Abstract
Objectives
Platelet-rich fibrin (PRF) has gained tremendous momentum in recent years as a natural autologous growth factor derived from blood capable of stimulating tissue regeneration. Owing to its widespread use, many companies have commercialized various centrifugation devices with various proposed protocols. The aim of the present study was to compare 3 different commercially available centrifuges at both high and low g-force protocols.
Materials and methods
PRF was produced on three commercially available centrifuges including the IntraSpin Device (IntraLock), the Duo Quattro (Process for PRF), and Salvin (Salvin Dental). Two separate protocols were tested on each machine including the original leukocyte and platelet-rich fibrin (L-PRF) protocol (~ 700 RCF max (~ 400 RCF clot) for 12 min) as well as the advanced platelet-rich fibrin (A-PRF+) protocol (~ 200 g RCF max (~ 130 g RCF clot) for 8 min). Each of the tested groups was compared for cell numbers, growth factor release, scanning electron microscopy (SEM) for morphological differences, and clot size (both weight and length/width).
Results
The present study found that PRF clots produced utilizing the low-speed centrifugation speeds (~ 200 g for 8 min) produce clots that (1) contained a higher concentration of evenly distributed platelets, (2) secreted higher concentrations of growth factors over a 10 day period, and (3) were smaller in size. This was irrespective of the centrifugation device utilized and consistently observed on all 3 devices. The greatest impact was found between the protocols utilized (up to a 200%). Interestingly, it was further revealed that the centrifugation tubes used had a much greater impact on the final size outcome of PRF clots when compared to centrifugation devices. It was found that, in general, the Process for PRF tubes produced significantly greater-sized clots when compared to other commercially available tubes. The Salvin Dental tubes also produced significantly greater PRF clots when compared to the IntraLock tubes on each of the tested centrifugation devices.
Conclusions
The present study demonstrated the reproducibility of a scientific concept (reduction in RCF produces PRF clots with more evenly distributed cells and growth factors) utilizing different devices. Furthermore, (and until now overlooked), it was revealed for the first time that the centrifugation tubes are central to the quality production of PRF. Future research investigating tube characteristics thus becomes critically important for the future optimization of PRF.
Clinical relevance
This is the first study to reveal the marked impact of centrifugation tubes on the final production of PRF. Future study thus becomes markedly important to further optimize the quality of PRF-based matrices. It was further found that little variability existed between the centrifugation devices if optimized centrifugation protocols (lower centrifugation speeds) were utilized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Platelet concentrates have been utilized in many fields of medicine over the past decade due to their ability to rapidly stimulate efficient revascularization of tissues owing to their supra-physiological dose of platelets and growth factors derived from blood [1]. They were first introduced in the late 1990s following studies demonstrating their marked impact on tissue regeneration and have exponentially been utilized in regenerative dentistry [2,3,4,5,6,7,8]. These first protocols (termed platelet-rich plasma; PRP) contained anti-coagulants which aided in the separation of platelets from whole blood by preventing coagulation during a 30+-min dual-spin centrifugation protocol. Despite its large and widespread clinical use, concerns were raised regarding the use of bovine thrombin and various other anti-coagulants [2, 9, 10]. These were thought to prevent wound healing since clotting is one of the first (and key) steps during the healing process [2,3,4,5,6]. Due to the ability for the clinician to rapidly collect peripheral blood and concentrate blood-derived growth factors following centrifugation, platelet concentrates have long been considered a low-cost and easy-to-obtain source of natural growth factors with continued ongoing research on the topic [11, 12].
A second-generation platelet concentrate was developed with the aim of removing anti-coagulants termed leukocyte and platelet-rich fibrin (L-PRF with a most commonly utilized protocol of ~ 700 g (at the RCF max; equivalent to ~ 400 g at the RCF clot) for 12 min). Since anti-coagulants were removed, blood is subject to clotting over time and a short and quick centrifugation protocol is therefore needed to separate blood layers prior to clotting. Following centrifugation, a fibrin clot is found in the “platelet-rich” layer well described in the literature [13]. Typically, cells contained include platelets and leukocytes entrapped within this fibrin matrix containing growth factors and cytokines derived from blood. The fibrin matrix has since been shown to favor the slow and gradual release of growth factors over time when compared to PRP [14].
