Abstract
Background
The use of 3D laparoscopic systems is expanding. The European Association of Endoscopic Surgery (EAES) initiated a consensus development conference with the aim of creating evidence-based statements and recommendations for the surgical community.
Methods
Systematic reviews of the PubMed and Embase libraries were performed to identify evidence on potential benefits of 3D on clinical practice and patient outcomes. Statements and recommendations were prepared and unanimously agreed by an international surgical and engineering expert panel which were presented and voted at the EAES annual congress, London, May 2018.
Results
9967 abstracts were screened with 138 articles included. 18 statements and two recommendations were generated and approved. 3D significantly shortened operative time (mean difference 11 min (8% [95% CI 20.29–1.72], I2 96%)). A significant reduction in complications was observed when 3D systems were used (RR 0.75, [95 CI% 0.60–0.94], I2 0%) particularly for cases involving laparoscopic suturing (RR 0.57 [95% CI 0.35–0.90], I2 0%). In 69 box trainer or simulator studies, 64% concluded trainees were significant faster and 62% performed fewer errors when using 3D.
Conclusion
We recommend the use of 3D vision in laparoscopy to reduce the operative time (grade of recommendation: low). Future robust clinical research is required to specifically investigate the potential benefit of 3D laparoscopy system on complication rates (grade of recommendation: high).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Stereopsis is the perception of depth that arises from comparison of disparities in the images projected to two laterally separated eyes [1]. Most surgeons (except those who lack depth perception) use this visual effect in open surgery. Conventional two-dimensional (2D) minimally invasive surgery (MIS) using single-channel endoscopes and 2D screens is akin to monocular viewing.
Contemporary 3D MIS systems capture separate images using dual-channel laparoscopes consisting of either two separate rod lenses or two separate chips at the end of the scope to provide two vertically separated images. This produces different fixed distance perspectives of the operative field and simulates binocular imaging as if the viewer were positioned at the tip of the laparoscope [2]. In most modern commercially available 3D systems, users wear lightweight glasses that polarize alternate horizontal rows of pixels corresponding to the right- and left-eye images.
The first randomized controlled trial (RCT) investigating three-dimensional imaging was reported 20 years ago [3]. This used a now outdated system as technological developments have led to improved system quality and overcome many of the technical and resolution challenges seen in early platforms. Several studies have been published in recent years, both in experimental and clinical settings, suggesting 3D systems present a number of potential benefits to surgeons, trainees and patients [4]. Therefore, the European Association of Endoscopic Surgery (EAES) sponsored this consensus development conference on the use of 3D laparoscopy MIS systems. The aim of this consensus was to draw a number of statements based on the available evidence and develop recommendations for the MIS surgical community.
Materials and methods
The scope of this consensus on the use of 3D systems in laparoscopic surgery consisted of three main parts: general topics, organ-specific data and ongoing trials. The coordinating team (AA, YM and NV) formulated a list of questions related to each topic to be specifically addressed, which guided literature searches (Table 1) conducted in cooperation with a medical information specialist of the University of Torino. An initial literature search was conducted in order to identify any additional topics of interest. All searches were performed in both PubMed and Embase electronic libraries on 22 September 2017 with no limitation regarding year of publication or language. Search strings are displayed in Table 2. Study inclusion criteria were (a) trials on 3D technology in the selected topic (Table 2); (b) RCTs, prospective and retrospective observational comparative studies. Case reports or series of less than 10 patients were excluded.
Endpoints
The two primary endpoints were the impact of 3D on operative time and complications (both intra- and post-operative). Eligible organ-specific studies were also merged into a single dataset for operative time assessment in addition to separate subgroup analyses. Complications were analysed together and also underwent planned subgroup analysis where only those that appeared directly related to surgery were included. RCTs and prospective studies were used for the analysis of all outcomes but complications also included data retrieved from retrospective studies.
Research team
The coordinators invited 13 surgeons and engineering members of the EAES executive and technology committee with recognized expertise on the topic of 3D vision to join the panel of experts. Each was asked to nominate at least one young surgical researcher to participate. An international research team of 14 young surgical researchers was formed to review and evaluate the existing literature on the use of 3D technology in laparoscopy. Young researchers were mentored by a senior expert. The final list of topics was approved by the experts and subsequently divided among the teams (Table 3).
All search hits were screened by topic and reviewed by two team members for eligibility, based on title and abstract. If considered eligible, full-text articles were reviewed and summarized. In cases of disagreement, the coordinators acted as referee and made the final decision. Standardized data extraction forms were used across all topics. A PRISMA chart was completed for each literature search according to recommendations [5]. The methodological quality of included RCT was assessed using the Cochrane risk of bias score [6]. All included studies were evaluated with the GRADE system [7,8,9]. GRADE is a systematic and explicit approach to judging quality of evidence and strength of recommendations. GRADE specifically assesses methodological flaws, consistency of results across different studies, generalizability of research results and treatment effectiveness. The original Centre for Evidence-Based Medicine levels of evidence (LoE) system was used [9].
The highest levels of identified evidence were assessed first. If there was a systematic review of sufficient quality, it was used to answer the research question with a statement. When data were considered sufficient, consensus statements were prepared by each team and scored with a grade of recommendation (GoR).
Consensus development process
A face-to-face consensus meeting was held in London on 20 January 2018 to present all findings and drafted consensus statements and recommendations, which were finalized during two further virtual meetings. A modified Delphi method was used, as anonymity was not applicable in our situation [10,11,12]. All statements and recommendations were shared with the proposed LoE and subjected to voting for agreement or disagreement. In case of 100% consensus, the statements and recommendations were accepted. Where there was lack of consensus, the research team responsible for that topic presented the underlying evidence and rationale for their statement. After discussion, further voting rounds were conducted until an agreement was reached.
All finalized recommendations and statements with LoE and GoR were presented at a dedicated session during the 26th EAES congress London 2018. Fifty delegates attended and voted on each statement in two aspects: (a) Do you agree with the above-mentioned recommendation? (b) Will this recommendation change your practice?
Statistical analyses
All analyses were performed by a specialist biostatistician (RP), according to the original treatment allocation (intention-to-treat analysis). For binary outcome data, the relative risk (RR) and 95% confidence interval (CI) were estimated using the Mantel–Haenszel method; a RR < 1 favoured 3D vision. For continuous outcome data, the mean differences (MD) and 95% CI were estimated using inverse variance weighting with negative MD values favouring 3D vision. When means and/or standard deviation were not reported they were estimated from reported medians, ranges and sample size as described by Hozo [13]. A fixed-effects model was used in all meta-analyses, repeating the same analyses using a random-effects model as described by DerSimonian and Laird [14].
Risk of bias assessment
Publication bias was assessed by generating a funnel plot and performing the rank correlation test of funnel plot asymmetry. Heterogeneity was assessed by the I2 measure of inconsistency. Potential sources of heterogeneity were explored by different sensitivity analyses: comparing fixed- versus random-effects models (incorporating heterogeneity by using the random-effect method); checking the results of cumulative (sequentially including studies by date of publication) and influential meta-analyses (calculating pooled estimates by omitting one study at a time) and performing subgroup analyses. All analyses were performed with the R package (v3.5.0 Package Meta, The R Foundation for Statistical Computing, Vienna-A. http://www.R-project.org) [15] and Review Manager (RevMan 5.3; The Cochrane Collaboration).
Results
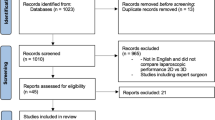
The literature searches yielded 9967 hits. In total, 138 articles were included and reviewed in detail to define 13 consensus statements and two recommendations. Nearly all studies presented in this review used dual-channel 3D laparoscopes and HD passive polarizing systems.
General topics
The impact of 3D vision on basic laparoscopy
Statement: 3D vision improves outcomes for junior trainees performing standardized box trainers tasks using properly setup 3D HD systems and passive polarized glasses (LoE: high).
The evidence behind this statement was derived from 14 studies, of which half were RCTs, comparing outcomes of basic experiments using either 2D or 3D vision systems [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Most RCTs were well designed. Primarily due to insufficient reporting, selection bias could not be excluded. Only three studies were considered to have a high randomisation bias risk with three considered to be at low risk of bias for participant blinding and 29% for outcome assessment blinding.
