pre-treatment, lignin and hemicellulose are solubilized and/or decomposed in the aqueous phase, while cellulose remain in the solid fraction. Aromatic compounds like furans and phenols are prone to undergo condensation reactions at room temperature (Burtscher et al. 1987) and at high temperatures (Klinke et al. 2002; Shevchenko et al. 2000), resulting in dark humic or tannin-like precipitates.
Formation of degradation products
The degradation products formed by pre-treatment of lignocellulose depend on both the biomass and the pre-treatment conditions such as temperature, time, pressure, pH, redox conditions, and addition of catalysts. In high temperature pre-treatments, the formation of fermentable carbohydrates and degradation products is dependent on a combined severity factor, including reaction temperature, time, and pH (Chum et al. 1990; Tengborg et al. 1998).
Sugar degradation products—i.e., furfural (from pentoses) and hydroxymethyl furfural (HMF) (from hexoses)—are formed in high concentrations during severe acidic pre-treatment conditions (Dunlop 1948; Taherzadeh et al. 1997a). Acetic acid is ubiquitous in hemicellulose hydrolysates from all lignocellulosics, where the hemicellulose and to some extent lignin is acetylated (Fengel and Wegener 1989; Sarkanen and Ludwig 1971; Torssell 1997). Hydroxycarboxylic acids such as glycolic acid and lactic acid are common degradation products from alkaline carbohydrate degradation (Alén et al. 1990; Sjöström 1991). Formic acid is a product from sugar and lignin degradation (Klinke et al. 2002), while levulinic acid is formed by 5-HMF degradation (Palmqvist and Hahn-Hägerdal 2000b). Other carboxylic acids can also be found in hemicellulose hydrolysates, including aromatic acids as reviewed by McMillan (1994a).
Aromatic degradation products from sugar degradation are predominantly furans: 2-furfural, 5-HMF, 2-furoic acid and to a minor extent phenols formed by solubilization and hydrolytic or oxidative cleavage of lignin. The aromatic compounds present in hydrolysates are dependent on the type of pre-treatment and the H/G/S ratio of the lignin contained in the biomass material. An overview of the phenols identified from pre-treatment of lignocellulosic materials is presented in Table 2. The most versatile phenols found were 4-hydroxybenzaldehyde, 4-hydroxybenzoic acid, vanillin, dihydroconiferyl alcohol, coniferyl aldehyde, syringaldehyde, and syringic acid. Phenol monomers have been quantified in lignocellulosic hydrolysates from pine (Clark and Mackie 1984; Tran and Chambers 1986), oak (Buchert et al. 1990; Tran and Chambers 1985), willow (Jönsson et al. 1998), spruce (Larsson et al. 1999a), wheat straw (Klinke et al. 2002), bagasse (Martin and Jonsson 2003), poplar (Ando et al. 1986), corn stover and switch grass (Fenske et al. 1998). An overview of quantified phenols found in hemicellulose fractions of pre-treated lignocellulose is provided in Table 3. The phenols were divided into three groups by their degree of methoxylation (H, G, S) and their functionality (aldehydes, ketones, acids, other). Softwood materials almost exclusively produce G (guaiacyl) phenols, while hardwoods and herbaceous materials produce H, G and S phenols consistent to the biomass composition (Table 1). The H (hydroxy) phenol concentration in hardwood acid hydrolysates was high for willow due to benzenediols (Jönsson et al. 1998) and for poplar due to 4-hydroxybenzaldehyde and 4-hydroxybenzoic acid (Ando et al. 1986). These compounds are thought to be extractive components rather than lignin components (Baeza and Freer 1997; Jönsson et al. 1998). Aspen and willow belong to the same family Salicaceae, and aspen lignin is esterified with 4-hydroxybenzoic acid, which is the main phenol monomer produced by steam pre-treatment (Bardet and Robert 1985). Phenol oligomers (polyphenols) were formed by steaming of aspen (Bardet and Robert 1985) or acid hydrolyzed spruce (Larsson et al. 1999b) and alkaline wet oxidation of wheat straw (Klinke et al. 2002). Phenol monomer concentrations in hydrolysates were lower than expected from the amount of lignin removed from the solid fraction by wet oxidation of wheat straw, due to further oxidation to carboxylic acids (Klinke et al. 2002). Syringyl lignin units have been shown to be more susceptible to hydrothermal degradation than guaiacyl lignin units (Jönsson et al. 1998). Thus, relative to untreated birch more syringyl type phenols than guaiacyl type phenols were found in hemicellulose hydrolysates (Buchert et al. 1990).
The hydrolysis conditions during the pre-treatment are important for the functionality of the degradation products, e.g., the formation of phenol aldehydes has been shown to be favored at oxidative acidic conditions (Klinke et al. 2002). Phenylpropane derivatives are generally formed by acid hydrolysis of biomass. Dihydroconiferyl alcohol has been found in hemicellulose fractions from acid hydrolysis of pine (Sears et al. 1971; Tran and Chambers 1986), spruce (Larsson et al. 1999b), switch grass (Fenske et al. 1998), oak (Tran and Chambers 1985) and birch (Buchert et al. 1990). Hibbert’s ketones are phenylpropane derivatives with one or two keto groups on the 1 or 2 position of the propanyl group (Baeza and Freer 1997). Hibbert’s ketones have been observed in hydrolysates from acidic pre-treatment conditions (Buchert et al. 1990; Fenske et al. 1998; Larsson et al. 1999b; Tran and Chambers 1986). From soda pulping of wheat straw the main phenols p-coumaric acid and ferulic acid are produced by hydrolysis of esterified hemicellulose and lignin (Lawther and Sun 1996a). Alkaline wet oxidation of wheat straw also produces these cinnamic acid derivatives. However due to oxidative cleavage of the conjugated double bonds, 4-hydroxybenzoic acid and vanillic acid are formed (Klinke et al. 2002). Phenol dimers were formed in low concentrations during steaming of willow (Jönsson et al. 1998), but in steamed and enzyme hydrolyzed birch the phenol dimers accounted for 61% of the total phenols formed (Buchert et al. 1990).