In 2014, Ghanaati and colleagues modified centrifugation protocols. Since high centrifugation speeds tend to push cells towards the bottom of PRF tubes, it was revealed that slower centrifugation speed (from an RCF max of ~ 700 to ~ 200 g) resulted in a PRF matrix with more concentrated cells and growth factors throughout the PRF matrix. This work was performed on the original PC-O2 centrifugation device (Today, the IntraSpin device; IntraSpin, Boca Raton, FL). Since then, others have followed on this research utilizing various other centrifugation devices [15,16,17] and have further shown that optimized platelet-rich fibrin could be achieved by not only a reduction in centrifugation speed but also time [15] (advanced platelet-rich fibrin (A-PRF+) with a protocol of ~ 200 g for 8 min).
Over the past few years, several commercially available centrifuges have been brought to market. These vary in both protocols, RCF values, tube-rotor angulation, rotor radius size, and tube composition. Each of these plays a role in the final obtained PRF clot, referred to by some as the “biological signature” of the PRF [18]. While much commercial debate exists on the topic, to date, there exists no comparative study that has investigated these various centrifuges at various centrifugation protocols utilizing a systemic approach aimed at better understanding the critical components/characteristics/protocols to optimize PRF. Therefore, the aim of the present study was to compare 3 different commercially available centrifuges at both high and low g-force protocols. Each group was then compared for growth factor analysis up to 10 days, scanning electron microscopy (SEM) for morphological patterns, and clot size (both weight and length/width) following centrifugation.
Materials and methods
Preparation of PRF
Blood samples were collected with the informed consent from 6 volunteer donors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. No ethical approval was required for this study because human samples were not identified, as previously described [19]. The factors that affect fibrin clot formation and structure include genetic factors, acquired factors (such as abnormal concentration of thrombin and factor XIII in plasma, blood flow, platelet activation, oxidative stress, hyperglycemia, hyper-homocysteinemia, medications, and cigarette smoking), and other parameters (such as microgravity, pH, temperature, reducing agents, and concentration of chloride and calcium ions) [20]. All patients with any of the above conditions were excluded. All patients were included if systemically healthy, non-smoking, and not taking any medications.
The following 3 centrifugation devices were utilized in this study including the IntraSpin Device (IntraLock, Boca Raton, FL, USA), the Duo Quattro (Process for PRF, Nice, France), and Salvin (Salvin Dental, Charlotte, NC, USA). Two separate protocols were tested on each machine including the original leukocyte and platelet-rich fibrin (L-PRF) protocol (~ 700 RCF max (~ 400 RCF clot) for 12 min) as well as the advanced platelet-rich fibrin (A-PRF+) protocol (~ 200 g RCF max (~ 130 g RCF clot) for 8 min). In total, 6 settings were utilized. A calculation was provided for the RCF values at the RCF max in Fig. 1and Table 1.
Each of the 6 volunteers donated 2 blood collection tubes for each of the 6 tested groups for a total of 12 tubes per participant. Each pair of tubes was utilized to balance the centrifuge during the spin cycle. The tubes were randomly drawn in pairs on each of the participants and spun according to group protocol. Figure 2 depicts the experimental setup including 6 centrifuges. Two centrifuges from each company were utilized with 1 centrifuge set to the low speed (~ 200 g for 8 min at the RCF max) and a second set at a standard original protocol at ~ 700 g for 12 min at the RCF max. In total, 72 samples were drawn from the 6 participants.
Blood draw was carried out into each of the provided company tubes including the 9-mL silica-coated plastic tubes provided by IntraLock, the 10-mL glass tubes provided by Process for PRF, and the 10-mL BD tubes provided by Salvin Dental. Following centrifugation, the PRF clots were removed; the red clot attached to the yellow PRF clot was separated with gentle scraping as previously described [21,22,23]. The entire PRF clots were utilized for each experiment. Each PRF clot was then weighed and measured both in width and height with a Vernier caliper as previously described [24]. A total surface area of each of the 72 clots was calculated by multiplying the width and height measurements of each individual PRF clot as previously described [24]. Thereafter, clot sizes and weights were compared from each of the protocols and thereafter sent for further analysis.
