Abstract
Objectives
Research across many fields of medicine now points towards the clinical advantages of combining regenerative procedures with platelet-rich fibrin (PRF). This systematic review aimed to gather the extensive number of articles published to date on PRF in the dental field to better understand the clinical procedures where PRF may be utilized to enhance tissue/bone formation.
Materials and methods
Manuscripts were searched systematically until May 2016 and separated into the following categories: intrabony and furcation defect regeneration, extraction socket management, sinus lifting procedures, gingival recession treatment, and guided bone regeneration (GBR) including horizontal/vertical bone augmentation procedures. Only human randomized clinical trials were included for assessment.
Results
In total, 35 articles were selected and divided accordingly (kappa = 0.94). Overall, the use of PRF has been most investigated in periodontology for the treatment of periodontal intrabony defects and gingival recessions where the majority of studies have demonstrated favorable results in soft tissue management and repair. Little to no randomized clinical trials were found for extraction socket management although PRF has been shown to significantly decrease by tenfold dry sockets of third molars. Very little to no data was available directly investigating the effects of PRF on new bone formation in GBR, horizontal/vertical bone augmentation procedures, treatment of peri-implantitis, and sinus lifting procedures.
Conclusions
Much investigation now supports the use of PRF for periodontal and soft tissue repair. Despite this, there remains a lack of well-conducted studies demonstrating convincingly the role of PRF during hard tissue bone regeneration. Future human randomized clinical studies evaluating the use of PRF on bone formation thus remain necessary.
Clinical relevance
PRF was shown to improve soft tissue generation and limit dimensional changes post-extraction, with little available data to date supporting its use in GBR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regenerative therapy in dentistry involves the replacement and/or regeneration of oral tissues altered as a result of disease or injury. One of the reported aspects complicating this endeavor has been the complex nature of the tissues found in the oral cavity. These include both mineralized tissues such as the cementum, alveolar bone, and dentin, as well as soft tissues connected by ligaments (periodontal ligament), each comprising distinct cell populations from various tissue origins (ectodermal and mesodermal). These cell populations reside in specialized extracellular matrices organized in complex fashions [1, 2]. In the past, a variety of regenerative procedures utilizing highly sophisticated biomaterials were introduced to attempt their regeneration. These included ambitious attempts with barrier membranes to perform guided tissue/bone regeneration and the use of a variety of bone grafting materials from human, animal and synthetic sources, as well as bioactive growth factors such as bone morphogenetic proteins (BMPs) and enamel matrix derivative (EMD). Other investigators proposed that the use of three-dimensional scaffolds fabricated from the patient’s own peripheral blood could be utilized [2]. This new approach is based on the concepts that were introduced over a decade ago consisting of a platelet concentrate without the use of anticoagulants. Platelet-rich fibrin (PRF) was therefore developed as an improved formulation of the previously utilized platelet-rich plasma (PRP) [3].
Unlike PRP, which requires the addition of anticoagulants such as bovine thrombin during initial blood collection, PRF is obtained simply by centrifugation without anticoagulants and is therefore strictly autologous. This fibrin matrix contains platelets and leukocytes as well as a variety of growth factors and cytokines including transforming growth factor-beta1 (TGF-β1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), interleukin (IL)-1β, IL-4, and IL-6 [4]. Furthermore, fibrin that forms during the final stages of the coagulation cascade, combined with cytokines secreted by platelets, makes PRF a highly biocompatible matrix especially in damaged sites where the fibrin network acts also as a reservoir of tissue growth factors [5]. These factors act directly on promoting the proliferation and differentiation of osteoblasts, endothelial cells, chondrocytes, and various sources of fibroblasts [6, 7]. Despite this, many questions remain about the actual clinical performance of PRF. Therefore, the purpose of the present systematic review is to report the current state of knowledge and clinical potential of PRF in regenerative dental therapy when compared to both standardized controls and well-established standard regenerative biomaterials from human clinical randomized trials.
Brief history of platelet concentrates
The original concept leading towards the preparation of platelet concentrates was that concentrated platelets and autologous growth factors could be collected in plasma solutions that could then be utilized in a surgical site to promote local healing [8, 9]. It was given the popular working name “platelet-rich plasma” (PRP), introduced in the late 1990s [10,11,12]. PRP is composed of over 95% platelets, a cell type that actively secretes growth factors for initiating wound healing and secreting factors responsible for enhancing cell adhesion, proliferation, and migration of various cell types [10, 13]. Around the same time period, Anitua et al. formulated a second platelet concentrate also utilizing anticoagulants termed platelet-rich growth factor (PRGF) [14, 15].
Despite this, several factors have been shown to limit the use of PRP and PRGF. Their preparation requires the additional use of bovine thrombin or CaCl2 in addition to coagulation factors. Furthermore, the preparation must be centrifuged in two separate stages in order to increase platelet concentration without incorporation of leukocytes (sometimes requiring 1 h). It has further been reported that the liquid nature of PRP also complicates its handling and reduces its potential application since it must be utilized in combination with other biomaterials. Lastly, the clinical potential for bone regeneration with PRP is limited having a very short release of growth factor profile [16,17,18]. All these limitations have led to the emergence of a second-generation platelet concentrate termed PRF fabricated from 100% autologous sources [19].
Advantages of PRF over PRP
PRF differs from its predecessor (PRP/PRGF) by several parameters which can be summarized as follows: the simplicity of its preparation and its implementation. The time of preparation and cost of preparation are both significantly lower as PRF does not necessitate the direct activation with additional factors such as bovine thrombin or extrinsic anticoagulants [3]. Because of its fibrous structure, PRF retains a larger number of cytokines and growth factors in a supportive three-dimensional fibrin scaffold for cell migration [20]. In tissue, PRF dissolves more slowly than PRP, forming a solid fibrin matrix slowly remodeled in the style of a natural blood clot. Platelets and cytokines are then effectively retained and released gradually over time [18]. The PRF scaffold allows a continuous slow release of growth factors and cytokines over a period of 10 days, in contrast to PRP which has been shown to release the majority of its growth factors within the first day [18]. Therefore, migrating cells in near proximity to PRF scaffolds are in an environment with fibrin and growth factors throughout their entire growth cycle [21].