Removal of degradation products
Detoxification of the hemicellulose fraction from pre-treated high feed stock concentrations is needed in order to achieve reasonable fermentation of the soluble sugars to ethanol. Removal of inhibitory components can be done by extraction (Clark and Mackie 1984), ion exchange (Buchert et al. 1990), active coal (Gong et al. 1993), overliming (Larsson et al. 1999b; Martinez et al. 2001) or laccase and peroxidase treatment (Jönsson et al. 1998; Larsson et al. 1999b). Other methods have also been reported (McMillan 1994a; Palmqvist and Hahn-Hägerdal 2000a). Detoxification methods result in removal of different types of fermentation inhibitory components: steam stripping or evaporation at low pH remove volatile inhibitors such acetic acid and furans (Buchert et al. 1990; Dierssen et al. 1956). Over-liming (addition of Ca(OH)2 to pH 11) removes the volatile and non-volatile inhibitors such as furans and phenols (Larsson et al. 1999b). The effectiveness of different detoxification procedures has been compared in spruce hydrolysates where over-liming and enzyme treatment with laccase produced the best results (Larsson et al. 1999b). The positive effect of detoxification on fermentation was primarily ascribed to lowered furfural and phenol concentrations in the hydrolysates.
Fermentation inhibitors from hemicellulose hydrolysates
Microbial inhibition
Microorganisms differ in their ability to adapt and grow in the hydrolysates, and the fermentative performances of microorganisms in lignocellulosic hydrolysates also depend on raw material and pre-treatment (Olsson and Hahn-Hägerdal 1996). There are several measures of fermentability: growth, ethanol yield, ethanol productivity (rate), and specific ethanol productivity that should be taken into account when comparing data from literature (Hahn-Hägerdal et al. 1994). Ethanol yield can be reported as the ethanol produced relative to the initial fermentable sugar concentration (total ethanol yield) or relative to the consumed sugar concentration (ethanol yield). In testing microbial inhibition, the hydrolysate medium is supplemented with essential growth factors and sterilized prior to inhibition assays. Precautions must be taken when using autoclavation (121°C, 10–20 min) as sterilization method has been found to increase the inhibitory effect of hydrolysates compared to sterile filtration of wet oxidized wheat straw (Klinke et al. 2003) or low temperature sterilization of acid hydrolyzed oak (Lee et al. 1999). Concentrations of phenol acids, formic acid, glycolic acid and malic acid increased by 30–40% and of acetic acid by 75% during autoclavation of wheat straw hydrolysates (Klinke et al. 2003). Total inhibition of Saccharomyces cerevisiae occurred in autoclaved acid hydrolysates of birch (Barber et al. 2000) and mixed waste paper (Rivard et al. 1996).
Growth and ethanol production of S. cerevisiae was strongly inhibited by acid hemicellulose hydrolysates from spruce (Larsson et al. 1999b), bagasse (Martin et al. 2002b; Martin and Jonsson 2003), alder and aspen (Taherzadeh et al. 1997a). Hemicellulose hydrolysates from acid hydrolyzed bagasse inhibited growth and fermentation of Escherichia coli LY01 (Martinez et al. 2001) and Candida sp. (Gong et al. 1993). However, xylose and glucose fermenting adapted Zymomonas mobilis strains were able to produce ethanol in dilute acid hydrolysates from yellow poplar (McMillan et al. 1999).
The performance of S. cerevisiae in lignocellulosic hydrolysates was compared and correlated to the content of acetic acid, formic acid, furfural, 5-HMF and phenol monomers (Table 4). Poor fermentability of dilute acid wood hydrolysates by S. cerevisiae correlated to high concentrations of furfural, 5-hydroxymethylfurfural and acetic acid (Taherzadeh et al. 1997a). Phenol monomer content was also shown to be important for the fermentabilty of spruce hemicellulose hydrolysates (Larsson et al. 1999b). The inhibitory potential of hemicellulose fractions is due to the combined effects of acetic acid, furans and phenols. The hemicellulose fraction from alkaline wet oxidized wheat straw had similar phenol concentrations to acid pre-treated spruce, SO2 steamed willow and steam exploded poplar. However, the ethanol productivity was at least 3.5 times higher in alkaline wet oxidized wheat straw hemicellulose fraction, resulting in reduced fermentation time. The better fermentability was probably due to the absence of furfural, which previously has been shown to inhibit fermentation synergistically with phenols in E. coli (Zaldivar et al. 1999, 2000) and acetic acid in S. cerevisiae (Palmqvist et al. 1999). Acid catalyzed hydrolysis done using SO2 has shown similar efficiency as with H2SO4. However SO2 pre-treated material shows better fermentability than H2SO4 pre-treated material (Martin et al. 2002a; Tengborg et al. 1998). Spruce hydrolysates from H2SO4 pre-treatment were fermented with higher ethanol yield in a genetically engineered S. cerevisiae strain expressing laccase to detoxify phenols (Larsson et al. 2001) than by Bakers yeast (Larsson et al. 1999b) (Table 4). Addition of acetaldehyde has been shown to alleviate inhibition of acid pre-treated birch hemicellulose hydrolysates in S. cerevisiae with no prior detoxification step (Barber et al. 2000).