Ten papers focused on specific tasks with all but one demonstrating a significant reduction in errors. In five papers, this was assessed on novices. Seven papers (three studying novices) assessed task completion time with all demonstrating a reduction in operative time with 3D.
The primary endpoints of included articles varied from task completion time, quality of task performance, enacted errors and subjective questionnaire assessment of comfort and headache. Some studies also used completely different imaging systems for 2D versus 3D [20, 30]. Few studies standardized specific conditions of 3D setup (monitor height, monitor distance and viewing angle) [27, 29] or tested participants for their stereovision abilities which could impact outcomes [28, 29].
Impact of 3D vision on training
Statement: The use of 3D imaging systems improves laparoscopic box trainer task completion time and error rate but this benefit has not been studied in clinical practice (LoE moderate).
Evidence from 72 primary studies in 19 countries across four continents supported this statement [3, 19,20,21, 24, 28,29,30, 32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]. Publication dates varied from 1996 to 2017 suggesting that a spectrum of 3D systems were used although 57% of the publications were reported in 2014 onwards. Studies included 2452 participants: 1367 (55.8%) were laparoscopically naïve, primarily medical students, with 644 trainees (26.3% [486 junior (26.3%), senior 186 (7.6%)]) and 404 expert surgeons studied. Primary endpoints were operative time (95.8%), enacted errors (62.5%), task-specific score (22.2%), instrument path length (13.9%), repetitions performed (5.6%) and instrument movement speed (4.2%).
The vast majority of included studies were single centre and utilized crossover designs where participants completed the same tasks in 2D and 3D. 68 studies (94.4%) were performed in box trainer simulators with three animal experiments (two ex-vivo, one live [91]). Only two studies included operative room performance with both using laparoscopic cholecystectomies [3, 80]. Participants performed an average of three tasks (IQR 2–4, range 1–10). Only 36% of studies used previously validated tasks mainly taken from the Fundamentals of Laparoscopic Surgery (FLS) tasks, McGill Inanimate System for Training and Evaluation of Laparoscopic Skills (MISTELS) or European training in basic laparoscopic urological skills (E-BLUS) systems. The selection of the tasks within each simulation was not fully explained and only three studies assessed all tasks from their chosen program.
Of the 33 identified RCTs, primarily due to insufficient reporting, selection bias could not be excluded [6]. Only 30% of the studies were considered to have a low randomization bias risk, with 15% of studies maintaining allocation concealment. Blinding of participants to imaging modality is challenging although one group had students wear 3D glasses before entering the testing room. Only two studies were assessed as low risk of outcome assessment bias [47, 80]. One reviewed deidentified 2D videos of performance and one used automated tracking technology to record instrument metrics. All other assessments were performed by directly observing performance and therefore not blinded to allocation. The use of laboratory-based studies meant attrition bias was low but inadequate reporting meant that selective reporting and other bias could not be fully assessed. Overall compliance with the CONSORT statement was low across all included trials.
Pooling all 145 endpoints from included studies, 3D was significantly better in 90 (62.1%). 3D imaging was associated with a significant reduction in task completion time in 44 of 69 studies (63.8%). In the 45 studies using errors as the primary outcome, 28 (62.2%) observed a significant reduction when using 3D. Task-specific scores were significantly higher in the 3D arm in 56.3% of the 16 studies. Instrument path length, repetitions needed and instrument movement speeds were significantly improved by 3D in 50%, 50% and 100% of studies, respectively. It is noteworthy that both clinical studies did not show any time or error count differences between 2D and 3D modalities. Across all studies and endpoints, 2D was seen to be significantly quicker in two studies only (1.4% of all endpoints).
Impact of 3D vision on cognitive load
Statement: 3D laparoscopy does not introduce a higher cognitive workload and may result in decreased experienced cognitive workload provided that the viewing setup is optimal (LoE: moderate).
The systematic search identified 1684 hits with seven eligible for inclusion [3, 27, 52, 55, 68, 91, 97]. Three studies were randomized trials [3, 27, 52]. Only one study was of high quality according to Cochrane risk of bias tool [27].
Buia et al. stated that the perceived cognitive workload in 3D laparoscopy was not higher. This was shared by Lin et al. and Feng et al. [27, 52, 91]. Sakata and colleagues found cognitive workload using 3D laparoscopy was lower provided the viewing setup was optimal [98]. Benefits other than lowering cognitive workload were reported by Foo et al. and Kong et al.; however, they reported no conclusion on cognitive workload [55, 97]. The only adverse effects of 3D vision were reported by Hanna et al. [3] although this used an outdated 3D system [3].
Pitfalls of 3D vision in laparoscopy
Statement: 3D systems may increase visual fatigue, discomfort and headaches when setup is not optimal (LoE: moderate).
Out of 481 hits found, only 3 articles were included.
3D laparoscopic systems provide an improved stereoscopic vision which facilitates tasks performance, especially in inexperienced subjects [99]. Nevertheless, signs of visual discomfort, such as headache and visual fatigue, dry eyes or double vision, are reported in all studies [1, 99, 100]. Interestingly Zhou et al. [100] found out that whereas individuals experienced the above-mentioned discomfort symptoms, objective visual functional parameters (distance and near exophoria and esophoria, fusion range, accommodative convergence/accommodation, and tear film breakup time) did not worsen during 3D laparoscopy. Sakata et al. [1] suggested that looking at the screen from eccentric positions causes variable degrees of double vision, whereas an optimal position results when the centre of the screen is aligned with the eyes of the viewer.
Cost-effectiveness
No prospective study or RCT directly investigating the cost of the 3D laparoscopy could be found. Hence, no statement made relating to the cost-effectiveness of 3D systems compared to 2D systems can be made.
Pooled evidence on potential clinical benefits from 3D imaging
Operative time
Statement: 3D laparoscopy shortens the operative time across all analysed surgical specialities (general surgery, urology and gynaecology) (LoE: high).
We included 18 primary studies, reporting data about operative time, from nine countries across three continents [3, 26, 101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116] (Fig. 1). All but one study was published after 2013 suggesting a relatively limited variability of modern 3D systems were used. Studies included 1729 individuals: 487 (25.5%) solid organ operations; 1289 (74.5%) hollow organ procedures; 875 (50.6%) cases contained laparoscopic suturing while 794 papers (45.9%) did not specify the type of surgery. 647 procedures were general surgical with others consisting of urology and gynaecology procedures. The seven identified RCTs were assessed for bias using the Cochrane risk tool [6] (Table 4). Primarily due to insufficient reporting, selection bias could not be excluded. All were deemed to have a low randomization bias risk with 57% of studies maintaining allocation concealment. Blinding of participants to imaging modality was not possible. Overall compliance with the CONSORT statement was low.

Reproduced with permission from Moher et al. [117]
PRISMA 2009 flow diagram—22 September 2018: operative time.
Pooling data derived from the 18 included studies, and 3D significantly shortened operative time. A mean difference (MD) of 11 min (8% [95%CI 1.72–20.29]) in favour of 3D with a high heterogeneity was seen (I2 96%, Fig. 2). Similarly, operative time in procedures including laparoscopic suturing a MD of 15 min was seen (11% [95%CI 2.70–27.2], I2 87%, Fig. 3). This effect size was reduced in procedures not including laparoscopic suturing with a MD of 6 min (5% [95%CI 0.11–11.79], I2 80%, Fig. 4) and absent in hollow organ procedures with a MD of 2.7 min (3.8% [95 CI% 2.91–8.33], I2 80%, p = 0.34). The analysis of the operative time in procedures performed on solid organs showed a MD of 21.7 min (14% [95 CI% 6.45–36.94], I2 88%, Fig. 5). Finally, the analysis of the operative time in procedures performed by general surgeons shows a MD of 7.44 min (4% [95 CI% 0.66–14.23], I2 85%, Fig. 6).
Recommendation: We recommend the use of 3D vision in laparoscopy to reduce the operative time (GoR: Low).
The recommendation was voted by 38 delegates with 36 (95%) agreeing and 44% stating this recommendation was likely to change their practice. 8 (19%) were already using 3D, while 15 (37%) disagreed that this recommendation would influence their practice.
