Abstract
The FPS1 gene coding for the Fps1p aquaglyceroporin protein of an industrial strain of Saccharomyces cerevisiae was disrupted by inserting CUP1 gene. Wild-type strain, CE25, could only grow on YPD medium containing less than 0.45% (v/v) acetic acid, while recombinant strain T12 with FPS1 disruption could grow on YPD medium with 0.6% (v/v) acetic acid. Under 0.4% (v/v) acetic acid stress (pH 4.26), ethanol production and cell growth rates of T12 were 1.7 ± 0.1 and 0.061 ± 0.003 g/l h, while those of CE25 were 1.2 ± 0.1 and 0.048 ± 0.003 g/l h, respectively. FPS1 gene disruption in an industrial ethanologenic yeast thus increases cell growth and ethanol yield under acetic acid stress, which suggests the potential utility of FPS1 gene disruption for bioethanol production from renewable resources such as lignocelluloses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioethanol produced from renewable resources, such as lignocelluloses, is an attractive alternative to fossil fuels. However, some factors, like temperature, pH and by-products of fermentation, strongly affect alcoholic fermentation, and as a consequence, the yield of ethanol. One of the main by-products of fermentation is acetic acid. Acetic acid formed by acid-catalyzed hydrolysis of lignocelluloses or as the by-product of fermentation has deleterious consequence on both the growth rate and fermentation of ethanologenic Saccharomyces cerevisiae (Lee et al. 1999; Maiorella et al. 1983). The undissociated form of acetic acid which enters into yeast cells causes a decrease of pH in the cytoplasm and inhibits the activity of some enzymes, especially endolase, phosphoglyceromutase, aldolase and triosephosphate isomerase (Ferrari et al. 1992; Pampulha and Loureiro 1989) Meanwhile, the intracellular acetate ion is also toxic to yeast cells. Obviously, decreasing the uptake of acetic acid may be an effective way to improve acetic acid resistance of yeast.
As a member of major intrinsic protein (MIP), Fps1p is an aquaglyceroporin of the plasma membrane of yeast involved in some important physiological functions, such as glycerol uptake and efflux (Luyten et al. 1995), uptake of arsenite and antimonite (Wysocki et al. 2001), regulation of osmotolerance (Tamas et al. 1999) and acetic acid uptake. Deletion of FPS1 gene could not only decrease resistance of yeast to some chemicals like CdCl2, Calcofluor White, ethanol, rapamycin, etc., but also increase resistance of yeast to acetate, arsenic and sodium meta-arsenite (http://www.yeastgenome.org/cgi-bin/phenotype/phenotype.pl?dbid = S000003966 ). In low pH cultures, undissociated acetic acid enters the yeast cells primarily by facilitated diffusion through the Fps1p aquaglyceroporin channel. The acetic acid which enters the yeast cells generates an intracellular acetate anion [CH3COO−] pool. The anion pool then transiently stimulates the HOG signaling (Mollapour and Piper 2006), leading to activation of the Hog1p, a MAP kinase, which directly phosphorylates the Fps1p. This phosphorylation provides the signals for Fps1p endocytosis and degradation in the vacuole, eliminating the channel of acetic acid entry to the cell, enhancing acetic acid tolerance of haploid yeast (Mollapour and Piper 2007; Mollapour et al. 2008). Deletion of FPS1 could increase ethanol yield of haploid yeast under condition without acetic acid stress (Zhang et al. 2007). But most of the ethanologenic yeast are diploid, aneuploid or polyploidy and have different genetic background from the haploid yeast.