Pentose fermenting microorganisms are generally more inhibited by hemicellulose hydrolysates than hexose fermenting yeasts. Comparison of fermentability of acid hydrolysates from poplar, switchgrass and corn stover by Pichia stipititis was correlated to the phenol monomer content (Fenske et al. 1999). The performance of genetically engineered S. cerevisiae, Z. mobilis, and E. coli in lignocellulosic hydrolysates has been reviewed recently by Zaldivar et al. (2001) and Dien et al. (2003). In detoxified hydrolysates from corn fiber, all microorganisms were able to convert 80–98% of the fermentable sugars to ethanol. E. coli obtained a 85% ethanol yield from detoxified steam pre-treated pine hemicellulose sugars (Zaldivar et al. 2001). Thermophilic bacteria have also been shown to ferment lignocellulosic hydrolysates (Sommer 1998). An adapted xylanolytic anaerobic thermophilic bacterium Thermoanaerobacter mathranii ferment xylose in the hemicellulose fraction from alkaline wet oxidized wheat straw to ethanol with no prior detoxification (Ahring et al. 1996; Klinke et al. 2001).
Inhibitory effects of degradation products
Degradation products from chemical pre-treatment of biomass can be divided in the following classes: carboxylic acids, furans, phenols and inorganic salts—with phenols showing the most inhibitory effect to fermentations (McMillan 1994a). Low molecular weight (MW) organic compounds or salts are able to penetrate cell membranes, whereas fermentation inhibitors with high MW influence the expression and activity of sugar and ion transporters in the cell membrane. Mechanisms for inhibition on growth and ethanol production of weak acids, furans and phenols have been reviewed recently (Palmqvist and Hahn-Hägerdal 2000a). Low MW phenolics were shown to be more toxic to microorganisms than high MW polyphenolics (Clark and Mackie 1984; Sierra-Alvarez and Lettinga 1991) and also extractives (including phenolic components) were shown to inhibit fermentation (Ranatunga et al. 1997b; Tran and Chambers 1986). The furans and phenols are aromatic compounds that have different functional groups (e.g., acid, ketone, or aldehyde) and hence different potential inhibitory activity. The inhibitory effect of aromatic compounds on glucose fermentation and growth in hexose fermenting yeasts (S. cerevisiae, Candida shehatae) and bacterium (Z. mobilis) is shown in Table 5. The inhibitory effects of acids and aromatic compounds on xylose fermentation and growth in pentose fermenting yeasts (C. shehatae, P. stipititis) and bacteria (E. coli, T. mathranii, Z. mobilis) are shown in Table 6.
Effect of furans and phenols on glucose fermentation
The furans and phenols generally inhibited growth and ethanol production rate (QEtOH) but not the ethanol yields (YEtOH) in S. cerevisiae and Z. mobilis (Table 5). An exception was the lower ethanol yield of S. cerevisiae Hakken No. 1 because it was determined at the mid-exponential phase and not as the final fermentation yield (Ando et al. 1986). Caution should be taken when growth is monitored by optical density because additions of fermentation inhibitors can induce changes in cell morphology (Ranatunga et al. 1997a). In spite of different inocula and strains, there was a good coherence in the data reported from S. cerevisiae fermentations with the phenol acids. S. cerevisiae tolerance towards aldehydes was strain dependent and the CBS 1200 strain was less tolerant than both the ATCC 96581 strain and Bakers yeast. Z. mobilis had an intermediate aldehyde tolerance compared to the four S. cerevisiae strains. C. shehateae improved the ethanol production by 30% relative to the reference fermentation upon addition of furfural and 4-hydroxybenzoic acid. The inhibitory effect of 4-hydroxybenzoic acid and vanillic acid was about the same and syringic acid was not inhibitory at all. The phenylpropane unsaturated acids—4-hydroxycinnamic acid and ferulic acid—severely inhibited ethanol productivity at low concentrations in S. cerevisiae. The detoxification of phenol aldehydes by conversion to alcohols in anaerobic cultures have been shown in S. cerevisiae (de Wulf et al. 1986; Klinke et al. 2003; Larsson et al. 2000) and in Klebsiella pneumoniae (Nishikawa et al. 1988).
Effect of acids, furans and phenols on xylose fermentation
The tolerance towards inhibitors differed in the tested pentose fermenting strains (Table 6). The ethanol production of Z. mobilis CP4 (pZB5) was generally inhibited at much lower concentrations of acids and aldehydes than the other strains tested. E. coli and T. mathranii tolerated aliphatic and aromatic acids well, but ferulic acid inhibited ethanol production by E. coli at only 3 mM concentration. E. coli was very tolerant to furfural and 5-hydroxymethylfurfural compared to Z. mobilis, P. stipititis, and C. shehateae. T. mathranii tolerated phenol aldehydes better than Z. mobilis, P. stipititis, and C. shehateae, but not as well as E. coli. The acids inhibited growth more than ethanol production in E. coli, but this was not the case for the phenol aldehydes or other phenols.