Protein quantification with ELISA
In order to determine the amount of released growth factors from each protocol, one PRF clot from each patient was sent for further ELISA quantification. At 15 min, 60 min, 8 h, 1 day, 3 days, and 10 days, samples were placed into a shaking incubator at 37 °C to allow for growth factor release into phosphate-buffered solution (PBS) as previously described [14, 15, 19]. At each time point, the 5 mL of PBS was collected, frozen, and replaced with 5 mL of additional PBS. Protein quantification was carried out using ELISA. At desired time points, PDGF-AA (DY221, range = 15.60–1000 pg/mL), PDGF-AB (DY222, range = 15.60–1000 pg/mL), PDGF-BB (DY220, 31.20–2000 pg/mL), TGF-β1 (DY240, range = 31.20–2000 pg/ml), VEGF (DY293B, range = 31.20–2000 pg/ml), EGF (DY236, range = 3.91–250 pg/ml), and IGF-1 (DY291, range = 31.20–2000 pg/ml) were quantified using an ELISA kit according to manufacturer’s protocol (DuoSet, R&D Systems, Minneapolis, MN, USA) as previously described [14]. Absorbance was measured at 450 nm and 570 nm using a microplate reader (DTX880, Beckman Coulter, Brea, CA, USA) and subtract at 570 nm from the readings at 450 nm. All samples were measured in duplicate, and 3 independent experiments were performed for each platelet concentrate.
Sequential pipetting technique
In order to investigate the various cell types following centrifugation on the various machines/protocols, a sequential pipetting methodology was utilized (Fig. 3). In summary, blood layers were sequentially investigated from the top 1-mL layer down to the bottom 1-mL layer as depicted in Fig. 3 using non-clotting PET tubes. Noteworthy, during the harvesting of 1-mL layers, for each centrifugation protocol, one sample was collected between the plasma/buffy coat and red blood cell layer. This layer was marked within each figure to represent the location of the buffy coat and displayed accordingly. Each 1 mL of the 10-mL samples was then sent for a complete blood count (CBC).
Illustration demonstrating a novel method to quantify cell types following centrifugation of PRF. Currently, one of the limitations is that whole blood is compared to the total plasma concentration following centrifugation. This, however, does not give a proper representation regarding the location of cells following centrifugation. By utilizing the proposed technique in this study by sequentially pipetting 1 mL of volume from the top layer downwards, it is then possible to send each of the 10 samples for CBC analysis and accurately determine the precise location of each cell type following centrifugation at various protocols. Notice that one layer (in this case layer 5) will contain some yellow plasma and red blood cells. This is typically the location of the buffy coat where a higher concentration of platelets is typically located. (Reprinted with permission from Miron et. al [25])
SEM procedures
In order to evaluate the morphological appearance of the PRF clots with a scanning electron microscope (SEM), the PRF clots were fixed in 2.5% glutaraldehyde for 24 h at 4 °C. The PRF clots were then dehydrated in a sequence of 25%, 50%, 75%, 90%, and 100% ethanol solutions for 5 min each and then dried by critical point drying. The PRF clot were then coated with 20 nm gold and examined in a field-emission scanning electron microscope (NOVA NANOSEM430, FEI, the Netherlands). Images were captured with 15–20 kV using × 5k–10k magnifications. This experiment was performed in triplicate.
Centrifugation tube analysis
During the experiments, it was discovered that the tubes seemed to have significant impact on the ability for PRF to clot. As such, 18 tubes of blood were collected (180 mL total) from each volunteer for further analysis. For each participant, 2 tubes from each company (6 total) were collected in a random order and placed into the IntraSpin Device. Following centrifugation, analysis of clot sizes and weights was then calculated. From that same patient, an additional of 6 tubes was collected in a random order and placed in the Process for PRF machine and an additional of 6 tubes was centrifuged in the Salvin Dental device. Thereafter, clots produced from each device/tube were investigated for differences. This experiment was performed in duplicate with 3 independent volunteers.
Statistical analysis
All experiments were performed at least in triplicate. Means and standard errors were calculated, and data were analyzed for statistical significance using one-way analysis of variance with GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA; *p values < 0.05 were considered significant).