Yeast CUP1 gene, encoding a metallothionein, endows copper resistance in yeast (Fogel and Welch 1982; Hottiger et al. 1995), and has been widely used as selectable marker for yeast genetic transformation, especial for industrial yeast strains (Hottiger et al. 1995; He et al. 2003; Wang et al. 2007, 2008). In our early study, the FPS1 gene of haploid S. cerevisiae YS58 (MATα leu2-3,112 his4-519 trp1-789 ura3-52) (Teunissen et al. 1993) was disrupted using LEU2 and CUP1 as selection marker, respectively. The two types of YS58-derived haploid deletants of FPS1 showed the same acetic acid tolerance, which was increased by 31.6% when compared with strain YS58. Meanwhile, no obvious difference in fermentation performance was observed between the two types of fps1 deletants. In this work, to investigate effects of FPS1 deletion on acetic acid tolerance and fermentation performance of diploid ethanologenic yeast, the aquaglyceroporin encoding gene FPS1 was disrupted by integrating CUP1 gene. The acetic acid tolerance and fermentation performance of the recombinant strain under acetic acid stress are reported.
Materials and methods
Strains and plasmids
Escherichia coli DH5α (supE44 ΔlacU169(φ80lacZΔM15) hsdR17 recAl endAl gyrA96 thi-1) was used as the host for maintenance and propagation of plasmids. Saccharomyces cerevisiae CE25 (CGMCC 2.1418), an industrial ethanologenic yeast strain, was from China General Microbiological Culture Collection Center (CGMCC). YEp352 vector (Hill et al. 1993) was used for plasmid construction and propogation. Plasmid pYCUP (Zhang et al. 2005) was used to isolate CUP1 gene.
Cultivation conditions
Escherichia coli cells were cultivated at 37°C in Luria–Bertani (LB) medium. Yeast cells were grown at 28°C in yeast extract/peptone/dextrose (YPD) medium (Burke et al. 2000). Recombinant strains were selected on YPD containing 12 mM CuSO4. For acetic acid resistance analysis, the pH of YPD medium was adjusted to 4.5 with HCl, and then supplemented with different concentration of acetic acid. Acetic acid was added to the indicated level from an 8.7 M acetic acid stock solution titrated to pH 4.5 with NaOH (Mollapour and Piper 2007).
Fermentation experiments were performed in YPD10 medium containing 2% peptone, 1% yeast extract, 10% glucose (w/v) and different levels of acetic acid.
DNA manipulation
DNA manipulations in E. coli were performed as standard procedures. The isolation of yeast chromosomal DNA was carried out with the methods described by Burke et al. (2000).
Plasmids construction
The 2010 bp DNA fragment corresponding to the FPS1 gene was amplified from the genomic DNA of yeast strain CE25 via PCR using primers FPS1-L (5′-CCGAAGCTTATGAGTAATCCTCAAAAAGCT-3′, HindIII), and FPS1-R (5′-CCAGAGCTCTCATGTTACCTTCTTAGCATT-3′, SacI). The amplified FPS1 was digested with SacI and HindIII and then inserted into YEp352 vector to generate recombinant plasmid pYFPS. A 1080 bp KpnI–PstI fragment containing CUP1 gene from the plasmid pYCUP was subcloned into pYFPS to give recombinant plasmid pYFCUP, in which the coding region of FPS1 was disrupted by a 60-bp internal deletion and 1,080-bp of CUP1 insertion (Fig. 1).
Yeast transformation
The 3030 bp recombinant DNA fragment FPS1L-CUP1-FPS1R was isolated from plasmid pYFCUP by digestion with SacI and HindIII. S. cerevisiae CE25 cells were transformed with the recombinant DNA fragment by electroporation using a Bio-Rad Gene-Pulser Apparatus (1.5 kV, 50 μF, 200 Ω, 3 ms). The transformed yeast cells were firstly grown in YPD medium containing 1 mM Tris/HCl and 1 mM MgCl2 for 2 h, and then spread on YPD plate containing 12 mM CuSO4 and cultured at 28°C for 3 days.