The ethanol yields of both glucose and xylose fermenting microorganisms were generally improved by adding sub-inhibitory levels of phenols to the medium (Tables 5, 6), and this effect has also been shown for acetic acid and furfural due to reduced cell mass production (Palmqvist et al. 1999; Taherzadeh et al. 1997b). In addition, the capacity to metabolize monocyclic aromatic compounds as sole carbon source has been shown to be present in six genera of yeasts (Mills et al. 1971).
Structure activity relationship for predicting inhibitory potential
The inhibitory potential of the aromatic compounds shown in Tables 5, 6, was found to be dependent on their chemical structure. However, this structure-activity relationship (SAR) is very complex and was shown to be dependent on the microbial strain due to different features of cell membranes and metabolism (McMillan 1994a; Mikulásova et al. 1990; Zemek et al. 1979). It is long known that in yeast the inhibitory effects of compounds from wood hydrolysates are closely related to their type: terpenes > aldehydes > polyhydroxy aromatics, and formic acid > acetic acid (Leonard and Hajny 1945). Furfural is more inhibitory than 5-HMF (Sanchez and Bautista 1988) and this was also concluded from model inhibition experiments (Tables 5, 6).
Model inhibition assays of 20 phenols produced detailed information on SAP affecting growth and ethanol production in S. cerevisiae (Larsson et al. 2000). In an extensive survey of genetic engineered ethanol-producing E. coli, the inhibitory activity of furans and phenols on the ethanol production was closely related to the functionality of the aliphatic sidechain, e.g., aldehydes > acids > alcohols (Zaldivar et al. 1999, 2000; Zaldivar and Ingram 1999). Typical fermentation pH of yeasts is slightly acidic (pH 4–5), while for bacteria it is neutral (pH 7). The pKa value of the phenol hydroxyl group in phenol aldehydes and ketones (including Hibbert’s ketones) is 7.3–8.2, phenol acids 9–11, other phenols 9.5–10.3. The pKa value of the carboxyl-group in phenol acids is 3.4–4.6, and in alpha-oxy acids it is 1.6–2.6 (Maman et al. 1996; Ragnar et al. 2000). The pKa value of phenol acids is not dependent on methoxy group substituents on the aromatic ring; thus, there is not a significant difference between H, G, or S derivatives. The lower pKa value of the phenol hydroxyl group of aldehydes and the ketones mean that the phenolic proton is not completely dissociated at neutral pH. The inhibitory activity of fermentation inhibitors were also correlated to their partition coefficients in octanol-water (log P, e.g., hydrophobicity) in E. coli (Zaldivar et al. 2000). Hydrophobic parts of proteins, enzymes, or membrane transport systems are possible sites of inhibitory action. Methoxy substituents ortho to the phenol hydroxy group has been shown to decrease the toxicity of the phenols towards S. cerevisiae, e.g., the inhibitory activity of a phenol is H>G>S (Ando et al. 1986; Clark and Mackie 1984; Delgenes et al. 1996, Klinke et al. 2003). The introduction of methoxyl groups in the aromatic ring of a phenol drastically reduces the hydrophobicity (log P), which is less dependent of the functional group para to the phenol hydroxyl group (Table 7). There is very small difference in hydrophobicity when comparing different types of phenols with the same amount of methoxyl groups, hence the hydrophobicity can only be used as an indicative tool for comparison within a functional group. However, a correlation between the hydrophobicity and inhibition of volumetric ethanol productivity of S. cerevisiae was seen, when plotting series of separate functional groups of phenol aldehydes, ketones, and acids (Fig. 3). It can be concluded that the more hydrophobic the compound was, the more inhibition of the volumetric ethanol productivity QEtOH in S. cerevevisiae was evident. The degree of inhibition increased almost linear as function of log P, and was higher in phenolic aldehydes and ketones compared to phenolic acids.
Inhibition of S. cerevisiae‘s volumetric ethanol productivity by para-phenol (Ph) aldehydes, ketones and acids (10 mM), as function of the partition coefficients in octanol-water (log P), e.g., hydrophobicity (Klinke et al. 2003)
Additive or synergistic inhibition
The biological effect of certain compounds can be enhanced by the presence of other compounds. In the case of microbial inhibition, the effect can be additive or synergistic if the inhibition increases significantly more than expected from individual measurements, respectively. When tested in combination with acetic acid, aromatic aldehydes and alcohols, 2-furfural and furfuryl alcohol has shown to increase the inhibitory potential for these compounds, resulting in synergistic inhibition of growth and ethanol yield in E. coli (Zaldivar et al. 1999, 2000; Zaldivar and Ingram 1999). Synergistic inhibition of 2-furfural with acetic acid was also shown for S. cerevisiae (Palmqvist et al. 1999).
Hemicellulose fractions from alkaline wet oxidation of wheat straw were added nine phenols and 2-furoic acid to test if hydrolysate components other than furfural or 5-hydroxymethylfurfural were present to display synergistic inhibition. Synergistic inhibitory effects of 10-mM syringaldehyde and acetovanillone with wheat straw hydrolysate components were shown in S. cerevisiae (Klinke et al. 2003). Growth and ethanol production in T. mathranii A3M3 was not inhibited at 2-mM phenol additions to hydrolysate, but by increasing the concentration to 10-mM, synergistic inhibitory effects with hydrolysate components were evident for all nine phenols and 2-furoic acid compared to synthetic medium (Klinke et al. 2001). Removal of phenols from hemicellulose hydrolysates with high concentrations of Hibbert’s ketones improved fermentability (Buchert et al. 1990; Larsson et al. 1999b; Tran and Chambers 1986). The toxicity of Hibbert’s ketones has not been demonstrated, but the inhibitory potentials of other phenol ketones (acetophenone-type) were comparable with phenol aldehydes towards S. cerevisiae (Table 5) and T. mathranii (Table 6) (Klinke et al. 2001, 2003).