Results
Effect of both high- and low-RCF centrifugations on PRF-based matrices produced on 3 different centrifuges
In a first set of experiments, investigation was performed on the size and weight outcomes of PRF-based matrices when centrifuged at both high (~ 700 g for 12 min) and low (~ 200 g for 8 min) protocols (Fig. 4a). It was first revealed that, in general, the PRF clot weights were all significantly greater when centrifugation was performed at high-speed protocols. Interestingly, it was revealed that clots increased in size by 55% on the Salvin Dental, 72% on the Process for PRF, and 91% on the IntraSpin centrifuge when compared to PRF clots produced on the same equivalent system at low speed (Fig. 4a). Furthermore, while the weights of the clots produced on the Salvin and IntraSpin devices/tubes were comparable, the Process for PRF equipment produced significantly heavier and bigger clots (~ 70%) when compared to the other devices (Fig. 4a). A similar trend was also observed when the surface area of the PRF clots was measured (Fig. 4b). The difference between high and low-speed centrifugation was however less pronounced when compared to the weight of the clots (up to a 50% increase in comparison to 70%) (Fig. 4b).
Size outcomes including a) weight and b) Surface area of PRF based matrices when centrifuged on 3 different devices at either high (~700 g) or low (~ 200 g) protocols. The weight and sizes (surface area) of each of the groups demonstrated that higher centrifugation speeds typically produce larger clots (in both weight and size). * corresponds to p<0.05
Thereafter, the morphological pattern of the PRF clots produced with each centrifuge was investigated via SEM (Fig. 5). It was found that the fibrin network produced at high speed produced a denser fibrin-like morphology when compared to PRF clots produced at low speed (Fig. 5). Furthermore, a greater number of visible cells were revealed at the bottom (near the red blood clot layer within the buffy coat) layer of the PRF scaffold, especially when the high RCF protocols were utilized.
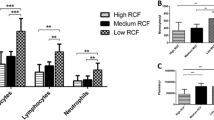
To investigate the cells found in the various PRF clots, a novel technique was developed that utilizes sequential pipetting 1 mL at a time to investigate the various cell types found in each layer following centrifugation (submitted for publication). Interestingly, it was found that the L-PRF protocol (IntraSpin device) accumulated the majority of platelets and the highest proportion of leukocytes specifically at layer 5 within the buffy coat (Fig. 6, arrows). Almost no cells were found in the first 4 layers (4 mL) of PRF. In contrast, a more even distribution of platelets was observed using the A-PRF+ protocol (Fig. 7). This was consistent irrespective of the device utilized (Supplemental Figures 1-4).
The concentration of cell types in each layer from 1 mL down to the 10-mL sample utilizing the solid L-PRF protocol (2700 RPM for 12 min; ~ 700 g). Notice that the majority of platelets accumulated directly within the 5th layer in the buffy coat. Furthermore, the highest concentration of leukocytes was also noted in this layer. The first 4 layers of this plasma layer, was typically devoid of all cells
The concentration of cell types in each layer from 1 mL down to the 10-mL sample utilizing the solid A-PRF+ protocol (1300 RPM for 8 min; ~ 200 g). Notice that specifically the platelets were more evenly distributed throughout the upper 5-mL plasma layer. Noteworthy, however, the majority of white blood cells (leukocytes, neutrophils, lymphocytes, and monocytes) were not found in the upper plasma layer
Lastly, growth factor analysis of various common growth factors found in blood was investigated over time from 15 min up to 10 days (Fig. 8). Once again, little difference was observed between centrifugation devices at each of the respected protocols. More importantly, a significantly greater release of growth factors was secreted utilizing lower centrifugation speeds and this was consistent on each of the tested devices (Fig. 8).
PDGF growth factor quantification analysis from PRF clots produced on 3 different devices at either high (~ 700 g) or low (~ 200 g) protocols. It was once again observed that PRF clots produced utilizing the low centrifugation protocols secreted a higher concentration of growth factors over time. (Asterisk depicts significant difference between each centrifuge at high vs low protocols)
In summary, the PRF clots produced at high centrifugal forces and times produced larger clots with a more dense fibrin network, however contained less concentrated cells (most notably found specifically at the base of the PRF clot). Less growth factor release over time was observed. The PRF clots produced using the low-speed centrifugation produced smaller clots that were more concentrated in platelets more evenly distributed throughout the PRF clot. These also secreted higher levels of growth factors over time. This was relatively independent of the centrifugation device utilized.