Genetic stability test of recombinant strain
Recombinant yeast strains were firstly transferred onto YPD slant for ten generations. Each generation was cultivated for 24 h at 28°C. Then plate streaking of the tenth generation yeast cells was performed. After 2 days cultivation at 28°C, 100 single colonies were chosen randomly and diluted in 0.5 ml of sterilized water. The cell suspension was kept at room temperature for 2 h, and a loopful of cell suspension was inoculated onto YPD plates containing 14 mM CuSO4 and cultivated at 28°C for 2 days.
PCR verification of recombinant strain
PCR analysis was performed using the primers FPS1-L, FPS1-R and CUP1-L (5′-CTTGGTACCTGGGCGCTATACGTGCATATG-3′). PCR was performed in 50 μl with 25 μl Taq Mastermix (Tiangen Biotech (Beijing) Co., Ltd, Beijing, China), 120 ng template DNA, and 0.2 μM primers. Cycle conditions were 94°C for 5 min followed by 30 cycles of 94°C for 30 s, 54°C for 30 s, and 72°C for 3 min 20 s, and finally 72°C for 10 min.
Acetic acid tolerance tests
To analyze the acetic acid tolerance of wild-type yeast strain and recombinant strains, yeast strains were firstly inoculated in 5 ml YPD and grown at 28°C with stirring at 150 rpm for 16 h. One ml culture of each strain was withdrawn, centrifuged, the pellets were washed twice with sterile water and resuspended in 1 ml sterile water. The cell suspension was serially diluted, kept at room temperature for 2 h, and then a loopful of suspension was inoculated on to YPD (pH 4.5) plates supplemented with the indicated level of acetic acid and cultivated at 28°C for 2 days.
Alcoholic fermentation under different acetic acid stress
For alcoholic fermentation, yeast cells were firstly grown in 5 ml liquid YPD at 28°C for 16 h under aerobic conditions. The cultures were inoculated into 200 ml of liquid YPD at the ratio of 1:100, respectively. After cultivation at 28°C for 18 h with agitation of 150 rpm, the culture was inoculated into 100 ml YPD10 media containing 0, 0.2, 0.3, 0.4% (v/v) of acetic acid in Erlenmeyer flasks. Initial cell concentration was set at OD600 of 1 after inoculation. The flasks were sealed by plastic wrap and the fermentation was carried out at low agitation of 100 rpm. All fermentation experiments were repeated at least three times.
Measurement of cell biomass, glucose, ethanol and glycerol
Samples (5 ml each) were withdrawn periodically. The biomass was determined as described previously (He et al. 2000). The concentrations of ethanol and glucose in medium were determined using a SBA-40C biosensor analyzer. The content of glycerol in the fermentation broth was determined by HPLC using differential refractive index detector and Agilent Zorbax carbohydrate column eluted by 75% (v/v) acetonitrile at 1 ml/min.
Results and discussion
Construction of recombinant yeast strain with FPS1 disruption
Recombinant plasmid pYFCUP was constructed, in which the FPS1 gene was disrupted by a 60-bp internal deletion and 1080-bp of CUP1 insertion (Fig. 1). Saccharomyces cerevisiae CE25 was transformed with the 3030-bp recombinant DNA fragment FPS1L-CUP1-FPS1R from plasmid pYFCUP. Transformants were screened for their resistance to CuSO4. Since the wild-type strain CE25 could only grow on the medium containing less than 10 mM CuSO4, transformants were screened on YPD medium containing 12 mM CuSO4.
Transformants were analyzed further for their resistance to CuSO4, and six transformants that could grow on YPD plate containing 14 mM CuSO4were obtained. The six transformants gave the same higher acetic acid tolerance than the strain CE25. Meanwhile, result of PCR analysis using primers FPS1L and FPS1R shown that the FPS1 gene was disrupted in all six transformants. The recombinant strain was name as T12, in which all two copy of FPS1 gene were disrupted by insertion of CUP1 gene (Fig. 2).