Once blood is collected (in the absence of anticoagulants), samples must be centrifuged immediately to avoid the activation of the coagulation cascade. During centrifugation, fibrinogen is concentrated to the top of the collection tube until the circulating thrombin transforms it into a fibrin network. This results in a fibrin clot rich in platelets, trapped between an acellular plasma layer and erythrocytes. The solid fibrin clot is found between the supernatant and the reddish background formed by red blood cells. The clot may then be removed immediately and condensed in a metal box so as to obtain a solid covering membrane or a filling cylinder (Fig. 1). The resulting exudate may be cut and used to hydrate graft materials if required (Fig. 1) [19, 20].
Implications of PRF in wound healing
Although leukocyte and platelet cytokines play an important role in the PRF healing capacity, it has often been suggested that it is the fibrin matrix supporting these elements which is actually responsible for its therapeutic potential [20]. The keys to tissue regeneration lie in their angiogenic potential, their immune system control, their potential to recruit circulating stem cells, and their ability to ensure undisturbed wound closure/healing by epithelial tissues [20]. The angiogenic properties of PRF may therefore be explained by the three-dimensional structure of the fibrin matrix which holds a number of growth factors and cytokines simultaneously embedded in the matrix including PDGF, TGF-β1, IGF, and VEGF. The regenerative potential of these cytokines has been abundantly studied in tissue wound healing and regeneration [4, 12, 22,23,24,25,26,27,28,29,30,31,32,33]. Furthermore, the fibrin matrix stimulates the expression of integrin avb3 which allows cells to bind to fibrin, fibronectin, and vitronectin [33]. This cascade of events is of utmost importance to initiate the process of angiogenesis and thus tissue wound healing [33].
Moreover, the fibrin degradation products directly stimulate neutrophil migration and facilitate transmigration into the vascular endothelium. This neutrophil activation causes secretion of proteases that facilitate their penetration in the basement membrane of blood vessels, in addition to their contribution to degrade the fibrin clot. Neutrophils trapped within the fibrin clot act to eliminate incoming bacteria and pathogens in the wound site by phagocytosis and the production of toxic free radicals and digestive enzymes. This contributes towards the prevention of bacterial contamination within the surgical site [34]. PRF also contains macrophages that are involved in the healing and repair process by playing a key role in the transition between inflammation and wound repair during osteogenesis [33, 34].
Methods
Development of a protocol
This review was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [35]. A protocol including all aspects of a systematic review methodology was developed prior to initiation of this review. This included definition of the focused question; a PICO (patient, intervention, comparison, outcome) question; a defined search strategy; study inclusion criteria; determination of outcome measures; screening methods, data extraction, and analysis; and data synthesis.
PICO question
-
P: Do patients in need of clinical bone, cementum, soft tissue, and/or PDL gain
-
I: Undergoing to dental treatments (i.e., guided bone/tissue repair/regeneration or pulp repair/regeneration) using defined non/surgical approaches combined with the use of PRF as sole/combined biomaterial
-
C: Defined regenerative/reparative approaches without the use of PRF
-
O: Soft and/or hard tissue reconstruction of the periodontium, alveolar bone, peri-implant tissues, or tooth structure
Defining the focused question
The following focused question was defined: “What indications has platelet rich fibrin (PRF) been shown effective for tissue repair/regeneration of either soft or hard tissues in dentistry?”
Search strategy
Electronic and manual literature searches were conducted independently by two authors (RJM and MFK) in several databases, including MEDLINE (OVID), EMBASE (OVID), Cochrane Central Register of Controlled Trials (Cochrane Library), Cochrane Oral Health Group Trials Register (Cochrane Library), Web of Science (Thomson Routers), and SciVerse (Elsevier). The electronic literature was searched for articles published up to and including May 14, 2016: combinations of several search terms and search strategies were applied to identify appropriate studies (Supplemental Tables 1–4). These include search strategies to identify the effects of PRF on (1) intrabony defect regeneration, (2) furcation defect regeneration, (3) management of gingival recessions, (4) guided bone regeneration and extraction socket healing, and (5) sinus floor elevation procedures. Reference lists of review articles and of the included articles in the present review were screened. Finally, a hand search of the Journal of Clinical Periodontology, Journal of Dental Research, Journal of Periodontal Research, Journal of Periodontology, Clinical Oral Implants Research, Clinical Implant Dentistry and Related Research, Clinical Oral Investigations, and The International Journal of Periodontics and Restorative Dentistry was performed from January 2000 to May 2016.
Criteria for study selection and inclusion
Study selection considered only articles published in English, describing the human clinical evaluation of PRF for the above-indicated search strategies. Only human studies evaluating the comparative effects of PRF to an appropriate control or to another regenerative modality in human studies were included. All human studies evaluating PRF in a case report or case series were excluded if controls were not present. All animal and in vitro studies were also excluded.
Outcome measure determination
The primary outcome of interest was to determine the regenerative/reparative potential of PRF in a variety of clinical settings utilized in dentistry. For each of the investigated clinical indications, different primary outcomes were considered. For studies dealing with intrabony defect regeneration, probing pocket depth (PPD) and clinical attachment levels (CAL) were measured. For studies dealing with gingival recessions, root coverage was calculated as percentage. Studies investigating the use of PRF for furcation defect regeneration quantified CAL gains as a primary outcome measure. For studies dealing with bone regeneration, dimensional change/density of hard tissues was compared. Similarly, sinus floor elevation procedures quantified new bone formation and/or implant success rates following sinus lifting procedures with/without PRF. Outcomes were summarized in Supplemental Tables 1–4 for the various clinical studies accordingly.
Screening method
Titles and abstracts of the selected studies were independently screened by three reviewers (RJM, MFK, and VG). The screening was based on the question: “Does platelet rich fibrin (PRF) have the ability to affect primary outcomes measured across a variety of procedures commonly performed in dentistry?” Full-text articles were obtained if the response to the screening question was “yes” or “uncertain.” The level of agreement between reviewers was determined by kappa scores. Disagreement regarding inclusion was resolved by discussion between authors. For necessary missing data, the authors of the studies were contacted.
Data extraction and analysis
The following data were extracted: general characteristics (authors and year of publication), defect type, number of patients, healing period, treatment groups, primary outcome measurements, and significant value. Due to the size of the study and the number of treatment procedures compared using PRF, no meta-analysis was performed. Instead, the data is reported in a systematic fashion with an overview of all studies fitting the search descriptions. Thereafter, data was extracted from the collection of articles and summarized in separate tables and discussed accordingly.