Investigation of the role of centrifugation tubes on the final size-effect of PRF-based matrices
Owing to the finding that the PRF clots produced using the Process for PRF centrifugation device seemed to consistently produce the largest clots (even though identical RCF values were utilized with comparable centrifugation angles), our research group began to question the role of the centrifugation tubes on the final size outcomes of PRF matrices. From a single patient, 6 tubes of blood were then collected (2 from each manufacturer) in a random order and inserted into a single centrifugation device (IntraSpin). Following centrifugation, it was once again found that the clots produced within the Process for PRF tubes generated significantly greater sizes when compared to both the Salvin and the IntraLock tubes and that even the Salvin Dental tubes produced significantly larger PRF clots when compared to the IntraLock tubes. This was quite surprising granted centrifugation was carried out on the same machine and blood was collected from the same patient (blood collection performed at the same time). This finding demonstrated convincingly that the tubes had a pronounced impact on the final size outcomes of PRF-based matrices.
We then decided to investigate the final size outcomes of PRF-based matrices on each of the 3 centrifuges utilizing tubes collected from each patient. In order to perform this experiment, 6 tubes (2 from each manufacturer) were collected in a random order and inserted into each of the 3 centrifuges (18 tubes total from each donor) and centrifugation was performed at the same G-force utilizing the standard high centrifugation protocol (700 RCF max for 12 min). Following centrifugation, it was revealed that the Process for PRF tubes generated up to a 250% significant increase in the size outcomes of PRF when compared to the IntraLock tubes—even though centrifugation was carried out on a single device with blood collected from the same patient (Fig. 9a). Furthermore, the Salvin Dental tubes also produced significantly greater PRF clots when compared to the IntraLock tubes. Figure 9b depicts the same data displayed differently. Notice how the IntraSpin centrifuge produced the largest clots when compared to the other centrifuges though changes were minimal (roughly 15% better than the Process for PRF device). It was also interesting to note that the overall best results were obtained utilizing an IntraSpin centrifuge with Process for PRF tubes (highlighting the necessity for further optimization of each of the tested devices). Interestingly as well, it was revealed that though the Salvin Dental centrifuge produced the smallest sized clots when all tubes were tested (Fig. 9b), notice that the size of the PRF clot produced on a Salvin Dental centrifuge with a Salvin tube were actually bigger when compared to the IntraLock centrifuge+tubes, despite the fact that the IntraSpin centrifuge produced the greatest sized clots when compared to all devices (Fig. 9b). This experiment revealed for the first time that in fact, the centrifugation tubes utilized to produce PRF clots have a much greater impact than the actual centrifugation device.
Final size outcome of PRF matrices produced utilizing 3 different centrifugation tubes in each of the 3 different centrifugation devices (a total of 9 tested groups). Notice that in general, the IntraSpin centrifugation device produced the largest sized clots, whereas the Process for PRF tubes produced the largest clots
One final interesting finding observed in the present study was the time (in seconds) required to fill each of the PRF tubes. Figure 10 reveals the average time required to fill each of the tubes up to their desired levels. Notice that the IntraLock and Process for PRF tubes were filled within roughly 15 s, whereas the BD tubes took roughly 25 s to fill (Fig. 10). This is critically important since PRF does not contain anti-coagulants which is discussed later in the discussion.
Discussion
PRF has gained tremendous momentum in recent years as a regenerative agent capable of promoting tissue regeneration primarily of soft tissues. Despite its widespread use, it is interesting to note that very little scientific data exists investigating the parameters of various centrifugation devices on the final size outcomes of PRF-based matrices. Therefore, the aim of the present study was to investigate in detail the characteristics of both centrifugation speed and time on the final PRF outcomes utilizing high and low-speed protocols on 3 commercially available centrifuges. To the best of the authors’ knowledge, this is the first comparative study to investigate different centrifugation protocols on various centrifugation systems.