The genetic stability of the recombinant strain T12 was analyzed as described in materials and methods. After growing recombinant strain T12 under non-selective condition for ten generations, 100 random single colonies of the 10th generation could grow on YPD containing 14 mM copper sulfate. Meanwhile, the desired disruption cassette can be PCR-amplified from ten randomly selected colonies. These results suggested that recombinant strain T12 was genetically stable.
Acetic acid tolerance test
Mid-growth cultures of wild-type strain CE25 and recombinant T12 were diluted serially. The serial dilutions were incubated on YPD solid media (pH 4.5) containing acetic acid from 0 to 0.6% (v/v). Both of T12 and CE25 could grow on YPD (pH 4.5) plate containing less than 0.45% acetic acid. However, at 0.5% acetic acid, recombinant strain T12 still could grow but strain CE25 could not. Recombinant strain T12 could resist 0.6% acetic acid in YPD medium (pH 4.5). The results in Fig. 3 demonstrated that the acetic acid tolerance of yeast strain was improved by disruption of the FPS1 gene.
Alcoholic fermentation
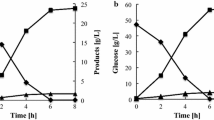
The fermentation profiles of recombinant strain T12 and the wild-type strain CE25 were compared under different acetic acid stresses. When fermentations were conducted in YPD10 medium without acetic acid, biomass and ethanol increased during the first 9 h with a corresponding decline of glucose. After 12 h, the glucose was consumed completely to produce 43.7 ± 2.1 and 40 ± 1.8 g/l of ethanol by T12 and CE25, respectively. The specific ethanol productivity (g/g DCW) of T12 was 9.2% higher than that of CE25 (Fig. 4a). Under the above condition, the extracellular glycerol yields of T12 and CE25 were 2.3 ± 0.1 and 2.9 ± 0.1 g/l, respectively (Fig. 5). With the increase of acetic acid in media, cell growth and ethanol production of the two strains were all inhibited but these of T12 were less inhibited than those of CE25. Under 0.2% acetic acid stress (pH 4.54), the ethanol production rates of T12 and CE25 were 3.2 ± 0.1 and 2.9 ± 0.1 g/l h (Fig. 4b). When fermentation was conducted in medium containing 0.3% acetic acid (pH 4.37), the ethanol production rates of T12 and CE25 were 2.3 ± 0.1 and 1.7 ± 0.1 g/l h, and the cell growth rates of T12 and CE25 were 0.092 ± 0.004 and 0.079 ± 0.003 g/l h (Fig. 4c). When acetic acid was increased to 0.4% (pH 4.26), distinct differences in both cell growth rate and ethanol production rate between the two stains were observed (Fig. 4d). Recombinant strain T12 consumed all the glucose after 24 h to produce 41.7 ± 2 g ethanol/l, while strain CE25 consumed the same glucose after 30 h to produce 35.7 ± 1.9 l ethanol/l. The ethanol production rate of T12 was increased by 46% as compared with that of CE25. The cell growth rates of T12 and CE25 were 0.061 ± 0.003 and 0.048 ± 0.003 g/l h. Hence, recombinant strain T12 had advantage in ethanol production over the wild-type strain CE25, and the substantial effect of FPS1 disruption was observed under 0.4% acetic acid stress.
Comparison of fermentation performances between recombinant strain T12 and wild-type S. cerevisiae CE25 under different acetic acid stress. a Fermentation without acetic acid stress. b Fermentation with 0.2% acetic acid. c Fermentation with 0.3% acetic acid. d Fermentation with 0.4% acetic acid. Wild-type S. cerevisiae CE25 (filled square Biomass, filled triangle Ethanol, filled circle Residual glucose), recombinant strain T12 (open square Biomass, open triangle Ethanol, open cirlce Residual glucose). Values are means of three replications ± SD
As an aquaglyceroporin of the plasma membrane of yeast, Fps1p is an important glycerol-export channel protein. Inactivity of Fps1p would reduce the glycerol efflux. In this study, the extracellular glycerol yield of T12 with FPS1 disruption was decreased by 21% when compared with wild-type strain CE25. The block of glycerol efflux would lead to intracellular glycerol accumulation which would increase cell turgor. It has been reported that mutants lacking Fps1p grew poorly in YPD media (Tamas et al. 1999; Zhang et al. 2007). In our research, compared with the original strain CE25, cell growth of strain T12 had a slight decrease and ethanol yield was improved by 9.3% under condition without acetic acid stress (Fig. 4a), which is in agreement with the results of haploid fps1 mutant (Zhang et al. 2007).