Results
Search outcomes
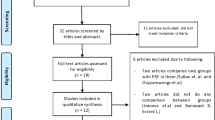
In total, the initial search strategies generated 152 articles that were separated accordingly into intrabony, furcation, gingival recession, guided bone regeneration (GBR)/extraction socket, and sinus lift accordingly (Fig. 2). Of the initial searches, 45 abstracts were retained for further investigation. In total, 10 articles were excluded primarily based on their lack of controls or appropriate endpoints matching our search criteria. In total, 35 articles were kept for further evaluation. This section aims to present viable treatment options utilizing PRF and evaluate its performance according to published studies.
Intrabony defect regeneration with PRF
One of the main uses for PRF has been for the repair/regeneration of periodontal intrabony defects [36,37,38,39,40,41,42,43,44,45]. To date, 11 randomized clinical trials (RCTs) have reported the use of PRF for intrabony defect regeneration, most often comparing PRF to open flap debridement alone (Table 1). Clinical improvements were reported via PPD reductions as well as CAL gains following regenerative periodontal therapy. All seven studies found that the additional use of PRF increased PPD reductions and CAL gains when compared to open flap debridement (OFD) alone (Table 1). One study comparing the regeneration of intrabony defects utilizing either PRF or a bone grafting material (demineralized freeze-dried bone allograft (DFDBA)) found no significant differences between treatment groups [42]. Two studies reported the effectiveness of PRF in combination with a bone grafting material when compared to bone grafting material alone [36, 38]. In both these studies, the additional use of PRF enhanced the filling of intraosseous defects. Most recently, Panda et al. most recently found that the supplemental use of PRF for intrabony defect regeneration in combination with a barrier membrane also led to statistically better results [39]. In summary, the collected RCTs have demonstrated that the use of PRF leads to statistically superior periodontal repair of intrabony defects when compared to OFD alone and may further be combined with regenerative biomaterials such as bone grafts or collagen barrier membranes to further enhance periodontal regeneration of intrabony defects. Despite the widespread use of PRF demonstrating the reduction of PPD and CAL gains, it remains of interest to note that no histological findings have yet been utilized to demonstrate true histological periodontal regeneration in human subjects. Therefore, future research to characterize intrabony defect regeneration versus repair utilizing PRF as a biomaterial remains necessary.
Furcation defect regeneration with PRF
Similarly, PRF has also been utilized in three studies investigating periodontal regeneration of class II furcation defects (Supplemental Table 5) [46,47,48]. In all studies, PRF was compared to OFD alone, thereby fully characterizing its regenerative potential utilizing appropriate well-designed controls in all human clinical studies. In all three studies conducted by Sharma et al. 2011, Bajaj et al. 2013, and Pradeep et al. 2016, the use of PRF led to a significant improvement in CAL gains when compared to controls [46,47,48]. These findings report a gain in vertical CAL of 2.33, 2.87, and 4.17 mm in test PRF groups when compared to 1.28, 1.37, and 1.82 mm, respectively, in OFD controls [46,47,48]. These results demonstrate the potential for tissue repair utilizing PRF for furcation defects. One remaining issue to address is that the results have not confirmed the regenerative potential of PRF via histological evaluation and therefore the process can solely be defined as tissue “repair.” Furthermore, to date, no study has compared the use of PRF to other effective regenerative materials such as bone grafting materials or other regenerative bioactive growth factors. In the future, its clinical performance could be better assessed if compared to other leading regenerative agents.
Root coverage of gingival recessions with PRF
PRF has also been widely utilized as a bioactive matrix in numerous studies for root coverage of gingival recessions (Table 2, Fig. 3) [49,50,51,52,53,54,55,56,57,58,59,60,61]. Of the 13 listed studies, six studies compared the use of coronally advanced flap (CAF) to CAF + PRF. Of these studies, three found that PRF induced a significant increase in root coverage [49, 51, 58] whereas the other three found no significant differences [52, 54, 60]. Of the remaining studies, one study compared PRF to EMD and found no differences in the reported root coverage [56]. Four studies compared CAF + PRF to CAF + connective tissue graft (CTF) and also found no difference in average root coverage [50, 53, 55, 61]. One study compared CAF + CTG with CAF + CTG and PRF and found a significant increase in root coverage for the combination approach utilizing both CTG with PRF [57]. Rajaram et al. utilized a double lateral sliding bridge flap with and without PRF for the treatment of gingival Miller class II defects and found no significant differences between control and test groups [59]. These results seem to hint at the fact that the use of PRF favors a slight gain in root coverage when compared to CAF alone but does not lead to better results when compared to EMD or to CTG. Furthermore, several reports show that CAF in combination with CTG leads to more width in keratinized tissue when compared to PRF.
a Multiple gingival recessions from the canine to the molar in the upper jaw. A frontal view. B–D Lateral views (case performed by Dr. Giovanni Zucchelli). b Surgical technique: A A flap for multiple gingival recessions has been elevated with a split-full-split thickness approach. B A-PRF prepared. C A-PRF has been applied to cover all teeth affected by gingival recessions. Multiple layers have been applied. D, E Lateral view showing the thickness of A-PRF material applied to the root exposures. F, G Lateral view showing the flap coronally advanced and covering completely the A-PRF material. H Frontal view showing the flap covering in excess all gingival recessions (case performed by Dr. Giovanni Zucchelli). c Six months follow-up. A Complete root coverage with increase in keratinized tissue height has been achieved in all treated gingival recessions. B–D Lateral view showing the increase in gingival thickness at all teeth previously affected by gingival recessions (case performed by Dr. Giovanni Zucchelli)
Interestingly, two reports commented on the added advantage of PRF in pain management [55, 56]. Jankovic et al. found in two separate studies comparing PRF to either EMD or CTG that PRF led to lower morbidity and faster wound healing [55, 56]. In a similar study investigating the wound healing properties of PRF, the palatal donor site of the epithelialized CTG was treated with PRF or a gelatin sponge on the healing of palatal donor sites [62]. It was reported that the PRF-enriched palatal bandage significantly accelerated palatal wound healing and reduced the patient’s morbidity [62].