Prior to beginning the first set of experiments, the various sizes/angulation of the rotors on each of the three centrifuges were investigated (Fig. 1). As can be observed, each of the centrifugation rotors has different angles (33–45°) with various radius diameters that are then affected the RCF min, RCF clot, and RCF max. While the international guidelines for reporting RCF values during centrifugation are most commonly reported at the RCF max, some authors have also expressed over the years the RCF values at the RCF clot [26]. Prior to commencing this study, all centrifugation parameters were calibrated to conduct centrifugation on each device at both low (~ 200 RCF max for 8 min) and high (~ 700 RCF max for 12 min) protocols according to Table 1.
In the present study, it was first found that PRF clots produced with the high RCF protocols consistently produced larger clots. This was irrespective of the centrifugation device utilized. Previously, Ghanaati and co-workers have shown that as centrifugation speed is decreased, the relative separation layers of PRF is also minimized, and as a result, PRF clots are also smaller [27, 28]. In the present study, we also observed this phenomenon irrespective of the centrifugation device utilized. Interestingly, it was found that though all sizes were greater utilizing low centrifugation protocols, it varied substantially between the different centrifuges (between 50 and 90%). This was most interesting as the G-force and separation of layers tended to be consistent. It was therefore one of the first clues that the tubes utilized to produce PRF clots may in fact have an impact on the final size outcomes of PRF matrices. Furthermore, while the PRF tubes of the IntraSpin system fill to 9 mL as opposed to 10 mL in the other tubes (representing a ~ 10% difference), the final size outcomes varied significantly (sometimes by more than 200% size differences), highlighting that this was not the main cause for these significantly differences in size outcomes. Therefore, as the experiments progressed, it became more critically apparent that the centrifugation tubes are key components of the final PRF clots produced.
The present study also confirmed previous studies demonstrating that the PRF clots produced utilizing high centrifugation speed tend to produce a clot denser in fibrin structure and network. Dohan and colleagues previously found that the original L-PRF protocols tend to produce these denser PRF clots owing to the increased centrifugation speeds [18]. We also observed consistent findings in the present study. Noteworthy, however, Kubesch et al. also showed that lower centrifugation speeds allow for the faster revascularization of PRF-based matrices owing to the ability for cells to more readily penetrate through PRF [16], potentially owing to the lower fibrin density of the PRF clot. These lower centrifugation speeds (~ 200 g) have also been shown in a series of studies to release more growth factors over time when compared to the original high g-force protocols (~ 700 g) [14, 15]. In the present study, we demonstrated convincingly utilizing this novel sequential pipetting technique that PRF produced at lower g-forces had more evenly distributed and concentrated platelets throughout the PRF clot when compared to high centrifugation speeds. We confirm also in the present study that the low-speed centrifugation concept is applicable to all tested centrifuges in the present study and most likely many others.
One interesting finding in the present study was the marked impact of centrifugation tubes on the final size outcomes of PRF-based matrices. Both the Process for PRF tubes as well as the Salvin Dental centrifugation tubes produced significantly greater sized clots—most notably on the IntraSpin centrifuge. Interestingly, the IntraSpin tubes in general consistently performed inferiorly when compared to each of the other tubes investigated (Fig. 9). Furthermore, it was revealed for the first time that the impact of the centrifugation tube was much more pronounced when compared to the centrifugation device. For instance, the IntraSpin centrifuge in general produced 15% greater-sized clots when compared to the Process for PRF centrifuge (for all tested clots), yet the IntraSpin tubes produced on average 200% smaller clots. These PRF clots were basically unusable at the low centrifugation speeds (images not shown) and confirm that optimization is required based on the centrifugation tubes utilized. Therefore, we reveal for the first time (1) the marked impact that centrifugation tubes on the final outcomes of PRF-based matrices and (2) that the majority of current commercially available centrifugation devices lack optimization.
It is also interesting to note that while a plethora of research has shown that utilizing the low centrifugation speed protocols produces significantly higher concentrations of leukocytes and growth factor release [14,15,16,17, 27,27,28,30], it was surprising to note that the PRF clot produced using the IntraSpin tubes produced using lower centrifugation protocols could not be achieved predictably owing to the inability for their current tubes to induce clotting following centrifugation. Therefore, a higher centrifugation protocol must therefore be clinically recommended at present until further optimization of their tubes is conducted. Furthermore, it was interesting to note that the combination of the Salvin centrifugation (lowest performing centrifuge of the tested groups) + Salvin tube still produced greater-sized clots when compared to the IntraSpin centrifuge + IntraSpin tubes (Fig. 9). This finding was surprising granted the fact that PRF has been commercially available for over a decade and that this is the first study to report the marked importance and impact of PRF centrifugation tubes.