Complications
Statement: The pooling of data from different settings seems to suggest a lowering in the overall rate of complications after surgical procedures involving suturing in 3D laparoscopy (LoE: low).
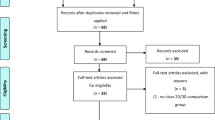
We included 18 primary prospective and retrospective studies from eight countries [26, 101,102,103, 105, 106, 108,109,110,111,112, 114, 118,119,120,121,122,123]. The flowchart is described in Fig. 7 with risk of bias reported in Table 5. All studies were published after 2013 suggesting a relatively limited variability of 3D systems used. Studies included 1733 individuals. 12 prospective studies included 1039 patients and six retrospective included 694 patients. 10 papers containing 713 patients regarded procedures including laparoscopic suturing with nine general surgical papers of 958 patients and all others consisting of urology and gynaecology procedures. Not all reported complications were considered for the analysis, as some appeared unrelated to the surgical procedure (Table 6).

Reproduced with permission from Moher et al. [117]
PRISMA 2009 flow diagram—22 September 2018: complications.
Overall compliance with the CONSORT statement was low across all five included RCTs, which were also assessed using the Cochrane risk tool [6]. Due to insufficient reporting, selection bias could not be excluded. All were deemed to have a low randomisation bias with 60% of studies also maintaining allocation concealment. Blinding of surgeons to imaging modality was not possible.
Using data pooled from the 18 included studies, a significant overall reduction complications was observed (RR 0.75, [95 CI% 0.60–0.94], I2 0%). However, no significant difference was observed when considering only general surgical procedures (RR 0.78, 95CI 0.60–1.02, I2 0%). When considering procedures including laparoscopic suturing, 3D showed a significant reduction in complications (RR 0.57 [95 CI% 0.35–0.90], I2 0%, Fig. 8). Omitting one study each time, the RR varied from 0.54 to 0.61 but without any statistically significant variation for the I2, demonstrating that no trial was a potential source of inconsistency. This was also confirmed in a subgroup analysis. When including only RCTs and prospective studies, the reduction in complications increased (RR 0.50 [95 CI% 0.25–0.97], I2 0, Fig. 9).
Recommendation: Future research is recommended to specifically investigate the potential benefit of the use of 3D laparoscopy system on complication rate (GoR: high).
This recommendation was voted by 45 delegates with 39 (87%) agreeing. 30 (72%) stated this recommendation was likely to change their practice while 6 members disagreed (14%) and 6 members (14%) reported already using 3D systems.
Organ specific
Cholecystectomy
Statements: There is no evidence that 3D vision is superior or inferior to 2D in laparoscopic cholecystectomy in terms of intra- and post-operative complications (LoE: moderate).
Less experienced surgeons could benefit from 3D imaging resulting in shorter operative time in laparoscopic cholecystectomy (LoE: low).
We analysed 402 hits. Six studies met inclusion criteria containing 309 elective patients (four prospective studies [80, 116, 125, 126] and two RCTs [3, 115]). Publication dates varied from 1998 to 2017, although 84% were reported from 2013 onwards.
All studies used operative time as their primary endpoint. Three studies demonstrated a reduction in operative time in favour of 3D [115, 116, 126], but in two studies, this was reported only in novices while no difference was noticed in the expert group. In all studies, the rate of conversion to open cholecystectomy and intra- and post-operative complication rate were not different in 3D. Four studies analysed the error rates during laparoscopic procedures with no significant difference seen [3, 80, 115, 126].
Colorectal surgery and appendectomy
Statement: 3D visualization shortens operative time in right colectomy compared to 2D (LoE: moderate).
We analysed a total of 552 hits of which six papers met the inclusion criteria [122,123,124, 127,128,129], with one RCT [124]. The procedures analysed were all resectional surgery but heterogeneous. Meta-analysis of these trials highlights a significant reduction in operative time (− 13.4 min; CI95% − 26.05, − 0.83, p < 0.01) but no significant difference in complications or lymph node yield. The RCT was performed by Curro et al. [124] and includes 40 right colectomies, 40 sigmoidectomies and 40 anterior resections, showing no difference in terms of complications or operative time.
For the topic of appendectomy, a total of 75 articles were identified; however, no article met the inclusion criteria.
Upper GI and bariatrics
Statements: 3D systems shorten operative time in hiatal hernia repair and mini gastric by-pass procedures (LoE: moderate).
There are no significant advantages in 3D for gastrectomy and sleeve gastrectomy (LoE: moderate).
Literature search identified 656 abstracts. After screening, six studies were included [120, 121, 130], of which three were RCTs [112,113,114]. The surgical procedures were hiatal hernia repair, bariatric surgery and two gastric cancer and two oesophagectomy studies. Operative time was investigated by all three RCTs with two showing a statistically significant advantage for 3D. There was significant time reduction in the 3D group for both bariatric surgery and hiatal hernia repair compared to 2D, but not for gastric cancer or oesophageal cancer surgery. Surgical complications were reported by all six studies with no significant differences seen.
Liver, pancreas, spleen and adrenal surgery
Literature search identified 666 hits for liver, 320 for pancreas, 190 for spleen and 152 for adrenal gland. Only one single prospective comparative study was found, focusing on different kinds of anatomical liver resections. The study [111] shows a significant reduction of blood loss (1255 ml versus 654 ml) and complications (33% vs. 14%) in favour of 3D. No prospective study or RCT dealing directly with the pancreas, spleen and adrenal surgery could be found. Hence, no statement made relating to the use of 3D systems in liver, pancreas, spleen or adrenal surgery could be made.
Abdominal wall
No prospective study or RCT dealing directly with 3D laparoscopic abdominal wall or hernia surgery was found. Hence, no statement could be made.
Gynaecology
Statements: 3D laparoscopy could be of benefit in terms of operative time in more complex procedures (LoE: moderate).
The literature search identified 1273 hits. Five articles met the inclusion criteria [107,108,109,110, 131] containing three RCTs [107, 109, 131] and a total of 693 patients: 371 in 2D group and 322 in 3D group. All procedures required suturing in the reconstructive phase. Two studies reported a shorter operative time in the 3D group [108, 110], but overall no statistically significant difference was observed (MD 2.12, [CI95% − 24.87 to 29.11], I2 81%, p = 0.88). No difference in terms of blood loss was seen. Meta-analysis of complications showed no differences. One study reported a significantly shorter hospital stay in favour of 3D surgery [108].
Urology
Statements: 3D laparoscopy significantly reduces the operative time but not perioperative complications in prostatectomy and renal lodge surgeries (LoE: high).
The literature search identified 1653 hits. Seven studies met inclusion criteria [26, 103,104,105,106, 132, 133] with four RCTs [26, 104,105,106]. A total of 460 patients were included: 224 in 3D and 236 2D. The urological procedures included 122 donor nephrectomies, 121 radical prostatectomies, 93 partial nephrectomies, 54 simple nephrectomies, 40 pyeloplasties, 21 radical nephrectomies, six radical cystectomies and three other laparoscopic surgeries. The operative time was significantly shorter in two of the four RCTs [104, 105], but in meta-analysis a statistically significant was seen (MD − 25.6 min, [95 CI% − 46.45 to − 4.75], I2 88%, p = 0.02). Blood loss was significantly lower in two of the three RCTs reporting it [26, 104]. Two of the three RCTs demonstrated shorter suturing time in 3D [26, 104]. When considering complications in suturing cases, a total of 11/179 and 18/223 for the 3D and 2D group were recorded, respectively. Meta-analysis did not show any difference in complications when including all studies or radical prostatectomy alone.
Ongoing trials
At the time of writing, searching registries of privately and publicly funded clinical studies for the terms “3D” and “laparoscopy”, we found 18 ongoing RCTs [134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151] registered on ClinicalTrials.gov and ISRCTN registries: 12 in Europe, three in the United States and three in Asia. Five have completed recruiting, while three are in setup. Four deal with cholecystectomy, three colorectal surgery, two gastrectomy, two hernia repair, six gynaecology surgery and one radical prostatectomy. In 10 studies the primary outcome is operative time, three error count, two complications, two patient reported outcomes (pain assessment and quality of life) and one lymph-node yield. Common secondary outcomes are conversion rate, assessment of fatigue, blood loss, readmission, mortality, oncologic safety and specimen quality, nerve sparing and functionality.