In summary, the use of PRF for the treatment of gingival recessions is limited. Evidence from another systematic review from 2016 concluded that the additional use of PRF for the treatment of gingival recessions did not lead to any additional benefit in root coverage or CAL (P = 0.57 and P = 0.50, respectively) [63]. Furthermore, the reported keratinized mucosa width (KMW) gain was significantly greater in the subgroup treated with CTG when compared to PRF for studies greater or equal to 6 months in duration [63]. The results of that meta-analysis suggest that the use of PRF does not improve the root coverage, KMW, or CAL of Miller class I and II gingival recessions compared with other treatment modalities including EMD or CTG but can be obtained easily at low cost when compared to other regenerative modalities [63].
Guided bone regeneration and extraction socket management with PRF
One area of research that has gained tremendous popularity in recent years is the management of dimensional changes of the alveolar bone directly following tooth extraction [64,65,66]. These changes have been reported to occur within 8 weeks following extraction [67] as a consequence of decreased blood supply following tooth removal (periodontal ligament absence). Several advantages have been reported when filling extraction sockets with PRF (Supplemental Table 6) [68,69,70,71]. Hauser et al. found in a study of 23 patients that PRF reduced dimensional changes prior to implant placement when compared to natural socket healing [71]. Furthermore, it was reported that raising a peri-mucosteal flap reduced the effectiveness of PRF [71]. Girish Rao et al. found that following third molar extractions, the filling of sockets with PRF led to a non-significant increase in bone volume [68]. Hoaglin et al. reported that filling third molar extraction sockets with PRF led to a nearly tenfold decrease in osteomyelitis infections when compared to natural healing. This study was conducted bilaterally in 200 patients, thus providing some of the highest scientific evidence for the reduced rate of infection following use of PRF [70]. Lastly, Suttapreyasri et al. found that PRF reduced dimensional changes in premolar extraction sites when compared to blank controls [69].
Despite the limited number of studies, the use of PRF acts as an ideal material post-extraction by improving bone healing/regeneration, preserving the quality and density of the residual ridge, reducing infection, and decreasing the time of surgery when compared to the use of a covering membrane. These benefits are increasingly associated with a low cost of operation and a minimal risk of infection. PRF may further be utilized around immediate implant placement to pack gaps or additionally to speed soft tissue wound healing (Fig. 4). There remains however a great necessity to further evaluate dimensional changes utilizing PRF in various clinical situations using appropriately designed studies. Future clinical research is therefore necessary. Furthermore, it remains unknown what effect PRF may play in combination with GBR techniques. While a collagen barrier membrane is routinely used during such procedures, additional use or replacement with PRF may provide further regenerative advantages when compared to collagen barrier membranes alone. Future studies are thus necessary to validate these potential advantages.
Immediate implant placement in combination with the use of PRF. a, b Maxillary right cuspid fracture, extraction. c PRF placed into fresh extraction socket. d Dental implant placement with PRF fragments visible following placement (arrow in e). f Three-week follow-up, excellent soft tissue wound healing. g Three-year follow-up (case performed by Dr. Michael A. Pikos)
Sinus elevation procedures with PRF
The use of PRF for sinus elevation is relatively new with little comparative studies or standardized protocols available. Although the success rate of surgeries utilizing the addition of PRF is very high, it is difficult to compare the results between various treatment methods. Three authors using PRF alone as a graft material for sinus lift concluded that PRF significantly promoted bone healing with bone gains of 7.52 mm [72], 10.1 mm [73], and 10.4 mm [74] between the sinus floor and the top of the alveolar ridge. No controls were utilized in these studies. Nevertheless, no implants were lost at 6 months, 1 year, and 6 years in their respective studies [72,73,74]. Some authors further claim that the use of PRF alone may be a valid treatment protocol for the majority of sinus lift cases although the lack of controls and limited number of studies have thus far limited its use [73].
Other studies compared the use of a bone grafting material with and without the additional use of PRF [75,76,77,78]. The results show that despite the large bone gains observed with PRF, no statistically significant differences were reported. One reported plausible advantage of combining PRF with a bone grafting material seems to result in a decrease in the overall healing time and better graft material handling [75,76,77,78]. A recent systematic review on the topic published in 2016 by Ali et al. found that of 290 initial publications searched, only eight met the inclusion criteria with approximately half not utilizing controls [79]. It was reported that the identified studies showed great heterogeneity regarding surgical technique, grafting material, implant placement time, surgical protocols, outcome measures, healing time for biopsy, implant placement, and follow-up periods [79]. In summary, although the results do not seem to confirm that PRF is better than other biomaterials, its ease of use, combined with its minimal costs and high success rates, seems to illustrate that high success rates with minimal costs can be obtained by using PRF during sinus lifting procedures. Nevertheless, much further research is needed to support the beneficial effect of PRF.
Discussion
In this systematic review, all randomized clinical studies using PRF in dentistry were selected without discrepancies as compared to controls or commonly utilized surgical methods. The aim was to evaluate the current literature with respect to the clinical indications where PRF has been investigated for wound healing and/or tissue regeneration/repair. As was observed in the analysis of its clinical applications, the performance of PRF was often compared either to conventional treatments such as OFD during intrabony/furcation defects or to naturally healing “blank” defects during extraction socket management. The analysis of the generated publications revealed a great heterogeneity of results with a general lack of conclusive evidence in large part due to the lack of study number with appropriate controls. Therefore, only guidelines can be drawn from the sum of these general conclusions with an obvious need for further research.
One factor that has been frequently reported in this systematic literature search was the ability for PRF to stimulate regeneration over a wide range of tissues. PRF has been shown to quickly stimulate tissue healing by significantly increasing the recruitment and proliferation of a variety of cells including endothelial cells, gingival fibroblast, chondrocytes, and osteoblasts, thereby heavily promoting tissue repair and angiogenesis at the site of injury [80, 81]. These processes are regulated by local concentrations of cytokines and growth factors trapped within the fibrous scaffolding, most notably derived from autologous sources. In comparison, the growing use of two of the main growth factors approved by the FDA are PDGF and BMP derived from recombinant sources [82,83,84,85]. While these products sell for hundreds of dollars and are fabricated in mammalian cells or bacteria, the use of PRF are growth factors harvested purely from autologous sources via low-cost methods. It is therefore of interest to determine the benefit of using high supra-physiological concentrations of growth factors in recombinant form (i.e., BMP and PDGF) versus lower concentrations from autologous form (PRF). Although recombinant proteins have a regenerative potential well documented in the literature [86,87,88], many biological limitations to their use (swelling and edema), coupled with their low stability in vivo [89, 90], remain a limiting factor. Therefore, future research should target the comparison of the half-life and bioactivity of the growth factors found in PRF in comparison to commercially available recombinant growth factors.