One final surprising outcome observed with the Salvin Dental tubes however was the length of time required to fill their centrifugation tubes (approximately 25 s). Our group has recently shown that all centrifugation tubes should be ideally collected within a 60–90-s window [24]. If not, the PRF clots begin to reduce in size owing to the clotting that occurs within the tubes over time prior to centrifugation [24]. Under these guidelines, a clinician would only be eligible to collect only 3 Salvin tubes. Since usually more PRF clots are needed during surgery, this would drastically prohibit the production of a series of quality PRF clots utilizing these tubes. It is therefore recommended that should the Salvin tubes be utilized within the Salvin centrifuge, that a slightly higher RCF be utilized to further improve separation of layers when 4 or more tubes are harvested. Naturally, should the higher centrifugation protocol be utilized, a resulting lower number of cells and growth factors would also ensue. In our study, we routinely only collected 2 tubes per machine (roughly 50 s). Should the clinician utilize this centrifuge/tube combo for clinical applications, a greater centrifugation speed and time (such as 2000 RPM for 10 min) be recommended owing to the slow blood draw. Once again, this further confirms the necessity to further optimize each specific device according to the tubes utilized.
While there has been some controversy with respect to centrifugation devices utilized to produce [31, 32], in the present study, we reveal for the first time the importance of the centrifugation tubes on the final size outcomes of PRF matrices. It was also found that the centrifugation protocols utilized were far more important than the actual centrifugation utilized. Therefore, future optimization of the various tested centrifuges is necessary to further optimize the cell content, growth factor release, and size outcomes of these PRF-based matrices and further highlight the fact that further research is needed to further optimize PRF matrices.
Conclusion
The present study found that PRF clots produced utilizing the low-speed centrifugation speeds (~ 200 g for 8 min) produces clots that contained a higher number of platelets/leukocytes, secreted higher concentrations of growth factors over a 10-day period, yet were smaller in size when compared to high-speed centrifugation protocols. This was irrespective of the centrifugation device utilized. While the IntraSpin device produced slightly larger PRF clots when compared to the other systems (roughly 15%), much greater importance was the optimization of PRF tubes utilized and the protocols utilized (up to a 200% improvement for either of those factors). Future research investigating tube characteristics thus becomes critically important for the future optimization of PRF.
References
Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL, Chandad F, Nacopoulos C, Simonpieri A, Aalam AA, Felice P, Sammartino G, Ghanaati S, Hernandez MA, Choukroun J (2017) Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig 21:1913–1927. https://doi.org/10.1007/s00784-017-2133-z
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
Cai YZ, Zhang C, Lin XJ (2015) Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg 24(12):1852–1859. https://doi.org/10.1016/j.jse.2015.07.035.Epub 2015 Oct 9. Review
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD (2016) Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 32(3):495–505. https://doi.org/10.1016/j.arthro.2015.08.005
Singh B, Goldberg LJ (2016) Autologous platelet-rich plasma for the treatment of pattern hair loss. Am J Clin Dermatol 17:359–367. https://doi.org/10.1007/s40257-016-0196-2
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen MJJocp (2017) Regenerative potential of leucocyte-and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. J Clin Periodontol 44(2):225–234. https://doi.org/10.1111/jcpe.12658
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen MJJocp (2017) Regenerative potential of leucocyte-and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. J Clin Periodontol 44(1):67–82. https://doi.org/10.1111/jcpe.12643
Anfossi G, Trovati M, Mularoni E, Massucco P, Calcamuggi G, Emanuelli G (1989) Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot Essent Fat Acids 36:1–7
Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, Roos D (1990) Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods. Transfusion 30:634–638
Chow TW, McIntire LV, Peterson DM (1983) Importance of plasma fibronectin in determining PFP and PRP clot mechanical properties. Thromb Res 29:243–248