Discussion
Over the past two decades, the rapid advancement in 3D imaging technology appears to have successfully overcome previous barriers to its wide clinical use. It was important for the EAES to sponsor this consensus conference in order to define recommendations, based on available evidence to provide guidance to clinicians regarding the impact of 3D laparoscopy on their practice. This project has generated consensus statements and recommendations, demonstrating that 3D laparoscopy has some advantages in reducing operating times, cognitive load and possibly complications.
This consensus follows the recent health technology objective assessment report [4] by the EAES-affiliated Italian Society of Endoscopic Surgery and new technologies (SICE). An expert consensus was now felt appropriate as a combination of structured review of the evidence with leading multi-disciplinary technological and surgical expertise to generate statements and recommendations for clinical practice. We used a modified Delphi approach to reach consensus on the statements and grade of evidence adhering to the PRISMA, Cochrane risk of bias and GRADE principles. Agreement was reached on 13 proposed consensus statements (Table 7). Paucity of available evidence limited us to two recommendations underpinned by the meta-analyses.
This project also involved pooling the evidence from multiple studies to allow meaningful conclusions by increasing the sample size. Meta-analysis of the included clinical studies across all specialities showed 3D systems reduced operative time by a mean of 11 min representing 8% of case time. This effect size was larger in procedures involving laparoscopic suturing. Box trainer task time was also shorter with 3D.
We found a large body of evidence investigating the role of 3D on the performance on box training. Results of the laboratory experimental trials showed 3D was associated with significantly better performance than 2D in the majority of technical tasks. Consideration for the learning effect when repeating identical tasks with a different imaging modality should be made as performance could be higher irrespective of imaging used. While affected by the risk of bias and methodological flaws, our findings suggest that 3D technology improves laparoscopic box trainer simulator task performance. This could speed time to competency with fewer enacted error events in laparoscopically naïve or junior participants. There are no data, however, on whether these benefits translate to operating room performance or patient outcomes. A Cochrane review concluded that the benefits of box training have not been shown to translate to real-world performance [152]. As improved operating performance represents the goal of all minimal access training, further dedicated translational and longitudinal studies are clearly indicated.
The major output of this consensus is represented by a clear advantage of 3D in the overall rate of complications, in particular after surgical procedures involving suturing. The data should be considered cautiously due to the variety of procedures; however, this is likely to increase the generalizability of the results. Nevertheless, this difference was even more evident when only complications strictly related to vision, such as intraoperative injuries, leaks and fistulas, were considered. When including only RCTs and prospective studies, the benefit of the use of 3D increased showing a halving of complications, while maintaining no heterogeneity. This could be explained by the increasing task complexity with laparoscopic suturing. It appears that presence of stereoscopy seems to be crucial due to a more accurate appreciation of depth perception during this technique. This is consistent with the other studies which demonstrated superiority with 3D suturing technique [153]. Nevertheless, this benefit of 3D was observed as either a secondary endpoint in the vast majority of the studies or in small cohorts. Further robust research is required to confirm these findings and specifically investigate the true benefit of this technique on clinical outcomes.
Limitations
Our findings are in line with the available 2D/3D systematic reviews on this topic [75, 154, 155] which reported similar methodological concerns. In identified studies including a number of RCTs, significant heterogeneity was observed which limited the number of recommendations made. There is a clear need for further randomized studies that use validated and reproducible tasks or standardization of intervention delivery. Wherever possible equipment, viewing distance, table height and ergonomics should also be standardized. Compliance with the CONSORT statement, A-priori sample size calculations, homogenous participant groups, surgical experience, stereopsis visual assessment, validated blinded assessment methods and robust randomisation tools are additional considerations that would strengthen results and add to our understanding. Although we have identified that 3D imaging can assist surgical efficiency, no study attempted to assess quality of surgery or cost-effectiveness of this technology. Limited by study reporting, we could not investigate the effective of varying stereopsis abilities on outcome data.
It is well acknowledged that there is approximately 1–30% percent of the general population, and 9.7% of surgeons are stereo blind and would not be expected to benefit from 3D [156, 157]. This may limit the generalizability of our proposed recommendations and testing for stereo blindness is strongly advocated before clinical practice or research involving 3D systems.
Future research
This exercise has highlighted areas of equipoise as well as clinically important gaps in the literature and can serve as a guide for future clinical studies. Currently, there are 18 ongoing clinical trials that are related to this topic and already registered. Health economic analysis is required to evaluate the cost-effectiveness of 3D systems and the observed reduction in operative time and complication data. Dedicated research assessing the impact of 3D systems on clinical outcomes is indicated.
Conclusion
3D laparoscopic systems reduce procedure time in both the operating room and box trainer settings. 3D vision may also reduce perioperative complications particularly in procedures involving laparoscopic suturing.
References
Sakata S, Grove PM, Hill A, Watson MO, Stevenson AR (2016) The viewpoint-specific failure of modern 3D displays in laparoscopic surgery. Langenbecks Arch Surg 401(7):1007–1018
Schwab K, Smith R, Brown V, Whyte M, Jourdan I (2017) Evolution of stereoscopic imaging in surgery and recent advances. World J Gastrointest Endosc 9(8):368–377
Hanna GB, Shimi SM, Cuschieri A (1998) Randomised study of influence of two-dimensional versus three-dimensional imaging on performance of laparoscopic cholecystectomy. Lancet 351(9098):248–251
Vettoretto N, Foglia E, Ferrario L, Arezzo A, Cirocchi R, Cocorullo G et al (2018) Why laparoscopists may opt for three-dimensional view: a summary of the full HTA report on 3D versus 2D laparoscopy by S.I.C.E. (Societa Italiana di Chirurgia Endoscopica e Nuove Tecnologie). Surg Endosc 32(6):2986–2993
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S et al (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490
Goldet G, Howick J (2013) Understanding GRADE: an introduction. J Evid Based Med 6(1):50–54
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C (2011) Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE 6(6):e20476
Dalkey N, Helmer O (1963) An experimental application of the DELPHI method to the use of experts. Manag Sci 9(3):458–467