Another interesting aspect that requires further study is to determine the regenerative/reparative potential of PRF on soft versus hard tissue formation. Thus far, this systematic review seems to point to the fact that the reparative potential of PRF favors soft tissue formation/ligament regeneration. Periodontitis is known to be one of the most common diseases with breakdown at the periodontium, causing destruction of the cementum and periodontal ligament and intrabony defects [91]. The use of PRF specifically for intrabony defect repair showed significantly higher PPD reductions and CAL gains when compared to control OFD in all seven studies. Furthermore, a bone grafting material (DFDBA) could be combined with PRF to further generate statistically better CAL gains and PPD reductions [36]. Therefore, these findings demonstrate that PRF is able to support periodontal ligament repair as effective or potentially more effectively than commonly utilized biomaterials. Despite these positive findings, it remains of interest to determine if the reparative potential of PRF leads to true periodontal regeneration in humans. Therefore, future human histological studies are needed.
With respect to treatment and management of gingival recessions, PRF has been studied in 13 randomized human clinical studies. In general, it was found that PRF had similar advantages in root coverage of Miller class I and II defects when compared to CTG. Noteworthy, it was however commonly reported that significantly higher keratinized tissue width was found with CTG when compared to PRF. While it is difficult to evaluate significant differences in these treatment procedures due to the high success rates (generally observed over 80% root coverage for all treatment groups), one area of research that remains to be determined is precisely under which clinical situations should one expect similar results between PRF and CTG. Since CTG procedures are associated with high patient morbidity, it may be that in the future, such procedures could be substituted with PRF to prevent high morbidity. Furthermore, the technical ability of the clinician plays a more prominent role during CTG harvesting when compared to PRF. Future studies are therefore needed to present updated clinical guidelines.
Another reported advantage to the use of PRF is its ability to decrease bacterial infections following surgery such as osteomyelitis commonly reported following third molar extractions [70]. In that study, a 9.5-fold significant decrease in reported cases of osteomyelitis was observed in a clinical trial with 100 patients [70]. Therefore and most likely due to the increase in white blood cells and macrophages capable of fighting infection, the use of PRF offers some antibacterial defense against incoming pathogens. Furthermore, macrophages have been shown to be key implicators in new bone formation both during bone modeling and remodeling, as well as in association with bone biomaterials [92,93,94]. Despite this, it remains interesting to point out that no human clinical study to date has investigated the effects of PRF in a controlled manner during GBR procedures, and only three studies on extraction socket healing have investigated dimensional changes following tooth extraction utilizing PRF (Supplemental Table 6). A similar trend investigating sinus lift procedures with PRF was also observed whereby a lack of well-designed studies with appropriate controls or endpoints was commonly found. Therefore, the effect of PRF on pure bone regeneration remains questionable and requires more validating studies. Similarly, various reports have now supported the use of PRF for pulp regeneration, cystic bone defect, and papilla augmentation under various clinical indications to improve healing [95,96,97,98,99,100,101,102,103]. While these reports are rare and anecdotal, future research aimed at better characterizing the regenerative potential of PRF for various other dental procedures remains necessary.
Very recently, a team of researchers has convincingly shown that lower centrifugation speeds and time resulted in higher leukocyte concentrations and release of growth factors [104,105,106,107,108]. Ghanaati et al. demonstrated via histological processing of PRF scaffolds that at higher centrifugation speeds, the majority of leukocytes were found at the bottom of PRF scaffolds [104]. By reducing centrifugation g-force, leukocytes were more evenly distributed throughout the PRF scaffolds [104]. In addition, regenerative growth factors released and gingival fibroblast activity are increased when utilizing slower centrifugation speed and time [108]. While reports from these studies support modifications to centrifugation protocols, the impact these may have on clinical outcomes in the various indications highlighted throughout this review article remains to be investigated. Future clinical study is therefore needed.
In conclusion, this systematic review demonstrates the widespread use of PRF in dentistry in various clinical settings. Although this regenerative modality remains unfamiliar to many clinicians, the evidence supporting its use has accumulated over the years, demonstrating its ability to improve tissue regeneration. The combination of PRF with regenerative therapy has been shown to be most promising for periodontal repair of intrabony and furcation defects, as well as soft tissue root coverage of gingival recessions. Furthermore, evidence from the literature suggests that PRF is able to decrease infection following tooth extraction and may further limit dimensional changes following tooth loss. It was also concluded that regeneration of bone defects (GBR procedures and sinus elevation) necessitates more study with focused endpoints. Nevertheless, its ease of use, combined with its low cost and autologous source, makes it an ideal biomaterial worth further investigation across a variety of surgical procedures in dentistry.