Salt inhibition
Alkali salts and heavy metal salts are present in lignocellulosic hemicellulose hydrolysates. The biomass, chemicals added during pre-treatment, and metals released from the walls of the pre-treatment equipment are the main sources of inorganic salts. Recirculation of process water in a bioethanol process will also result in higher salt concentrations. The varying quality of molasses or worts utilized by breweries, wineries or distilleries are due to different mineral concentrations and other organic components affecting fermentation yields. Much attention has been given to understand the effects of inorganic nutrients required for cell growth and ethanol production in yeast (Jones and Greenfield 1984; Jones 1986).
Trace minerals or metals are transported across the cell membrane by either active or passive mechanisms, and differences in toxicity have been demonstrated for the cations Ca2+, Mg2+, K+, Na+, NH4+, and anions Cl−, SO42−, HPO4− in S. cerevisiae (Maiorella et al. 1984). Chloride (Cl−) has been shown to have detrimental effects in concentrations above 6 g/l in sugarcane molasses on ethanol formation in Z. mobilis(Doelle et al. 1990). Magnesium is important for many metabolic and physiological functions in yeast and bacteria (Alexandre and Charpentier 1998), while calcium has toxic effect when it is present in high amount (Maiorella et al. 1984). High concentrations of calcium in molasses have been shown to be the main inhibitory salt to S. cerevisiae (Tajima et al. 1966). If inorganic salts with the same valence are present in wrong ratios (e.g., Ca/Mg-ratio), they slow down fermentation. Fermentation was improved by addition of magnesium to beet molasses (Wolniewicz et al. 1988) and to barley wort (Bromberg et al. 1997). Heavy metals Zn, Cu, Fe, Co are micronutrients with a relatively narrow optimum concentration range for the organisms, and at higher concentrations they have toxic effects. Sluggish fermentation was correlated to higher cupper content in raisin (Akrida-Demertzi et al. 1988). Recently, it was shown that high mineral salt concentration rather than high ethanol concentration is the main inhibitor of xylose fermentation by the thermophilic bacterium T. thermosaccharolyticum (Lynd 2001). Generally, thermophiles show lower tolerance towards high sugar and ethanol concentrations than mesophiles. This may be due to the 30–40°C higher growth temperature where inorganic salts and other organic components have higher solubility and osmotic pressure (Herrero and Gomez 1980; Lynd 1989).
Conclusion
The aromatic compounds formed by pre-treatment display different inhibitory potential according to their structure. Formation of certain degradation products such as 2-furfural should be minimized because of its synergistic inhibitory effects with other degradation products present in lignocellulosic hydrolysates. Aromatic and carboxylic acids are generally not inhibitory to either pentose or hexose fermenting microorganisms, but phenols, phenol aldehydes and phenol ketones (Hibbert’s ketones) are potent inhibitors and their formation during pre-treatment should be minimized.
In addition to high ethanol, sugar and salt tolerance, ethanol-producing strains only perform well in hemicellulose hydrolysates if they are tolerant towards inhibitors. Screening for inhibitor resistance will be needed for future selection and development of microbial strains. Genetically engineered microorganisms that can utilize all sugars in the hemicellulose hydrolysates are being developed. These organisms have not yet been reported to ferment lignocellulosic hydrolysates without prior detoxification. From model inhibition experiments E. coli and T. mathranii seems to be potential candidates for future work. S. cerevisiae has recently been genetically modified to produce ethanol in severely inhibiting spruce hemicellulose hydrolysates by heterologous expression of laccase that detoxifies the phenolic inhibitors during fermentation. However, at the present wild-type adapted microbial strains perform better in lignocellulosic hydrolysates.