Hasson F, Keeney S, McKenna H (2000) Research guidelines for the Delphi survey technique. J Adv Nurs 32(4):1008–1015
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Schwarzer G (2007) Meta: an R package for meta-analysis. R News 7:40–45
Alaraimi BS, Sarker SJ, Elbakbak WS, Makkiyah S, Al-Marzouq A, Goriparthi RG et al (2013) Laparoscopic skills performance with stereoscopic vision as compared to the standard laparoscopic vision: a randomised control study. Int J Surg 11(8):593–594
Cicione A, Autorino R, Breda A, De Sio M, Damiano R, Fusco F et al (2013) Three-dimensional vs standard laparoscopy: comparative assessment using a validated program for laparoscopic urologic skills 82(6):1444–1450
Sinha R, Sundaram M, Raje S, Rao G, Sinha M, Sinha R (2013) 3D laparoscopy: technique and initial experience in 451 cases. Gynecol Surg 10:123–128
Storz P, Buess GF, Kunert W, Kirschniak A (2012) 3D HD versus 2D HD: surgical task efficiency in standardised phantom tasks. Surg Endosc Other Interv Tech 26(5):1454–1460
Herron DM, Lantis IJC, Maykel J, Basu C, Schwaitzberg SD (1999) The 3-D monitor and head-mounted display: a quantitative evaluation of advanced laparoscopic viewing technologies. Surg Endosc 13(8):751–755
Ashraf A, Collins D, Whelan M, O’Sullivan R, Balfe P (2015) Three-dimensional (3D) simulation versus two-dimensional (2D) enhances surgical skills acquisition in standardised laparoscopic tasks: a before and after study. Int J Surg 14:12–16
Leite M, Carvalho AF, Costa P, Pereira R, Moreira A, Rodrigues N et al (2016) Assessment of laparoscopic skills performance: 2D versus 3D vision and classic instrument versus new hand-held robotic device for laparoscopy. Surg Innov 23(1):52–61
Axt S (2016) Influence of the endoscope’s stereoscopic base on performance in standardized laparoscopic tasks: a prospective randomized controlled trial. Surg Endosc Other Interv Tech 30:S74
Morawala A, Almeida R, Merali N, Patel B (2016) Impact of 3-D laparoscopic surgical training on performance in standard 2-D laparoscopic surgery: a randomised prospective study. Surg Endosc Other Interv Tech 30:S190
Uemura M, Yamashita M, Tomikawa M, Obata S, Jimbo T, Matsuoka N et al (2016) Suggestion of novel measurement methodology for performance evaluation of medical equipment. Surg Endosc Other Interv Tech 30:S362
Ruan Y, Wang XH, Wang K, Zhao YY, Xia SJ, Xu DL (2016) Clinical evaluation and technical features of three-dimensional laparoscopic partial nephrectomy with selective segmental artery clamping. World J Urol 34(5):679–685
Buia A, Stockhausen F, Filmann N, Hanisch E (2017) 3D vs. 2D imaging in laparoscopic surgery—an advantage? Results of standardised black box training in laparoscopic surgery. Langenbeck’s Arch Surg 402(1):167–171
Poudel S, Kurashima Y, Watanabe Y, Ebihara Y, Tamoto E, Murakami S et al (2017) Impact of 3D in the training of basic laparoscopic skills and its transferability to 2D environment: a prospective randomized controlled trial. Surg Endosc Other Interv Tech 31(3):1111–1118
Sakata S, Grove PM, Hill A, Watson MO, Stevenson ARL (2017) Impact of simulated three-dimensional perception on precision of depth judgements, technical performance and perceived workload in laparoscopy. Br J Surg 104(8):1097–1106
Honeck P, Wendt-Nordahl G, Rassweiler J, Knoll T (2012) Three-dimensional laparoscopic imaging improves surgical performance on standardized ex-vivo laparoscopic tasks. J Endourol 26(8):1085–1088
Yalcin S, Kibar Y, Ozgok IY (2014) Which system is better for beginners’ laparoscopy training? glasses based full-hd 3D monitor systems or standard (full-hd 2D) monitor systems. J Endourol 28:A271
Ajao MO, Fuchs Weizman N, Goggins ER, Manoucheri E, Hur HC, Wang K et al (2015) Three-dimensional vision: does it improve acquisition of laparoscopic skills? J Minim Invasive Gynecol 22(6):S36
Alaraimi B, El Bakbak W, Sarker S, Makkiyah S, Al-Marzouq A, Goriparthi R et al (2014) A randomized prospective study comparing acquisition of laparoscopic skills in three-dimensional (3D) vs. two-dimensional (2D) laparoscopy. World J Surg 38(11):2746–2752
Autorino R, Cicione A, Breda A, De Sio M, Damiano R, Greco F et al (2013) Three-dimensional versus standard laparoscopy: comparative assessment using a validated program for laparoscopic urologic skills. J Endourol 27:A161–A162
Aykan S, Akin Y, Pelit ES, Gulmez H, Tuken M, Colakerol A et al (2017) Impact of motorized articulating laparoscopic devices with three-dimension visualizing system: a pilot study. J Endourol 31(2):174–179
Bucur P, Lusch A, Menhadji A, Liss MA, Okhunov Z, Landman J (2013) Evaluation of the impact of threedimensional vision on laparoscopic performance. J Endourol 27:A32
Chiu CJ, Lobo Prabhu K, Tan-Tam CCH, Panton ONM, Meneghetti A (2015) Using three-dimensional laparoscopy as a novel training tool for novice trainees compared with two-dimensional laparoscopy. Am J Surg 209(5):824–827
Cologne KG, Zehetner J, Liwanag L, Cash C, Senagore AJ, Lipham JC (2015) Three-dimensional laparoscopy: does improved visualization decrease the learning curve among trainees in advanced procedures? Surg Laparosc Endosc Percutan Tech 25(4):321–323
Davenport K, Burns A, Helo S, Bailey G, Peters C, Schenkman N (2012) Comparison of 3D stereoscope vs. standard 2D laparoscope for performance of two standard laparoscopic tasks by urology residents. J Urol 187(4):e611
Drosdeck JM, Renton DB (2014) The effect of three-dimensional versus two-dimensional imaging displays on task performance by laparoscopy-naïve subjects. Surg Endosc Other Interv Tech 28:324
Feng C, Rozenblit JW, Hamilton AJ (2010) A computerized assessment to compare the impact of standard, stereoscopic, and high-definition laparoscopic monitor displays on surgical technique. Surg Endosc Other Interv Tech 24(11):2743–2748
Feng X, Morandi A, Imvised T, Ure B, Kuebler JF, Lacher M (2015) Three-dimensional versus two-dimensional imaging in adult versus pediatric laparoscopy: a simulator box study. J Adv Surg Tech 25(12):1051–1056
Ghedi A, Donarini E, Lamera R, Sgroi G, Turati L, Ercole C (2015) 3D vs 2D laparoscopic systems: development of a performance quantitative validation model. In: Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference, pp 6884-6887
Guanà R, Ferrero L, Garofalo S, Cerrina A, Cussa D, Arezzo A et al (2017) Skills comparison in pediatric residents using a 2-dimensional versus a 3-dimensional high-definition camera in a pediatric laparoscopic simulator. J Surg Educ 74(4):644–649
Han KN, Kim HK, Lee HJ, Choi YH (2015) Simulation of single port endoscopic surgery: comparative study of two-with three-dimensional video system. Surg Endosc Other Interv Tech 29:S434
Elbakbak W, Alaramim B, Bouhelal A, Sarker SJ, Patel B (2013) Does 3D imaging improve laparoscopic intracorporeal suturing skill acquisition in novices and trainee surgeons? Int J Surg 11(8):706
Harada H, Kanaji S, Nishi M, Otake Y, Hasegawa H, Yamamoto M et al (2018) The learning effect of using stereoscopic vision in the early phase of laparoscopic surgical training for novices. Surg Endosc 32(2):582–588
Jones DB, Brewer JD, Soper NJ (1996) The influence of three-dimensional video systems on laparoscopic task performance. Surg Laparosc Endosc 6(3):191–197
Kan YM, Lee CL, Cheah WK, Seow CS, Tan DE, Foo JS (2013) 3D imaging in laparoscopy: improving training & skill aquistition for junior trainees. Surg Endosc Other Interv Tech 27:S418
Kommu S, Finnigan T, Cartlidge D, Golash A, Luscombe C, Sarg S (2009) Tandem two dimensional versus three dimensional viewing in learning curve for ex vivo skill acquision for laparoendoscopic single site surgery (LESS). J Endourol 23:A1
Lagrange CA, Clark CJ, Gerber EW, Strup SE (2008) Evaluation of three laparoscopic modalities robotics versus three-dimensional vision laparoscopy versus standard laparoscopy. J Endourol 22(3):511–516