Delaini F, Poggi A, Donati MB (1982) Enhanced affinity for arachidonic acid in platelet-rich plasma from rats with Adriamycin-induced nephrotic syndrome. Thromb Haemost 48:260–262
Ehrenfest DMD, Rasmusson L and Albrektsson TJTib (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF) 27:158–167
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20:2353–2360. https://doi.org/10.1007/s00784-016-1719-1
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J (2017) Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol 88:112–121. https://doi.org/10.1902/jop.2016.160443
Kubesch A, Barbeck M, Al-Maawi S, Orlowska A, Booms PF, Sader RA, Miron RJ, Kirkpatrick CJ, Choukroun J and Ghanaati S (2018) A low-speed centrifugation concept leads to cell accumulation and vascularization of solid platelet-rich fibrin: an experimental study in vivo. Platelets:1–12. doi: https://doi.org/10.1080/09537104.2018.1445835
Wend S, Kubesch A, Orlowska A, Al-Maawi S, Zender N, Dias A, Miron RJ, Sader R, Booms P, Kirkpatrick CJ, Choukroun J, Ghanaati S (2017) Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med 28:188. https://doi.org/10.1007/s10856-017-5992-6
Dohan Ehrenfest DM, Pinto NR, Pereda A, Jimenez P, Corso MD, Kang BS, Nally M, Lanata N, Wang HL, Quirynen M (2018) The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 29:171–184. https://doi.org/10.1080/09537104.2017.1293812
Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J (2017) Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig 21:2619–2627. https://doi.org/10.1007/s00784-017-2063-9
Nunes CR, Roedersheimer M, Simske S, Luttges M (1995) Effect of microgravity, temperature, and concentration on fibrin and collagen assembly. Microgravity Sci Technol 8(2):125–130
Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier J-B (2010) Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol 81:546–555
Yajamanya SR, Chatterjee A, Babu CN, Karunanithi D (2016) Fibrin network pattern changes of platelet-rich fibrin in young versus old age group of individuals: a cell block cytology study. J Indian Soc Periodontol 20(2):151–6. https://doi.org/10.4103/0972-124X.176390
Miron RJ and Choukroun J (2017) Platelet rich fibrin in regenerative dentistry: biological background and clinical indications. John Wiley & Sons,
Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A (2018) The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig 23:2179–2185. https://doi.org/10.1007/s00784-018-2673-x
Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. (2019) A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. https://doi.org/10.1002/jbm.a.36734
Miron R, Choukroun J, Ghanaati SJIJoGF and Dentistry SCi (2018) Controversies related to scientific report describing g-forces from studies on platelet-rich fibrin: necessity for standardization of relative centrifugal force values. 1:80
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J (2014) Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. The Journal of oral implantology 40:679–689. https://doi.org/10.1563/aaid-joi-D-14-00138
El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, Booms P, Dohle E, Sader R, Kirkpatrick CJ, Choukroun J, Ghanaati S (2017) Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg 45(3):467–479. https://doi.org/10.1007/s00068-017-0785-7
Choukroun J, Ghanaati S (2018) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg 44(1):87–95. https://doi.org/10.1007/s00068-017-0767-9
Dohle E, El Bagdadi K, Sader R, Choukroun J, James Kirkpatrick C, Ghanaati S (2018) Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J Tissue Eng Regen Med 12(3):598–610. https://doi.org/10.1002/term.2475
Miron RJ, Choukroun J, Ghanaati S (2018) Necessity for standardization of relative centrifugal force values in studies on platelet rich fibrin; response to letter to the editor. J Periodontol. https://doi.org/10.1002/jper.18-0329
Pinto N, Quirynen M (2018) Letter to the editor regarding Fujioka-Kobayashi et al. 2017 (JOP-16-0443.R1). J Periodontol. https://doi.org/10.1002/jper.18-0175
Funding
This work was supported by the funds of the National Key R&D Program of China (2018YFC1105300 to Yufeng Zhang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
No ethical approval was required for this study, as human samples were not identified.
Informed consent
For this study, informed consent was provided prior to blood draw to conduct the outlined experiments.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Miron, R.J., Xu, H., Chai, J. et al. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~ 700 g) and low (~ 200 g) relative centrifugation forces. Clin Oral Invest 24, 1171–1182 (2020). https://doi.org/10.1007/s00784-019-02981-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02981-2