Another role of Fps1p is to regulate acetic acid uptake. The intake of acetic acid not only makes cell acidification but also generates an intracellular acetate anion [CH3COO−] pool, which has deleterious consequence on cell growth and metabolic activity. The inhibitory effect of acetic acid on cell growth is more effective than on fermentation performance (Phowchinda et al. 1995). Inactivity of Fps1p may decrease acetic acid entering into cells and enhance acetic acid tolerance of S. cerevisiae. The results obtained in this study indicate, for the first time, that the acetic acid tolerance of industrial ethanologenic S. cerevisiae in fermentation can be improved by disruption of the FPS1 gene. Under the acetic acid stress, the cell growth is more affected than the ethanol production. When acetic acid was increased to 0.4% (v/v) from zero, the biomass of recombinant T12 decreased from 2.1 ± 0.1 to 1.5 ± 0.1 g/l, the ethanol yield of recombinant T12 decreased from 43.7 ± 2.1 to 41.7 ± 2.0 g/l, while the biomass of wild-type strain CE25 decreased from 2.2 ± 0.1 to 1.5 ± 0.1 g/l and the ethanol yield of CE25 decreased from 40 ± 1.9 to 35.7 ± 1.6 g/l. The ethanol production rate of T12 was increased by 46% as compared with that of CE25 under 0.4% acetic acid stress. In short, the acetic acid tolerance and ethanol fermentation performance of industrial diploid S. cerevisiae is successfully improved by FPS1 disruption. This work offers the possibility for the application of S. cerevisiae in bioethanol production from renewable resources such as lignocelluloses.
Glycerol is a major by-product during anaerobic production of ethanol by S. cerevisiae. Regulation of glycerol synthesis and transport in S. cerevisiae leads to improvement of ethanol production (Nissen et al. 2000; Zhang et al. 2007). Recently, deletion of genes GPD1 and GPD2 encoding NAD-dependent glycerol-3-phosphate dehydrogenase led to the complete elimination of glycerol production in S. cerevisiae and inability to grow anaerobically for lack of NADH reoxidation (Medina et al. 2010). However, when the E. coli mhpF gene encoding the acetylating NAD-dependent acetaldehyde dehydrogenase was expressed in the above deletant, the engineered yeast strain could use acetic acid as the electron acceptor to reduce acetic acid to ethanol via NADH-dependent reactions. Because acetic acid is available at significant amounts in lignocellulosic hydrolysates, improvement of the conversion of acetic acid to ethanol is significant for industrial production of bioethanol from biomass. The study of Medina et al. (2010) inspires us to improve not only acetic acid tolerance but also the conversion rate of acetic acid to ethanol by using metabolic engineering strategy in the further research.