Lin CJ, Cheng CF, Chen HJ, Wu KY (2017) Training performance of laparoscopic surgery in two- and three-dimensional displays. Surg Innov 24(2):162–170
Lu J, Hu J, Tan WB, Lomanto D (2015) Does 3D vision make a difference in laparoscopic skills acquisition? A randomized controlled trial. Surg Endosc Other Interv Tech 29:S437
Lusch A, Bucur PL, Menhadji AD, Okhunov Z, Liss MA, Perez-Lanzac A et al (2014) Evaluation of the impact of three-dimensional vision on laparoscopic performance. J Endourol 28(2):261–266
Kong SH, Oh BM, Yoon H, Ahn HS, Lee HJ, Chung SG et al (2010) Comparison of two- and three-dimensional camera systems in laparoscopic performance: a novel 3D system with one camera. Surg Endosc Other Interv Tech 24(5):1131–1143
Mashiach R, Mezhybovsky V, Ziv A, Gutman M, Goldenberg M (2013) Three-dimensional imaging improves surgical performance for both experienced and novice laparoscopic surgeons. J Minim Invasive 20(6):S17–S18
Matsunaga R, Nishizawa Y, Saito N, Kobayashi A, Ohdaira T, Ito M (2017) Quantitative evaluation of 3D imaging in laparoscopic surgery. Surg Today 47(4):440–444
Mistry M, Roach VA, Wilson TD (2013) Application of stereoscopic visualization on surgical skill acquisition in novices. J Surg 70(5):563–570
Morawala A, Alaraimi B, Patel B (2015) Validation of 3D (dimensional) models for training in laparoscopic surgery based on mistels for training and evaluation of laparoscopic skills. Surg Endosc Other Interv Tech 29:S300–S301
Nagao Y, Uemura M, Ishii H, Ohuchida K, Ieiri S, Morimasa T et al (2012) The effect of 3D monitoring system on single incision laparoscopic surgery. Surg Endosc Other Interv Tech 26:S403
Ng EK, Yip HC, Teoh AY (2016) A randomized study comparing the performance and learning curve of laparoscopic suturing by the novice using either 3D versus 2D laparoscopy systems. J Gastroenterol Hepatol 31:435
Nolan GJ, Howell S, Hewett P (2015) Impact of three-dimensional imaging in acquisition of laparoscopic skills in novice operators. J Adv Surg Tech 25(4):301–304
Noureldin YA, Stoica A, Kaneva P, Andonian S (2016) Impact of training on three-dimensional versus two-dimensional laparoscopic systems on acquisition of laparoscopic skills in novices: a prospective comparative pilot study. Biomed Res Int. https://doi.org/10.1155/2016/4197693
Ozsoy M, Kallidonis P, Kyriazis I, Panagopoulos V, Vasilas M, Sakellaropoulos GC et al (2015) Novice surgeons: do they benefit from 3D laparoscopy? Lasers Med Sci 30(4):1325–1333
Patel HR, Ribal MJ, Arya M, Nauth-Misir R, Joseph JV (2007) Is it worth revisiting laparoscopic three-dimensional visualization? A validated assessment. Urology 70(1):47–49
Peitgen K, Walz MV, Walz MV, Holtmann G, Eigler FW (1996) A prospective randomized experimental evaluation of three-dimensional imaging in laparoscopy. Gastrointest Endosc 44(3):262–267
Rabischong B, Compan C, Botchorishvili R, Bourdel N, Canis M (2014) Interest of a three-dimensional vision system in laparoscopic suturing on pelvi-trainer: a prospective comparative study among naïve medical students. J Minim Invasive 21(6):S90
Sakata S, Grove PM, Watson MO, Stevenson AR (2017) The impact of crosstalk on three-dimensional laparoscopic performance and workload. Surg Endosc 31:4044–4050
Schoenthaler M, Schnell D, Wilhelm K, Schlager D, Adams F, Hein S et al (2016) Stereoscopic (3D) versus monoscopic (2D) laparoscopy: comparative study of performance using advanced HD optical systems in a surgical simulator model. W J Urol 34(4):471–477
Shetty S, Wilk S, Bhamidipati V, Shaikh I, Palesty AJ (2013) The role of 3d visualization in laparoscopic simulation training. Surg Endosc Other Interv Tech 27:S341
Silvestri M, Simi M, Cavallotti C, Vatteroni M, Ferrari V, Freschi C et al (2011) Autostereoscopic three-dimensional viewer evaluation through comparison with conventional interfaces in laparoscopic surgery. Surg Innov 18(3):223–230
Smith R, Day A, Rockall T, Ballard K, Bailey M, Jourdan I (2012) Advanced stereoscopic projection technology significantly improves novice performance of minimally invasive surgical skills. Surg Endosc Other Interv Tech 26(6):1522–1527
Smith R, Schwab K, Day A, Rockall T, Ballard K, Bailey M et al (2014) Effect of passive polarizing three-dimensional displays on surgical performance for experienced laparoscopic surgeons. Br J Surg 101(11):1453–1459
Sorensen SMD, Konge L, Bjerrum F (2017) 3D vision accelerates laparoscopic proficiency and skills are transferable to 2D conditions: a randomized trial. Am J Surg 214(1):63–68
Sorensen SM, Savran MM, Konge L, Bjerrum F (2016) Three-dimensional versus two-dimensional vision in laparoscopy: a systematic review. Surg Endosc 30(1):11–23
Spille J, Wenners A, von Hehn U, Maass N, Pecks U, Mettler L et al (2017) 2D versus 3D in laparoscopic surgery by beginners and experts: a randomized controlled trial on a pelvitrainer in objectively graded surgical steps. J Surg Educ 74(5):867–877
Sun CC, Chiu AW, Chen KK, Chang LS (2000) Assessment of a three-dimensional operating system with shill tests in a pelvic trainer. Urol Int 64(3):154–158
Tanagho YS, Andriole GL, Paradis AG, Madison KM, Sandhu GS, Varela JE et al (2012) 2D versus 3D visualization: impact on laparoscopic proficiency using the fundamentals of laparoscopic surgery skill set. J Adv Surg Tech 22(9):865–870
Thomsen MN, Lang RD (2004) An experimental comparison of 3-dimensional and 2-dimensional endoscopic systems in a model. Arthroscopy 20(4):419–423
Tung KL, Yang GP, Li MK (2015) Comparative study of 2-D and bichanneled 3-D laparoscopic images: is there a difference? Asian J Endosc Surg 8(3):275–280
Usta TA, Ozkaynak A, Kovalak E, Ergul E, Naki MM, Kaya E (2015) An assessment of the new generation three-dimensional high definition laparoscopic vision system on surgical skills: a randomized prospective study. Surg Endosc Other Interv Tech 29(8):2305–2313
van Bergen P, Kunert W, Bessell J, Buess GF (1998) Comparative study of two-dimensional and three-dimensional vision systems for minimally invasive surgery. Surg Endosc 12(7):948–954
Vilaca JM, Ferreira-Fernandes S, Leite M, Correia-Pinto J, Leão P (2016) Less surgery in a porcine model: comparative study of 3D vs 2D. Surg Endosc Other Interv Tech 30:S54
Votanopoulos K, Brunicardi FC, Thornby J, Bellows CF (2008) Impact of three-dimensional vision in laparoscopic training. World J Surg 32(1):110–118
Wagner OJ, Hagen M, Kurmann A, Horgan S, Candinas D, Vorburger SA (2012) Three-dimensional vision enhances task performance independently of the surgical method. Surg Endosc Other Interv Tech 26(10):2961–2968
Wilhelm D, Reiser S, Kohn N, Witte M, Leiner U, Muhlbach L et al (2014) Comparative evaluation of HD 2D/3D laparoscopic monitors and benchmarking to a theoretically ideal 3D pseudodisplay: even well-experienced laparoscopists perform better with 3D. Surg Endosc 28(8):2387–2397
Wilhelm P, Dietz N, Axt S, Storz P, Kunert W, Falch C et al (2016) Effect of stereoscopic vision on the learning curve of laparoscopic training: a prospective randomized controlled trial. Surg Endosc Other Interv Tech 30:S4
Bhayani SB, Andriole GL (2005) Three-dimensional (3D) vision: does it improve laparoscopic skills? An assessment of a 3D head-mounted visualization system. Rev Urol 7(4):211–214
Bittner JG, Hathaway CA, Brown JA (2008) Three-dimensional visualisation and articulating instrumentation: impact on simulated laparoscopic tasks. J Minim Access Surg 4(2):31–38
Cicione A, Autorino R, Laguna MP, De Sio M, Micali S, Turna B et al (2015) Three-dimensional technology facilitates surgical performance of novice laparoscopy surgeons: a quantitative assessment on a porcine kidney model. Urology 85(6):1252–1256