References
Dangaria SJ, Ito Y, Walker C, Druzinsky R, Luan X, Diekwisch TG (2009) Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation 78:79–90. doi:10.1016/j.diff.2009.03.005
Hollander A, Macchiarini P, Gordijn B, Birchall M (2009) The first stem cell-based tissue-engineered organ replacement: implications for regenerative medicine and society. Regen Med 4:147–148. doi:10.2217/17460751.4.2.147
Choukroun J, Adda F, Schoeffler C, Vervelle A (2001) Une opportunité en paro-implantologie: le PRF. Implantodontie 42:e62
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endod 101:e45–e50. doi:10.1016/j.tripleo.2005.07.009
Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, Kim ES, Choung PH (2011) Platelet-rich fibrin is a bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng A 17:349–359. doi:10.1089/ten.TEA.2010.0327
He L, Lin Y, Hu X, Zhang Y, Wu H (2009) A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endod 108:707–713. doi:10.1016/j.tripleo.2009.06.044
Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB (2009) In vitro effects of Choukroun’s PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endod 108:341–352. doi:10.1016/j.tripleo.2009.04.020
Anfossi G, Trovati M, Mularoni E, Massucco P, Calcamuggi G, Emanuelli G (1989) Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot Essent Fat Acids 36:1–7
Fijnheer R, Pietersz RN, de Korte D, Gouwerok CW, Dekker WJ, Reesink HW, Roos D (1990) Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods. Transfusion 30:634–638
Jameson C (2007) Autologous platelet concentrate for the production of platelet gel. Lab Med 38:39–42
Whitman DH, Berry RL, Green DM (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 55:1294–1299
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 14:529–535
Anitua E, Prado R, Troya M, Zalduendo M, de la Fuente M, Pino A, Muruzabal F, Orive G (2016) Implementation of a more physiological plasma rich in growth factor (PRGF) protocol: anticoagulant removal and reduction in activator concentration. Platelets 27:459–466. doi:10.3109/09537104.2016.1143921
Lucarelli E, Beretta R, Dozza B, Tazzari PL, O’Connel SM, Ricci F, Pierini M, Squarzoni S, Pagliaro PP, Oprita EI, Donati D (2010) A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater 20:13–23
Saluja H, Dehane V, Mahindra U (2011) Platelet-rich fibrin: a second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann Maxillofac Surg 1:53–57. doi:10.4103/2231-0746.83158
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. doi:10.1007/s00784-016-1719-1
Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB (2010) Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol 81:546–555. doi:10.1902/jop.2009.090531
Toffler M, Toscano N, Holtzclaw D, Corso M, Dohan D (2009) Introducing Choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. J Implant Adv Clin Dent 1:22–31
Tsay RC, Vo J, Burke A, Eisig SB, Lu HH, Landesberg R (2005) Differential growth factor retention by platelet-rich plasma composites. J Oral Maxillofac Surg 63:521–528. doi:10.1016/j.joms.2004.09.012
Carlson NE, Roach RB Jr (2002) Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc 1939(133):1383–1386
Andrades JA, Han B, Becerra J, Sorgente N, Hall FL, Nimni ME (1999) A recombinant human TGF-beta1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp Cell Res 250:485–498. doi:10.1006/excr.1999.4528
Lind M, Deleuran B, Thestrup-Pedersen K, Soballe K, Eriksen EF, Bunger C (1995) Chemotaxis of human osteoblasts. Effects of osteotropic growth factors. APMIS 103:140–146
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg, Oral Med Oral Pathol Oral Radiol Endod 101:e51–e55. doi:10.1016/j.tripleo.2005.07.010
Grando Mattuella L, Poli de Figueiredo JA, Nor JE, de Araujo FB, Medeiros Fossati AC (2007) Vascular endothelial growth factor receptor-2 expression in the pulp of human primary and young permanent teeth. J Endod 33:1408–1412. doi:10.1016/j.joen.2007.08.019
Troost E, Hold GL, Smith MG, Chow WH, Rabkin CS, McColl KE, El-Omar EM (2003) The role of interleukin-1beta and other potential genetic markers as indicators of gastric cancer risk. Can J Gastroenterol 17(Suppl B):8B–12B
Kishimoto T, Akira S, Narazaki M, Taga T (1995) Interleukin-6 family of cytokines and gp130. Blood 86:1243–1254
Kishimoto T (1989) The biology of interleukin-6. Blood 74:1–10
Waters JP, Pober JS, Bradley JR (2013) Tumour necrosis factor in infectious disease. J Pathol 230:132–147. doi:10.1002/path.4187
Murtaugh MP, Johnson CR, Xiao Z, Scamurra RW, Zhou Y (2009) Species specialization in cytokine biology: is interleukin-4 central to the T(H)1-T(H)2 paradigm in swine? Dev Comp Immunol 33:344–352. doi:10.1016/j.dci.2008.06.014
Li Z, Zhang Y, Sun B (2011) Current understanding of Th2 cell differentiation and function. Protein Cell 2:604–611. doi:10.1007/s13238-011-1083-5
Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e56–e60. doi:10.1016/j.tripleo.2005.07.011
Clark RA (2001) Fibrin and wound healing. Ann N Y Acad Sci 936:355–367
Swartz MK (2011) The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care 25:1–2. doi:10.1016/j.pedhc.2010.09.006
Agarwal A, Gupta ND, Jain A (2016) Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta Odontol Scand 74:36–43. doi:10.3109/00016357.2015.1035672
Ajwani H, Shetty S, Gopalakrishnan D, Kathariya R, Kulloli A, Dolas RS, Pradeep AR (2015) Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J Int Oral Health 7:32–37
Elgendy EA, Abo Shady TE (2015) Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J Indian Soc Periodontol 19:61–65. doi:10.4103/0972-124x.148639
Panda S, Sankari M, Satpathy A, Jayakumar D, Mozzati M, Mortellaro C, Gallesio G, Taschieri S, Del Fabbro M (2016) Adjunctive effect of autologus platelet-rich fibrin to barrier membrane in the treatment of periodontal intrabony defects. J Craniofac Surg 27:691–696. doi:10.1097/scs.0000000000002524
Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN (2015) Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 86:729–737. doi:10.1902/jop.2015.140646
Pradeep AR, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB (2012) Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 83:1499–1507. doi:10.1902/jop.2012.110705
Shah M, Patel J, Dave D, Shah S (2015) Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: a randomized controlled clinical study. J Indian Soc Periodontol 19:56–60. doi:10.4103/0972-124x.145803
Thorat M, Pradeep AR, Pallavi B (2011) Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol 38:925–932. doi:10.1111/j.1600-051X.2011.01760.x
Pradeep AR, Bajaj P, Rao NS, Agarwal E, Naik SB (2012) Platelet-rich fibrin combined with a porous hydroxyapatite graft for the treatment of three-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. doi:10.1902/jop.2012.110722