References
Burke D, Dawson D, Stearns T (2000) Methods in Yeast Genetics. A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Ferrari MD, Neirotti E, Albornoz C, Saucedo E (1992) Ethanol production from eucalyptus wood hemicellulose hydrolysate by Pichia stipitis. Biotechnol Bioeng 40:753–759
Fogel S, Welch JW (1982) Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci USA 79:5342–5346
He XP, Huai W, Tie C, Liu Y, Zhang BR (2000) Breeding of high ergosterol-producing yeast strains. J Ind Microbiol Biotechnol 25:39–44
He XP, Zhang BR, Tan HR (2003) Overexpression of a sterol C-24(28) reductase increases ergosterol production in Saccharomyces cerevisiae. Biotechnol Lett 25:773–778
Hill JE, Meyers AM, Koerner TJ, Tzagoloff A (1993) Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2:163–167
Hottiger T, Kuhla J, Pohlig G, Fürst P, Spielmann A, Garn M, Haemmerli S, Heim J (1995) 2-micron vectors containing the Saccharomyces cerevisiae metallothionein gene as a selectable marker: excellent stability in complex media, and high-level expression of a recombinant protein from a CUP1-promoter-controlled expression cassette in cis. Yeast 11:1–14
Lee YY, Iyer P, Torget RW (1999) Dilute-acid hydrolysis of lignocellulosic biomass. Adv Biochem Eng Biotechnol 65:93–115
Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S (1995) Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J 14:1360–1371
Maiorella B, Blanch HW, Wilke CR (1983) By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 25:103–121
Medina VG, Almering MJH, Maris AJA, Pronk JT (2010) Elimination of glycerol production in anaerobic cultures of a Saccharomyces cerevisiae strain engineered to use acetic acid as an electron acceptor. Appl Environ Microbiol 76:190–195
Mollapour M, Piper PW (2006) Hog1p mitogen-activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae. FEMS Yeast Res 6:1274–1280
Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27:6446–6456
Mollapour M, Shepherd A, Piper PW (2008) Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 25:169–177
Nissen TL, Hamann CW, Kielland-Brandt MC, Nielsen J, Villadsen J (2000) Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 16:463–474
Pampulha ME, Loureiro V (1989) Interaction of the effect of acetic acid and ethanol on inhibition of fermentation in Saccharomyces cerevisiae. Biotechnol Lett 11:269–274
Phowchinda O, Delia-Dupuy ML, Strehaiano P (1995) Effects of acetic acid on growth and fermenting activity of Saccharomyces cerevisiae. Biotechnol Lett 17:237–242
Tamas MJ, Luyten K, Sutherland FCW, Hernandez A, Albertyn J, Valadi H, Li H, Prior BA, Kilian SG, Ramos J, Gustafsson L, Thevelein JM, Hohmann S (1999) Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol 31:1087–1104
Teunissen ARH, Holub E, Hucht JVD (1993) Physical localization of the flocculation gene FLO1 on chromosome I of Saccharomyces cerevisiae. Yeast 9:1–10
Wang ZY, He XP, Zhang BR (2007) Over-expression of GSH1 gene and disruption of PEP4 gene in self-cloning industrial brewer’s yeast. Int J Food Microbiol 119:192–199
Wang DL, Wang ZY, Liu N, He XP, Zhang BR (2008) Genetic modification of industrial yeast strains to obtain controllable NewFlo flocculation property and lower diacetyl production. Biotechnol Lett 30:2013–2018
Wysocki R, Chery CC, Wawrzycka D, Hulle MV, Cornelis R, Thevelein JM, Tamas MJ (2001) The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol Microbiol 40:1391–1401
Zhang JN, He XP, Guo XN, Liu N, Zhang BR (2005) Genetically modified industrial brewing yeast with high-glutathione and low-diacetyl production. Chin J Biotechnol 21:942–946
Zhang A, Kong Q, Cao L, Chen X (2007) Effect of FPS1 deletion on the fermentation properties of Saccharomyces cerevisiae. Lett Appl Microbiol 44:212–217
Acknowledgment
The authors would like to acknowledge the financial support of Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX1-YW-11-C4).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, JG., Liu, XY., He, XP. et al. Improvement of acetic acid tolerance and fermentation performance of Saccharomyces cerevisiae by disruption of the FPS1 aquaglyceroporin gene. Biotechnol Lett 33, 277–284 (2011). https://doi.org/10.1007/s10529-010-0433-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0433-3