Feng X, Morandi A, Boehne M, Imvised T, Ure BM, Kuebler JF et al (2015) 3-Dimensional (3D) laparoscopy improves operating time in small spaces without impact on hemodynamics and psychomental stress parameters of the surgeon. Surg Endosc Other Interv Tech 29(5):1231–1239
Folaranmi SE, Partridge RW, Brennan PM, Hennessey IA (2016) Does a 3D image improve laparoscopic motor skills? J Laparoendosc Adv Surg Tech A 26(8):671–673
Hanna GB, Cuschieri A (2000) Influence of two-dimensional and three-dimensional imaging on endoscopic bowel suturing. World J Surg 24(4):444–448 (discussion 8–9)
Kawanishi Y, Fujimoto Y, Kumagai N, Takemura M, Nonaka M, Nakai E et al (2013) Evaluation of two- and three-dimensional visualization for endoscopic endonasal surgery using a novel stereoendoscopic system in a novice: a comparison on a dry laboratory model. Acta Neurochir 155(9):1621–1627
Nishi M, Kanaji S, Otake Y, Harada H, Yamamoto M, Oshikiri T et al (2017) Quantitative comparison of operative skill using 2- and 3-dimensional monitors during laparoscopic phantom tasks. Surgery 161(5):1334–1340
Taffinder N, Smith SG, Huber J, Russell RC, Darzi A (1999) The effect of a second-generation 3D endoscope on the laparoscopic precision of novices and experienced surgeons. Surg Endosc 13(11):1087–1092
Foo JL, Martinez-Escobar M, Juhnke B, Cassidy K, Hisley K, Lobe T et al (2013) Evaluating mental workload of two-dimensional and three-dimensional visualization for anatomical structure localization. J Adv Surg Tech 23(1):65–70
Sakata S, Grove PM, Watson MO, Stevenson ARL (2017) The impact of crosstalk on three-dimensional laparoscopic performance and workload. Surg Endosc Other Interv Tech 31:4044–4050
Gomez-Gomez E, Carrasco-Valiente J, Valero-Rosa J, Campos-Hernandez JP, Anglada-Curado FJ, Carazo-Carazo JL et al (2015) Impact of 3D vision on mental workload and laparoscopic performance in inexperienced subjects. Actas Urol Esp 39(4):229–235
Zhou J, Xu HJ, Liang CZ, Zhang L, Hao ZY, Feng LX (2015) A comparative study of distinct ocular symptoms after performing laparoscopic surgical tasks using a three-dimensional surgical imaging system and a conventional two-dimensional surgical imaging system. J Endourol 29(7):816–820
Velayutham V, Fuks D, Nomi T, Kawaguchi Y, Gayet B (2016) 3D visualization reduces operating time when compared to high-definition 2D in laparoscopic liver resection: a case-matched study. Surg Endosc Other Interv Tech 30(1):147–153
Agrusa A, di Buono G, Chianetta D, Sorce V, Citarrella R, Galia M et al (2016) Three-dimensional (3D) versus two-dimensional (2D) laparoscopic adrenalectomy: a case-control study. Int J Surg 28:S114–S117
Kyriazis I, Ozsoy M, Kallidonis P, Vasilas M, Panagopoulos V, Liatsikos E (2015) Integrating three-dimensional vision in laparoscopy: the learning curve of an expert. J Endourol 29(6):657–660
Patankar SB, Padasalagi GR (2017) Three-dimensional versus two-dimensional laparoscopy in urology: a randomized study. Indian J Urol 33(3):226–229
Wahba R, Kleinert R, Hellmich M, Heiermann N, Dieplinger G, Schlosser HA et al (2017) Optimizing a living kidney donation program: transition to hand-assisted retroperitoneoscopic living donor nephrectomy and introduction of a passive polarizing three-dimensional display system. Surg Endosc 31(6):2577–2585
Kinoshita H, Nakagawa K, Usui Y, Iwamura M, Ito A, Miyajima A et al (2015) High-definition resolution three-dimensional imaging systems in laparoscopic radical prostatectomy: randomized comparative study with high-definition resolution two-dimensional systems. Surg Endosc Other Interv Tech 29(8):2203–2209
Hoffmann E, Bennich G, Larsen CR, Lindschou J, Jakobsen JC, Lassen PD (2017) 3-dimensional versus conventional laparoscopy for benign hysterectomy: protocol for a randomized clinical trial. BMC Women’s Health 17(1):76
Raspagliesi F, Bogani G, Martinelli F, Signorelli M, Scaffa C, Sabatucci I et al (2017) 3D vision improves outcomes in early cervical cancer treated with laparoscopic type B radical hysterectomy and pelvic lymphadenectomy. Tumori J 103(1):76–80
Fanfani F, Rossitto C, Restaino S, Ercoli A, Chiantera V, Monterossi G et al (2016) How technology can impact surgeon performance: a randomized trial comparing 3-dimensional versus 2-dimensional laparoscopy in gynecology oncology. J Minim Invasive 23(5):810–817
Lara-Dominguez MD, Lopez-Jimenez A, Grabowski JP, Arjona-Berral JE, Zapardiel I (2017) Prospective observational study comparing traditional laparoscopy and three-dimensional laparoscopy in gynecologic surgery. Int J Gynaecol Obstet 136(3):320–324
Qiu D, Zhuang H, Han F (2017) Effect and influence factor analysis of intrahepatic Glisson’s sheath vascular disconnection approach for anatomical hepatectomy by three-dimensional laparoscope. J BUON 22(1):157–161
Lu J, Zheng CH, Zheng HL, Li P, Xie JW, Wang JB et al (2017) Randomized, controlled trial comparing clinical outcomes of 3D and 2D laparoscopic surgery for gastric cancer: an interim report. Surg Endosc Other Interv Tech 31(7):2939–2945
Curro G, La Malfa G, Caizzone A, Rampulla V, Navarra G (2015) Three-dimensional (3D) versus two-dimensional (2D) laparoscopic bariatric surgery: a single-surgeon prospective randomized comparative study. Obes Surg 25(11):2120–2124
Leon P, Rivellini R, Giudici F, Sciuto A, Pirozzi F, Corcione F (2017) 3D vision provides shorter operative time and more accurate intraoperative surgical performance in laparoscopic hiatal hernia repair compared with 2D vision. Surg Innov 24(2):155–161
Curro G, La Malfa G, Lazzara S, Caizzone A, Fortugno A, Navarra G (2015) Three-dimensional versus two-dimensional laparoscopic cholecystectomy: is surgeon experience relevant? J Laparoendosc Adv Surg Tech A 25(7):566–570
Bilgen K, Ustun M, Karakahya M, Isik S, Sengul S, Cetinkunar S et al (2013) Comparison of 3D imaging and 2D imaging for performance time of laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 23(2):180–183
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed1000097
Bove P, Iacovelli V, Celestino F, De Carlo F, Vespasiani G, Finazzi Agro E (2015) 3D vs 2D laparoscopic radical prostatectomy in organ-confined prostate cancer: comparison of operative data and pentafecta rates: a single cohort study. BMC Urol 15:12
Aykan S, Singhal P, Nguyen DP, Yigit A, Tuken M, Yakut E et al (2014) Perioperative, pathologic, and early continence outcomes comparing three-dimensional and two-dimensional display systems for laparoscopic radical prostatectomy-a retrospective, single-surgeon study. J Endourol 28(5):539–543
Li Z, Li JP, Qin X, Xu BB, Han YD, Liu SD et al (2015) Three-dimensional vs two-dimensional video assisted thoracoscopic esophagectomy for patients with esophageal cancer. World J Gastroenterol 21(37):10675–10682
Padin EM, Santos RS, Fernandez SG, Jimenez AB, Fernandez SE, Dacosta EC et al (2017) Impact of three-dimensional laparoscopy in a bariatric surgery program: influence in the learning curve. Obes Surg 27:2552–2556
Curro G, Cogliandolo A, Bartolotta M, Navarra G (2016) Three-dimensional versus two-dimensional laparoscopic right hemicolectomy. J Laparoendosc Adv Surg Tech A 26(3):213–217
Tao K, Liu X, Deng M, Shi W, Gao J (2016) Three-dimensional against 2-dimensional laparoscopic colectomy for right-sided colon cancer. Surg Laparosc Endosc Percutan Tech 26(4):324–327
Currò G, Lazzara S, La Malfa G, Giovanni P, De Leo E, Fortugno A et al (2016) Three-dimensional (3D) versus two-dimensional (2D) laparoscopic oncological colorectal surgery: a single-surgeon prospective randomized comparative study. Eur J Surg Oncol 42(10):S206