References
Ahring BK, Jensen K, Nielsen P, Bjerre AB, Schmidt AS (1996) Pretreatment of wheat straw and conversion of xylose and xylan to ethanol by thermophilic anaerobic bacteria. Bioresour Technol 58:107–113
Akrida-Demertzi K, Demertzis PG, Koutinas AA (1988) pH and trace-elements content in raisin extract industrial-scale alcoholic fermentation. Biotechnol Bioeng 31:666–669
Alexandre H, Charpentier C (1998) Biochemical aspects of stuck and sluggish fermentation in grape must. J Ind Microbiol Biotechnol 20:20–27
Alén R, Sjöström E, Suominen S (1990) Application of ion-exclusion chromatography to alkaline pulping liquors; separation of hydroxy carboxylic acids from inorganic solids. J Chem Tech Biotechnol 51:225–233
Ando S, Arai I, Kiyoto K, Hanai S (1986) Identification of aromatic monomers in steam-exploded poplar and their influences on ethanol fermentation by Saccharomyces cerevisiae. J Ferment Technol 64:567–570
Baeza J, Freer J (1997) Chemical characterization of wood and its components. In: Hon DNS, Shirashi N (eds) Wood and cellulosic chemistry. Dekker, New York, pp 275–374
Barber AR, Hansson H, Pamment NB (2000) Acetaldehyde stimulation of the growth of Saccharomyces cerevisiae in the presence of inhibitors found in lignocellulose-to-ethanol fermentations. J Ind Microbiol Biotechnol 25:104–108
Bardet M, Robert DR (1985) On the reactions and degradation of the lignin during steam hydrolysis of aspen wood. Svensk Papperst 6:61–67
Barquinero E, Cruz R, Mieres G, Dominguez H (1980) Caracterizacion quimica de efluentes de pulpeoquimico a la soda de bagazo. Revista Icidca 14:28–33
Bjerre AB, Olesen AB, Fernqvist T, Plöger A, Schmidt AS (1996) Pretreatment of wheat straw using combined wet oxidation and alkaline hydrolysis resulting in convertible cellulose and hemicellulose. Biotechnol Bioeng 49:568–577
Bobleter O (1994) Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19:797–841
Bromberg SK, Bower PA, Duncombe GR, Fehring J, Gerber L, Lau VK, Tata M (1997) Requirements for zinc, manganese, calcium and magnesium in wort. J Am Soc Brew Chem 55:123–128
Buchert J, Niemelä K, Puls J, Poutanen K (1990) Improvement in the fermentability of steamed hemicellulose hydrolysate by ion exclusion. Process Biochem Int pp 176–180
Burtscher E, Bobleter O, Schwald W, Concin R, Binder H (1987) Chromatographic analysis of biomass reaction products produced by hydrothermolysis of poplar wood. J Chromatogr 390:401–412
Campbell MM, Sederoff R (1996) Variation in lignin content and composition: mechanisms of control and implications for the genetic improvement of plants. Plant Physiol 110:3–13
Chum HL, Johnson DK, Black SK, Overend RP (1990) Pretreatment catalyst effects and the combined severity parameter. Appl Biochem Biotechnol 24/25:1–14
Clark TA, Mackie KL (1984) Fermentation inhibitors in wood hydrolysates derived from the softwood Pinus radiata. J Chem Tech Biotechnol 34B:101–110
Delgenes JP, Moletta R, Navarro JM (1996) Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb Technol 19:220–225
Dien BS, Cotta MA, Jeffries TW (2003) Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol 63:258–266
Dierssen GA, Holtegaard K, Jensen B, Rosen K (1956) Volatile carboxylic acids in molasses and their inhibitory action on fermentation. Int Sugar J 58:35–39
Doelle M, Greenfield PF, Doelle HW (1990) Effect of mineral ions on ethanol formation during sugar cane molasses fermentation using Zymomonas mobilis ATCC 39676. Process Biochem Int: 151–156
Dunlop AP (1948) Furfural formation and behaviour. Ind Eng Chem 40:204–209
Fan LT, Lee Y-H, Gharpuray MM (1982) The nature of lignocellulosics and their pretreatments for enzymatic hydrolysis. In: Fiechter A (ed) Microbial reactions. Springer, Berlin Heidelberg New York, pp 157–187
Fengel D, Wegener G (1989) Wood. Chemistry, ultrastructure, reactions. Walter de Gruyter, Berlin
Fenske JJ, Griffin DA, Penner MH (1998) Comparison of aromatic monomers in lignocellulosic biomass prehydrolysates. J Ind Microbiol Biotechnol 20:364–368
Fenske JJ, Hashimoto AG, Penner MH (1999) Relative fermentability of lignocellulosic dilute-acid prehydrolysates: application of a Pichia stipititis-based toxicity assay. Appl Biochem Biotechnol 73:145–157
Galbe M, Zacchi G (2002) A review of the production of ethanol from softwood. Appl Microbiol Biotechnol 59:618–628
Garrote G, Dominguez H, Parajó JC (1999) Hydrothermal processing of lignocellulosic materials. Holz Roh Werkstoff 57:191–202
Gong CS, Chen CS, Chen LF (1993) Pretreatment of sugar cane bagasse hemicellulose hydrolysate for ethanol by yeast. Appl Biochem Biotechnol 39/40:83–88
Hahn-Hägerdal B, Jeppsson H, Olsson L, Mohagheghi A (1994) An interlaboratory comparison of the performance of ethanol-producing micro-organisms in a xylose-rich acid hydrolysate. Appl Microbiol Biotechnol 41:62–72
Herrero AA, Gomez RF (1980) Development of ethanol tolerance in Clostridium thermocellum: effect of growth temperature. Appl Environ Microbiol 40:571–577
Holtzapple MT, Jun JH, Ashok G, Patibandla S, Dale BE (1991) The ammonia freeze explosion (AFEX) process—a practical lignocellulose pretreatment. Appl Biochem Biotechnol 28–29:59–74
Jones RP (1986) Effect of the relative concentration of ion species on yeast growth and ethanol-production. Process Biochem 21:183–187