Sharma A, Pradeep AR (2011) Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol 82:1705–1712. doi:10.1902/jop.2011.110075
Sharma A, Pradeep AR (2011) Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. J Periodontol 82:1396–1403. doi:10.1902/jop.2011.100731
Bajaj P, Pradeep AR, Agarwal E, Rao NS, Naik SB, Priyanka N, Kalra N (2013) Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. J Periodontal Res. doi:10.1111/jre.12040
Pradeep AR, Karvekar S, Nagpal K, Patnaik K, Raju A, Singh P (2016) Rosuvastatin 1.2 mg in situ gel combined with 1:1 mixture of autologous platelet-rich fibrin and porous hydroxyapatite bone graft in surgical treatment of mandibular class II furcation defects: a randomized clinical control trial. J Periodontol 87:5–13. doi:10.1902/jop.2015.150131
Agarwal SK, Jhingran R, Bains VK, Srivastava R, Madan R, Rizvi I (2016) Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: a comparative analysis. Eur J Dent 10:121–133. doi:10.4103/1305-7456.175686
Aleksic Z, Jankovic S, Dimitrijevic B, Divnic-Resnik T, Milinkovic I, Lekovic V (2010) The use of platelet-rich fibrin membrane in gingival recession treatment. Srp Arh Celok Lek 138:11–18
Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D (2009) Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol 80:244–252. doi:10.1902/jop.2009.080253
Dogan SB, Dede FO, Balli U, Atalay EN, Durmuslar MC (2015) Concentrated growth factor in the treatment of adjacent multiple gingival recessions: a split-mouth randomized clinical trial. J Clin Periodontol 42:868–875. doi:10.1111/jcpe.12444
Eren G, Atilla G (2014) Platelet-rich fibrin in the treatment of localized gingival recessions: a split-mouth randomized clinical trial. Clin Oral Investig 18:1941–1948. doi:10.1007/s00784-013-1170-5
Gupta S, Banthia R, Singh P, Banthia P, Raje S, Aggarwal N (2015) Clinical evaluation and comparison of the efficacy of coronally advanced flap alone and in combination with platelet rich fibrin membrane in the treatment of Miller class I and II gingival recessions. Contemp Clin Dent 6:153–160. doi:10.4103/0976-237x.156034
Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, Camargo P (2012) Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int J Periodontics Restorative Dent 32:e41–e50
Jankovic S, Aleksic Z, Milinkovic I, Dimitrijevic B (2010) The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. Eur J Esthet Dent 5:260–273
Keceli HG, Kamak G, Erdemir EO, Evginer MS, Dolgun A (2015) The adjunctive effect of platelet-rich fibrin to connective tissue graft in the treatment of buccal recession defects: results of a randomized, parallel-group controlled trial. J Periodontol 86:1221–1230. doi:10.1902/jop.2015.150015
Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, Sreedhar A (2013) A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller’s class-I and II recession defects. J Indian Soc Periodontol 17:631–636. doi:10.4103/0972-124x.119281
Rajaram V, Thyegarajan R, Balachandran A, Aari G, Kanakamedala A (2015) Platelet rich fibrin in double lateral sliding bridge flap procedure for gingival recession coverage: an original study. J Indian Soc Periodontol 19:665–670. doi:10.4103/0972-124x.164764
Thamaraiselvan M, Elavarasu S, Thangakumaran S, Gadagi JS, Arthie T (2015) Comparative clinical evaluation of coronally advanced flap with or without platelet rich fibrin membrane in the treatment of isolated gingival recession. J Indian Soc Periodontol 19:66–71. doi:10.4103/0972-124x.145790
Tunaliota M, Ozdemir H, Arabaciota T, Gurbuzer B, Pikdoken L, Firatli E (2015) Clinical evaluation of autologous platelet-rich fibrin in the treatment of multiple adjacent gingival recession defects: a 12-month study. Int J Periodontics Restorative Dent 35:105–114. doi:10.11607/prd.1826
Femminella B, Iaconi MC, Di Tullio M, Romano L, Sinjari B, D’Arcangelo C, De Ninis P, Paolantonio M (2016) Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol 87:103–113. doi:10.1902/jop.2015.150198
Moraschini V, Barboza Edos S (2016) Use of platelet-rich fibrin membrane in the treatment of gingival recession: a systematic review and meta-analysis. J Periodontol 87:281–290. doi:10.1902/jop.2015.150420
De Risi V, Clementini M, Vittorini G, Mannocci A, De Sanctis M (2015) Alveolar ridge preservation techniques: a systematic review and meta-analysis of histological and histomorphometrical data. Clin Oral Implants Res 26:50–68. doi:10.1111/clr.12288
Jambhekar S, Kernen F, Bidra AS (2015) Clinical and histologic outcomes of socket grafting after flapless tooth extraction: a systematic review of randomized controlled clinical trials. J Prosthet Dent 113:371–382. doi:10.1016/j.prosdent.2014.12.009
Moraschini V, Barboza ED (2016) Quality assessment of systematic reviews on alveolar socket preservation. Int J Oral Maxillofac Surg. doi:10.1016/j.ijom.2016.03.010
Chappuis V, Engel O, Reyes M, Shahim K, Nolte LP, Buser D (2013) Ridge alterations post-extraction in the esthetic zone: a 3D analysis with CBCT. J Dental Res 92:195s–201s. doi:10.1177/0022034513506713
Girish Rao S, Bhat P, Nagesh KS, Rao GH, Mirle B, Kharbhari L, Gangaprasad B (2013) Bone regeneration in extraction sockets with autologous platelet rich fibrin gel. J Maxillofac Oral Surgery 12:11–16. doi:10.1007/s12663-012-0370-x
Suttapreyasri S, Leepong N (2013) Influence of platelet-rich fibrin on alveolar ridge preservation. J Craniofac Surg 24:1088–1094. doi:10.1097/SCS.0b013e31828b6dc3
Hoaglin DR, Lines GK (2013) Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. Int J Dent 2013:875380. doi:10.1155/2013/875380
Hauser F, Gaydarov N, Badoud I, Vazquez L, Bernard JP, Ammann P (2013) Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling: a prospective randomized controlled study. Implant Dent 22:295–303. doi:10.1097/ID.0b013e3182906eb3
Tajima N, Ohba S, Sawase T, Asahina I (2013) Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants 28:77–83. doi:10.11607/jomi.2613
Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, Dohan Ehrenfest DM (2009) Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole grafting material: a radiologic and histologic study at 6 months. J Periodontol 80:2056–2064. doi:10.1902/jop.2009.090252
Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan Ehrenfest DM (2011) Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent 20:2–12. doi:10.1097/ID.0b013e3181faa8af
Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Scacco S, Inchingolo AD, Dipalma G, Vermesan D, Abbinante A, Cagiano R (2010) Trial with platelet-rich fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci 14:1075–1084
Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, Scacco S, Dipalma G, Pacifici L, Inchingolo F (2012) Platelet rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci 9:872–880. doi:10.7150/ijms.5119
Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X (2012) Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Cranio-Maxillo-Facial Surg 40:321–328. doi:10.1016/j.jcms.2011.04.020
Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Rad Endod 101:299–303. doi:10.1016/j.tripleo.2005.07.012
Ali S, Bakry SA, Abd-Elhakam H (2015) Platelet-rich fibrin in maxillary sinus augmentation: a systematic review. J Oral Implantol 41:746–753. doi:10.1563/aaid-joi-D-14-00167
Roy S, Driggs J, Elgharably H, Biswas S, Findley M, Khanna S, Gnyawali U, Bergdall VK, Sen CK (2011) Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen 19:753–766. doi:10.1111/j.1524-475X.2011.00740.x
Chen FM, Wu LA, Zhang M, Zhang R, Sun HH (2011) Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials 32:3189–3209. doi:10.1016/j.biomaterials.2010.12.032
Steed DL, Donohoe D, Webster MW, Lindsley L (1996) Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg 183:61–64
Wieman TJ, Smiell JM, Su Y (1998) Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 21:822–827
White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD (2007) Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop 31:735–741. doi:10.1007/s00264-007-0422-x
Miron RJ, Zhang YF (2012) Osteoinduction: a review of old concepts with new standards. J Dent Res 91:736–744. doi:10.1177/0022034511435260
Young CS, Ladd PA, Browning CF, Thompson A, Bonomo J, Shockley K, Hart CE (2009) Release, biological potency, and biochemical integrity of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) combined with Augment(TM) Bone Graft or GEM 21S beta-tricalcium phosphate (beta-TCP). J Control Release 140:250–255. doi:10.1016/j.jconrel.2009.06.030
Park YJ, Lee YM, Lee JY, Seol YJ, Chung CP, Lee SJ (2000) Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J Control Release 67:385–394
Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J (2000) Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Release 64:103–114
Delgado JJ, Evora C, Sanchez E, Baro M, Delgado A (2006) Validation of a method for non-invasive in vivo measurement of growth factor release from a local delivery system in bone. J Control Release 114:223–229
Oe S, Fukunaka Y, Hirose T, Yamaoka Y, Tabata Y (2003) A trial on regeneration therapy of rat liver cirrhosis by controlled release of hepatocyte growth factor. J Control Release 88:193–200
Sculean A, Gruber R, Bosshardt DD (2014) Soft tissue wound healing around teeth and dental implants. J Clin Periodontol 41(Suppl 15):S6–22. doi:10.1111/jcpe.12206
Adamson R (2009) Role of macrophages in normal wound healing: an overview. J Wound Care 18:349–351. doi:10.12968/jowc.2009.18.8.43636
Miron RJ, Bosshardt DD (2016) OsteoMacs: key players around bone biomaterials. Biomaterials 82:1–19. doi:10.1016/j.biomaterials.2015.12.017
Sinder BP, Pettit AR, McCauley LK (2015) Macrophages: their emerging roles in bone. J Bone Miner Res 30:2140–2149. doi:10.1002/jbmr.2735
Subash D, Shoba K, Aman S, Bharkavi SK (2016) Revitalization of an immature permanent mandibular molar with a necrotic pulp using platelet-rich fibrin: a case report. J Clin Diagn Res 10:Zd21–zd23. doi:10.7860/jcdr/2016/21793.8902
Rebentish PD, Umashetty G, Kaur H, Doizode T, Kaslekar M, Chowdhury S (2016) Platelet-rich fibrin: a boon in regenerative endodontics. Minerva Stomatol 65:385–392
Kim JH, Woo SM, Choi NK, Kim WJ, Kim SM, Jung JY (2017) Effect of platelet-rich fibrin on odontoblastic differentiation in human dental pulp cells exposed to lipopolysaccharide. J Endod 43:433–438. doi:10.1016/j.joen.2016.11.002
Bakhtiar H, Esmaeili S, Fakhr Tabatabayi S, Ellini MR, Nekoofar MH, Dummer PM (2017) Second-generation platelet concentrate (platelet-rich fibrin) as a scaffold in regenerative endodontics: a case series. J Endod 43:401–408. doi:10.1016/j.joen.2016.10.016
Pradeep K, Kudva A, Narayanamoorthy V, Cariappa KM, Saraswathi MV (2016) Platelet-rich fibrin combined with synthetic nanocrystalline hydroxy apatite granules in the management of radicular cyst. Niger J Clin Pract 19:688–691. doi:10.4103/1119-3077.188711
Mirkovic S, Djurdjevic-Mirkovic T, Pugkar T (2015) Application of concentrated growth factors in reconstruction of bone defects after removal of large jaw cysts--the two cases report. Vojnosanit Pregl 72:368–371
Meshram VS, Lambade PN, Meshram PV, Kadu A, Tiwari MS (2015) The autologous platelet rich fibrin: a novel approach in osseous regeneration after cystic enucleation: a pilot study. Indian J Dent Res 26:560–564. doi:10.4103/0970-9290.176915
Dar M, Hakim T, Shah A, Najar L, Yaqoob G, Lanker F (2016) Use of autologous platelet-rich fibrin in osseous regeneration after cystic enucleation: a clinical study. J Oral Biol Craniofac Res 6:S29–s32. doi:10.1016/j.jobcr.2016.04.004
Arunachalam LT, Merugu S, Sudhakar U (2012) A novel surgical procedure for papilla reconstruction using platelet rich fibrin. Contemp Clin Dent 3:467–470. doi:10.4103/0976-237x.107443
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Sader R, Kirkpatrick C, Choukroun J (2014) Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 40:679–689. doi:10.1563/aaid-joi-D-14-00138
Choukroun J, Ghanaati S (2017) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. doi:10.1007/s00068-017-0767-9
El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, Booms P, Dohle E, Sader R, Kirkpatrick CJ, Choukroun J, Ghanaati S (2017) Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). Eur J Trauma Emerg Surg. doi:10.1007/s00068-017-0785-7
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig 20:2353–2360. doi:10.1007/s00784-016-1719-1
Fujioka-Kobayashi M, Miron RJ, Hernandez M, Kandalam U, Zhang Y, Choukroun J (2017) Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol 88:112–121. doi:10.1902/jop.2016.160443
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Joseph Choukroun is the founder of Process of PRF company and the developer and inventor of PRF protocol in Nice, France. All other authors declare no conflict of interest.
Funding
This work was fully funded by the Cell Biology Laboratory at Nova Southeastern University, College of Dental Medicine.
Ethical approval
No ethical approval was required for this study as human participants or animals were not utilized in this study.
Informed consent
Informed consent was not required as no human or animal subjects were utilized in this study.
Rights and permissions
About this article
Cite this article
Miron, R.J., Zucchelli, G., Pikos, M.A. et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Invest 21, 1913–1927 (2017). https://doi.org/10.1007/s00784-017-2133-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2133-z