Avram IO, Koukoulas D, Olariu S, Avram MF (2017) Laparoscopic cholecystectomy using 3D-vision: are there any benefits? Surg Endosc Other Interv Tech 31(2):S72
Sahu D, Mathew MJ, Reddy PK (2014) 3D laparoscopy—help or hype; initial experience of a tertiary health centre. J Clin Diagn Res 8(7):NC01–NC0C3
Ji F, Liu X, Liu Z, Fang X (2014) Application of three-dimensional laparoscopic system in obturator lymph node dissection of progressive rectal cancer. Zhonghua wei chang wai ke za zhi = Chinese. J Gastrointest Surg 17(11):1121–1124
Ji F, Fang X, Fei B (2017) Comparative study of 3D and 2D laparoscopic surgery for gastrointestinal tumors. Zhonghua wei chang wai ke za zhi 20(5):509–513
Avram IO, Olariu S, Koukoulas D, Avram MF (2017) Colorectal surgery using 3D-vision: benefits and setbacks. Surg Endosc Other Interv Tech 31(2):S81
Kanaji S, Suzuki S, Harada H, Nishi M, Yamamoto M, Matsuda T et al (2017) Comparison of two- and three-dimensional display for performance of laparoscopic total gastrectomy for gastric cancer. Langenbeck’s Arch Surg 402(3):493–500
Kaufman Y, Sharon A, Klein O, Spiegel D, Auslander R, Lissak A (2007) The three-dimensional “insect eye” laparoscopic imaging system—A prospective randomized study. Surgery 4(1):31–34
Fujii Y, Kihara K, Yoshida S, Ishioka J, Matsuoka Y, Numao N et al (2014) A three-dimensional head-mounted display system (RoboSurgeon system) for gasless laparoendoscopic single-port partial cystectomy. Wideochirurgia I Inne Techniki Maloinwazyjne 9(4):1–6
Kihara K, Fujii Y, Masuda H, Saito K, Koga F, Matsuoka Y et al (2012) New three-dimensional head-mounted display system, TMDU-S-3D system, for minimally invasive surgery application: procedures for gasless single-port radical nephrectomy. Int J Urol 19(9):886–889
Aesculap AG (2019) Prostatectomies using Einstein Vision® 3D [updated May]. https://ClinicalTrials.gov/show/NCT02991794. Accessed 22 Sept 2017
Brigham WH (2016) Does 3D laparoscopy improve vaginal cuff suture time? [updated August]. https://ClinicalTrials.gov/show/NCT02192606. Accessed 22 Sept 2017
Casa di Cura Dott P (2017) 3D laparoscopy versus 2D laparoscopy [updated October]. https://ClinicalTrials.gov/show/NCT02841657. Accessed 22 Sept 2017
Catholic University of the Sacred Heart, Fagotti AMD, Fanfani FMD (2015) 2D versus 3D radical laparoscopic hysterectomy for endometrial cancer: a prospective randomized trial [updated March]. https://ClinicalTrials.gov/show/NCT02320565. Accessed 22 Sept 2017
Catholic University of the Sacred Heart, Fanfani FMD, Fagotti AMD (2015) 2D versus 3D radical laparoscopic hysterectomy for cervical cancer: a prospective randomized trial [updated March]. https://ClinicalTrials.gov/show/NCT02320578. Accessed 22 Sept 2017
Chinese PLAGH (2019) 3D versus 2D laparoscopic total gastrectomy with splenic hilum lymph nodes dissection [updated December. https://ClinicalTrials.gov/show/NCT02984787. Accessed 22 Sept 2017
Clinical Research Management Centre (2017) Does 3D visualisation improve performance of laparoscopic cholecystectomy by junior surgeons? [updated September 30]. https://ClinicalTrials.gov/show/NCT03143426. Accessed 22 Sept 2017
Fujian Medical University (2016) Randomized controlled trials comparing clinical outcomes of 3D versus 2D laparoscopic surgery for gastric cancer [updated June]. https://ClinicalTrials.gov/show/NCT02327481. Accessed 22 Sept 2017
Helsinki University Central Hospital (2017) 3D vs 2D HD laparoscopy in cholecystectomy [updated May 10]. https://ClinicalTrials.gov/show/NCT02357589. Accessed 22 Sept 2017
Helsinki University Central Hospital (2020) 3D vs 2D HD laparoscopy in inguinal hernia repair [updated January]. https://ClinicalTrials.gov/show/NCT02367573. Accessed 22 Sept 2017
Herlev Hospital (2017) 3D HD versus 2D HD in cholecystectomy [updated September]. https://ClinicalTrials.gov/show/NCT02396927. Accessed 22 Sept 2017
Herlev Hospital (2018) 3D HD versus 2D HD in laparoscopic inguinal hernia repair: a randomized controlled trial [updated December]. https://ClinicalTrials.gov/show/NCT02396940. Accessed 22 Sept 2017
Olympus Corporation of the Americas, International Urogynecology Associates (2018) Laparoscopic three-dimensional versus two-dimensional sacral colpopexy and paravaginal repair [updated March]. https://ClinicalTrials.gov/show/NCT02258230. Accessed 22 Sept 2017
Royal Surrey County Hospital (2014) Investigating three-dimensional versus two-dimensional imaging in laparoscopic cholecystectomies [updated October]. https://ClinicalTrials.gov/show/NCT01930344. Accessed 22 Sept 2017
Royal Surrey County Hospital (2017) 3D versus 4K laparoscopic cholecystectomy [updated August 29]. https://ClinicalTrials.gov/show/NCT02858986. Accessed 22 Sept 2017
Shanghai First Maternity, Infant Hospital (2018) Analysis of TU-LESS by 3D laparoscopy in the treatment of infertility [updated December]. https://ClinicalTrials.gov/show/NCT02948205. Accessed 22 Sept 2017
The Cleveland C (2018) Evaluation of 3D visualization for total colectomy [updated December]. https://ClinicalTrials.gov/show/NCT02370056. Accessed 22 Sept 2017
Zealand University Hospital (2018) 2-D and 3-D laparoscopic hysterectomy [updated December]. https://ClinicalTrials.gov/show/NCT02610985. Accessed 22 Sept 2017
Gurusamy KS, Nagendran M, Toon CD, Davidson BR (2014) Laparoscopic surgical box model training for surgical trainees with limited prior laparoscopic experience. Cochrane Database Syst Rev 3:Cd010478
Lim S, Ghosh S, Niklewski P, Roy S (2017) Laparoscopic suturing as a barrier to broader adoption of laparoscopic surgery. Jsls. https://doi.org/10.4293/JSLS.2017.00021
Sakata S, Watson MO, Grove PM, Stevenson AR (2016) The conflicting evidence of three-dimensional displays in laparoscopy: a review of systems old and new. Ann Surg 263(2):234–239
Cheng J, Gao J, Shuai X, Wang G, Tao K (2016) Two-dimensional versus three-dimensional laparoscopy in surgical efficacy: a systematic review and meta-analysis. Oncotarget 7(43):70979–70990
Bohr I, Read JC (2013) Stereoacuity with Frisby and revised FD2 stereo tests. PLoS ONE 8(12):e82999
Bosten JM, Goodbourn PT, Lawrance-Owen AJ, Bargary G, Hogg RE, Mollon JD (2015) A population study of binocular function. Vision Res 110(Pt A):34–50
Acknowledgements
We would like to acknowledge Nicoletta Colombi for helping us in determining the correct syntax for the search strategy.
Funding
This project was supported and funded by the EAES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Nader Francis and Nathan J Curtis received technical support consisting of 3D stacks from Karl Storz Endoscopy UK Ltd as a free research loan for their study “The role of 3D laparoscopic rectal surgery: a randomised controlled trial” (ISRCTN59485808). Alberto Arezzo, Nereo Vettoretto, Marco Augusto Bonino, Daniele Amparore, Simone Arolfo, Manuel Barberio, Luigi Boni, Ronit Brodie, Nicole Bouvy, Elisa Cassinotti, Thomas Carus, Enrico Checcucci, Petra Custers, Michele Diana, Marilou Jansen, Joris Jaspers, Gadi Marom, Kota Momose, Beat P Müller-Stich, Kyokazu Nakajima, Felix Nickel, Silvana Perretta, Francesco Porpiglia, Francisco Sánchez-Margallo, Juan A Sánchez-Margallo, Marlies Schijven, Gianfranco Silecchia, Roberto Passera and Yoav Mintz have no conflict of interest or financial ties to disclose.
Research involving human and animal participants
This article does not contain any original study with human or animal subjects.
Rights and permissions
About this article
Cite this article
Arezzo, A., Vettoretto, N., Francis, N.K. et al. The use of 3D laparoscopic imaging systems in surgery: EAES consensus development conference 2018. Surg Endosc 33, 3251–3274 (2019). https://doi.org/10.1007/s00464-018-06612-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-06612-x