Jones RP, Greenfield PF (1984) A review of yeast ionic nutrition. Part I. Growth and fermentation requirements. Process Biochem April:48–59
Jönsson LJ, Palmqvist E, Nilvebrant NO, Hahn-Hägerdal B (1998) Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl Microbiol Biotechnol 49:691–697
Klinke HB, Thomsen AB, Ahring BK (2001) Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. Appl Microbiol Biotechnol 57:631–638
Klinke HB, Ahring BK, Schmidt AS, Thomsen AB (2002) Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol 82:15–26
Klinke HB, Olsson L, Thomsen AB, Ahring BK (2003) Potential inhibitors from wet oxidation of wheat straw and their effect on ethanol production of Saccharomyces cerevisiae: wet oxidation and fermentation by yeast. Biotechnol Bioeng 81:738–747
Larsson M, Galbe M, Zacchi G (1997) Recirculation of process water in the production of ethanol from softwood. Bioresour Technol 60:143–151
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant NO (1999a) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159
Larsson S, Reimann A, Nilvebrant NO, Jönsson LJ (1999b) Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol 77–79:91–103
Larsson S, Quintana-Sáinz A, Reimann A, Nilvebrant N-O, Jönsson LJ (2000) Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae. Appl Biochem Biotechnol 84–86:617–632
Larsson S, Cassland P, Jönsson LJ (2001) Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulosic hydrolysates by heterologous expression of laccase. Appl Environ Microbiol 67:1163–1170
Lawther JM, Sun R (1996a) The fractional characterisation of polysaccharides and lignin components in alkaline treated and atmospheric refined wheat straw. Ind Crops Prod 5:87–95
Lawther JM, Sun R, Banks WB (1996b) Fractional characterization of alkali-labile lignin and alkaline-insoluble lignin from wheat straw. Ind Crops Prod 5:291–300
Lee WG, Lee JS, Shin CS, Park SC, Chang HN, Chang YK (1999) Ethanol production using concentrated oak wood hydrolysates and methods to detoxify. Appl Biochem Biotechnol 77–79:547–559
Leonard RH, Hajny GJ (1945) Fermentation of wood sugars to ethyl alcohol. Ind Eng Chem 37:390–395
Lynd LR (1989) Production of ethanol from lignocellulosic materials using thermophilic bacteria: critical evaluation of potential and review. In: Fiechter A (ed) Lignocellulosic materials. Springer, Berlin Heidelberg New York, pp 1–52
Lynd L (2001) Salt accumulation resulting from base added for pH control, and not ethanol, limits growth of Thermoanaerobacterium thermosaccharolyticum HG-8 at elevated feed xylose concentrations in continuous culture. Biotechnol Prog 17:118–125
Maiorella BL, Blanch HW, Wilke CR (1984) Feed component inhibition in ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 26:1155–1166
Maman O, Marseille F, Guillet B, Disnar JR, Morin P (1996) Separation of phenolic aldehydes, ketones and acids from lignin degradation by capillary zone electrophoresis. J Chromatogr A 755:89–97
Martin C, Jonsson LJ (2003) Comparison of the resistance of industrial and laboratory strains of Saccharomyces and Zygosaccharomyces to lignocellulose-derived fermentation inhibitors. Enzyme Microb Technol 32:386–395
Martin C, Galbe M, Nilvebrant NO, Jonsson LJ (2002a) Comparison of the fermentability of enzymatic hydrolyzates of sugarcane bagasse pretreated by steam explosion using different impregnating agents. Appl Biochem Biotechnol 98:699–716
Martin C, Galbe M, Wahlbom CF, Hahn-Hagerdal B, Jonsson LJ (2002b) Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb Technol 31:274–282
Martinez A, Rodriguez ME, Wells ML, York SW, Preston JF, Ingram LO (2001) Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol Prog 17:287–293
McMillan JD (1994a) Conversion of hemicellulose hydrolyzates to ethanol. In: Himmel ME, Baker JO, Overend RP (eds) Enzymatic conversion of biomass for fuels production. ACS Symp Ser, pp 292–324
McMillan JD (1994b) Pre-treatment of lignocellulosic biomass. In: Himmel ME, Baker JO, Overend RP (eds) Enzymatic conversion of biomass for fuels production. ACS Symp Ser, pp 411–437
McMillan JD, Newman MM, Templeton DW, Mohagheghi A (1999) Simultaneous saccharification and cofermentation of dilute-acid pretreated poplar to ethanol using xylose-fermenting Zymomonas mobilis. Appl Biochem Biotechnol 77–79:649–665
Mikulásova M, Pekarovicová A, Vodnú S (1990) Influence of phenolics on biomass production by Candida utilis and Candida albicans. Biomass 23:149–154
Mills SC, Child JJ, Spencer JFT (1971) The utilization of aromatic compounds by yeasts. Antonie Van Leeuwenhoek 37:281–287
Nishikawa NK, Sutcliffe R, Saddler JN (1988) The influence of lignin degradation products on xylose fermentation by Klebsiella pneumoniae. Appl Microbiol Biotechnol 27:549–552
Olsson L, Hahn-Hägerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb Technol 18:312–331
Palmqvist E, Hahn-Hägerdal B (2000a) Fermentation of lignocellulosic hydrolysates. I. Inhibition and detoxification. Bioresour Technol 74:17–24
Palmqvist E, Hahn-Hägerdal B (2000b) Fermentation of lignocellulosic hydrolysates. II. Inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Palmqvist E, Hahn-Hägerdal B, Galbe M, Zacchi G (1996) The effect of water-soluble inhibitors from steam-pretreated willow on enzymatic hydrolysis and ethanol fermentation. Enzyme Microb Technol 19:470–476
Palmqvist E, Grage H, Meinander NQ, Hahn-Hägerdal B (1999) Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol Bioeng 63:46–55
Ragnar M, Lindgren CT, Nilvebrant N-O (2000) pKa values of guaiacyl and syringyl phenols related to lignin. J Wood Chem Technol 20:277–305
Ranatunga TD, Jervis J, Helm RF, McMillan JD, Hatzis C (1997a) Identification of inhibitory components toxic toward Zymomonas mobilis CP4(pZB5) xylose fermentation. Appl Biochem Biotechnol 67:185–197
Ranatunga TD, Jervis J, Helm RF, McMillan JD, Hatzis C (1997b) Toxicity of hard-wood extractives toward Saccharomyces cerevisiae glucose fermentation. Biotechnol Lett 19:1125–1127
Rivard CJ, Engel RE, Hayward TK, Nagle NJ, Hatzis C, Philippidis GP (1996) Measurement of the inhibitory potential and detoxification of biomass pretreatment hydrolysate for ethanol production. Appl Biochem Biotechnol 57/58:183–191
Saka S (1997) Chemical composition and distribution. In: Hon DNS, Shirashi N (eds) Wood and cellulosic chemistry. Dekker, New York, pp 51–81
Sanchez B, Bautista J (1988) Effects of furfural and 5-hydroxymethylfurfural on the fermentation of Saccharomyces cerevisiae and biomass production from Candida guilliermondii. Enzyme Microb Technol 10:315–318
Sarkanen KV, Ludwig CH (1971) Lignins: occurrence, formation, structure and reactions. Wiley-Interscience, New York, pp 345–372
Sears KD, Beélik A, Casebier RL, Engen RJ, Hamilton JK, Hergert HL (1971) Southern pine prehydrolyzates: characterization of polysaccharides and lignin fragments. J Polym Sci, Part C 36:425–433
Shevchenko SM, Chang K, Robinson J, Saddler JN (2000) Optimization of monosaccharide recovery by post-hydrolysis of the water-soluble hemicellulose component after steam explosion of softwood chips. Appl Biochem Biotechnol 72:207–211
Sierra-Alvarez R, Lettinga G (1991) The methanogenic toxicity of wastewater lignins and lignin related compounds. J Chem Tech Biotechnol 50:443–455
von Sivers M, Zacchi G (1996) Ethanol from lignocellulosics: a review of the economy. Bioresour Technol 56:131–140
von Sivers M, Zacchi G, Olsson L, Hahn-Hägerdal B (1994) Cost ananlysis of ethanol production from willow using recombinant Escherichia coli. Biotechnol Prog 10:555–560
Sjöström E (1991) Carbohydrate degradation products from alkaline pretreatment of biomass. Biomass Bioenergy 1:61–64
Sommer P (1998) Conversion of hemicellulose and d-xylose into ethanol by the use of thermophilic anaerobic bacteria. Ph.D. Thesis, Technical University of Denmark, p 64
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Taherzadeh MJ, Eklund R, Gustafsson L, Niklasson C, Lidén G (1997a) Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind Eng Chem Res 36:4659–4665
Taherzadeh MJ, Niklasson C, Lidén G (1997b) Acetic acid—friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae. Chem Eng Sci 52:2653–2659
Tajima K, Yoshizumi H, Terashima Y (1966) Salt and sugar tolerances of yeast on alcoholic fermentation. I. The inhibition of fermentation by the highly concentrated salts in molasses. Jap Ferment J: 77–84
Tengborg C, Stenberg K, Galbe M, Zacchi G, Larsson S, Palmqvist E, Hahn-Hägerdal B (1998) Comparison of SO2 and H2SO4 impregnation of softwood prior to steam pretreatment on ethanol production. Appl Biochem Biotechnol 70–72:3–15
Torry-Smith M, Sommer P, Ahring BK (2003) Purification of bioethanol effluent in an UASB reactor system with simultaneous biogas formation. Biotech Bioeng 84:7–12
Torssell KBG (1997) Natural product chemistry: a mechanistic, biosynthetic and ecological approach. Apotekarsocieteten, Stockholm
Tran AV, Chambers RP (1985) Red oak wood derived inhibtors in the ethanol fermentation of xylose by Pichia stipititis CBS 5776. Biotechnol Lett 7:841–846
Tran AV, Chambers RP (1986) Lignin and extractives derived inhibitors in the 2,3-butanediol fermentation of mannose-rich prehydrolysates. Appl Microbiol Biotechnol 23:191–197
Umezawa T, Higuchi T (1991) Chemistry of lignin degradation by lignin peroxidases. In: Leatham GF, Himmel ME (eds) Enzymes in biomass conversion. ACS Symposium Series, Washington, pp 236–246
Wilkie AC, Riedesel KJ, Owens JM (2000) Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 19:63–102
Wolniewicz E, Letourneau F, Villa P (1988) Comportment of S. cerevisiae in relation to ions Ca++ and Mg++ on beet molasses wort. Biotechnol Lett 10:355–360
de Wulf O, Thonart P, Gainage P, Marlier M, Paris A, Paquot M (1986) Bioconversion of vanillin to vanillyl alcohol by Saccharomyces cerevisiae. Biotechnol Bioeng Symp 17:605–616
Zaldivar J, Ingram LO (1999) Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol Bioeng 66:203–210
Zaldivar J, Martinez A, Ingram LO (1999) Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 65:24–33
Zaldivar J, Martinez A, Ingram LO (2000) Effect of alcohol compounds found in hemicelllulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol Bioeng 68:524–530
Zaldivar J, Nielsen J, Olsson L (2001) Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl Microbiol Biotechnol 56:17–34
Zemek J, Kosikova B, Augustin J, Joniak D (1979) Antibiotic properties of lignin components. Folia Microbiologica 24:483–486
Acknowledgments
We thank the Danish Energy Program for support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klinke, H.B., Thomsen, A.B. & Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66, 10–26 (2004). https://doi.org/10.1007/s00253-004-1642-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1642-2